13.3
Impact Factor
Theranostics 2026; 16(2):712-735. doi:10.7150/thno.122577 This issue Cite
Review
Endocrine nanozymology: Nanozyme applications in diabetes, obesity, and hormonal disorders
1. Department of Geriatrics, Shenzhen Longgang Second People's Hospital, Shenzhen, Guangdong, China.
2. Internal Medicine Ward I, Shenzhen Longgang Second People's Hospital, Shenzhen, Guangdong, China.
3. Department of Neurosurgery, Institute of Translational Medicine, Shenzhen Second People's Hospital/The First Affiliated Hospital of Shenzhen University, Shenzhen, China.
4. Premium Research SL, 19003, Guadalajara, Spain.
5. Endocrine Disorders Group, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS)/ Hospital Clínic, Barcelona, Spain.
6. Endocrinology Department, Hospital Clínic, Barcelona, Spain.
7. Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Spain.
8. Department of Biochemistry and Molecular Genetics, Hospital Clínic, FRCB-IDIBAPS, Barcelona, Spain.
9. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Spain.
10. Department of Biomedicine, Faculty of Medicine and Health Science, University of Barcelona, 08036 Barcelona, Spain.
11. Institut de Ciència de Materials de Barcelona, ICMAB (CSIC), Campus UAB, Bellaterra, Spain.
12. Department of Fundamental and Clinical Nursing, Faculty of Nursing, University of Barcelona, L'Hospitalet de Llobregat, 08907 Barcelona, Spain.
* these authors contributed equally
Received 2025-7-28; Accepted 2025-9-27; Published 2026-1-1
Abstract
Nanozymes, engineered nanomaterials with enzyme-like catalytic activity, are emerging as versatile tools in biomedicine due to their catalytic tunability and higher chemical, thermal, and structural stability compared to natural enzymes. While widely studied in oncology and inflammation, their potential in endocrine disorders remains comparatively underexplored due to the historical focus of nanomedicine on cancer-related oxidative stress, the complexity and heterogeneity of endocrine signaling networks that hinder direct translation, and the scarcity of preclinical models that capture the dynamic and systemic nature of endocrine physiology. However, disruption of hormonal homeostasis by free radical imbalance points to the significant potential of nanozymes in endocrine disorders. By mimicking redox-active enzymes such as catalase, superoxide dismutase, and peroxidase, nanozymes regulate reactive oxygen species (ROS), thereby influencing hormone biosynthesis, receptor sensitivity, and redox signaling. They also offer advantages such as composite architectures, targeted delivery, and integration into smart platforms like hydrogels and biosensors. This review explores the expanding role of nanozymes in endocrine and metabolic diseases, including diabetes, obesity, thyroid and adrenal dysfunctions, and reproductive disorders. We highlight advances in glucose biosensing, hormone detection, redox-targeted therapies, and regenerative approaches. Despite promising preclinical data, there is a lack of clinical trials and long-term biosafety assessments of nanozymes, underscoring the need for further translational studies. By bridging nanotechnology and hormonal regulation, we outline future research directions toward integrating nanozymes into endocrine diagnostics and therapeutics.
Keywords: nanozymes, diabetes mellitus, thyroid, adrenal, metal-organic frameworks
1. Nanozymes in biomedicine: catalytic properties and classifications
The advent of nanotechnology has led to the emergence of nanozymes (NZs), a class of nanomaterials that mimic the catalytic activities of natural enzymes. Since their conceptual introduction in 2004 and the first demonstration of intrinsic enzyme-like activity in ferromagnetic nanoparticles in 2007 [1], NZs have attracted widespread attention for their robust catalytic activity, physicochemical stability, and cost-effectiveness. Unlike natural enzymes, which are often susceptible to environmental conditions such as temperature and pH, NZs provide a stable alternative for various catalytic processes, particularly in biomedical settings. Their multifunctionality and tunability have enabled their integration into fields ranging from biosensing and diagnostics to targeted therapy and imaging.
NZs are generally classified based on their elemental composition and structure, with each category possessing unique catalytic properties and mechanisms. The major categories include metal-based, carbon-based, metal sulfide-based, rare-earth element-based, and hybrid NZs. Table 1 summarizes their catalytic activities, key advantages, and associated risks.
Metal-based NZs are among the most extensively studied due to their diverse electronic configurations and redox potential. Iron oxide nanozymes (Fe3O4NZs), for instance, exhibit peroxidase-like activity by catalyzing the decomposition of hydrogen peroxide (H2O2) into reactive oxygen species (ROS), a property that has proven valuable in inducing oxidative stress in tumor microenvironments [1,2]. The mechanism involves the redox cycling between Fe2+ and Fe3+ states, which facilitates electron transfer during catalytic reactions. Gold nanozymes (AuNZs) present oxidase-like activity, which enables them to catalyze oxidation reactions without the need for H2O2, making them particularly suitable for biosensing and colorimetric assays [3]. The mechanism of action in AuNZs is influenced by their surface plasmon resonance and electron-rich surface. Cerium oxide nanozymes (CeO2NZs) mimic both catalase and superoxide dismutase (SOD) activities, attributed to the redox cycling between Ce3+ and Ce4+. This dual functionality allows CeO2NZs to scavenge ROS and reduce oxidative damage in neurodegenerative diseases and inflammatory conditions [4]. Copper oxide nanozymes (CuONZs) possess both oxidase and peroxidase-like activities. Their efficacy in antimicrobial applications stems from their ability to generate ROS and disrupt bacterial membranes [5].
Carbon-based NZs include materials such as graphene oxide and carbon nanotubes. Graphene oxide displays peroxidase- and oxidase-like activities due to its high surface area and electronic conductivity. These properties enhance their catalytic efficiency in redox reactions, especially in biosensing and environmental remediation [6]. Carbon nanotubes, owing to their tubular morphology and high aspect ratio, facilitate electron transport, thereby enhancing catalytic performance. Their application ranges from electrochemical sensors to nanocarriers for drug delivery [7].
Metal sulfide NZs are an emerging class of materials known for their semiconducting properties. Cadmium sulfide nanozymes (CdSNZs) exhibit light-dependent photocatalytic activity. Under visible light irradiation, CdSNZs can generate ROS through photoinduced electron-hole pair formation, making them effective agents in photodynamic therapy [8]. Molybdenum disulfide nanozymes (MoS2NZs), on the other hand, possess intrinsic peroxidase-like activity. Their two-dimensional layered structure and sulfur-rich surface facilitate catalytic decomposition of H2O2, enabling their application in biosensors and tumor therapy [9].
Rare-earth elements, such as lanthanum and europium, form NZs with distinctive electronic properties. Lanthanum-based NZs exhibit catalytic activity through electron transfer mechanisms, contributing to applications in medical imaging and targeted therapy [10,11]. Europium-containing nanoparticles are used in luminescent biosensing due to their strong photoluminescent properties, driven by intra-4f transitions. These materials can serve dual functions in diagnostics and imaging-guided therapy [11].
To enhance functionality, researchers have developed hybrid NZs that combine multiple catalytic centers or integrate organic and inorganic components. Metal-organic frameworks (MOFs), composed of metal nodes and organic linkers, offer tunable structures with intrinsic enzyme-like activity. Their porous nature and catalytic versatility make them suitable for applications in drug delivery and pollutant degradation [12]. Core-shell structured NZs, which include a catalytically active core surrounded by a protective or functional shell, allow for improved stability, biocompatibility, and targeted activity. These structures are particularly useful in theranostics, combining therapeutic and diagnostic functions in a single platform [13].
2. Diagnostic and therapeutic applications of nanozymes. Advancing toward endocrine applications
2.1. Main applications: from cancer to biosensing
The biomedical applications of NZs span a diverse spectrum of therapeutic and diagnostic fields. In cancer therapy, for instance, NZs have been harnessed to exploit the elevated oxidative stress within tumor microenvironments. Fe3O4 and CeO2 NZs are of particular interest in this regard. These nanomaterials catalyze the decomposition of H2O2, which is abundantly present in tumors, to generate ROS, causing selective oxidative damage in cancer cells without affecting the healthy tissues.
Catalytic Activity, Advantages, and Limitations of Nanozyme Types
| Nanozyme class | Dominant catalytic activities | Application advantages | Limitations or risks |
|---|---|---|---|
| Metal‑based | Fe₃O₄: peroxidase‑like (Fenton/Fenton‑like); Au: oxidase‑like; CeO₂: catalase‑ and SOD‑like (Ce³⁺/Ce⁴⁺ redox); CuO: oxidase and peroxidase; noble metals (Pt/Pd/Ru): strong peroxidase‑like | High catalytic turnover; robust redox cycling; straightforward surface functionalization; strong optical/electronic features for biosensing and imaging | Potential metal ion release; off‑target ROS generation; long‑term tissue accumulation; pH‑dependent; batch‑to‑batch heterogeneity in synthesis |
| Carbon‑based (graphene oxide, carbon nanotubes, carbon dots) | Peroxidase‑ and oxidase‑like via defect/edge sites and π‑electron networks | Large surface area and conductivity enhance electron transfer; easy hybridization with biomolecules/electrodes; good signal amplification for electrochemical or colorimetric sensing | Possible heteroatom/impurity variability; biocompatibility depends on surface chemistry; catalytic activity can be modest without doping or defects |
| Metal sulfide‑based (CdS, MoS) | CdS: light‑driven photocatalysis generating ROS; MoS₂: intrinsic peroxidase‑like; some show photo‑enhanced peroxidase | Semiconducting band structures enable controllable, light‑activated therapy; 2D morphologies favor high active‑site exposure | Phototoxic or ion toxicity concerns; may require light delivery and O2; stability and oxidative leaching under physiological conditions |
| Rare‑earth element‑based (Ce, La, Eu) | CeO₂: catalase/SOD‑like; La‑based: redox/electron‑transfer catalysis; Eu‑based: luminescent probes with auxiliary catalytic roles | Intrinsic antioxidant mimicry (redox buffering) plus strong, tunable luminescence for imaging/theragnostic platforms | Valence‑state control is sensitive to environment; dopant alters activity; potential long‑term retention; unclear clearance profiles |
| Hybrid/Composite (MOFs, core-shell, NP@MOF) | Multi‑enzyme cascades (e.g., GOx & peroxidase/catalase in one construct); porous frameworks can host catalytic centers | Programmable porosity and multivalent active sites; co‑loading of drugs/enzymes for synergistic therapy; improved stability and targeting via shells | Framework stability in biological fluids varies; metal nodes and ligand can contribute to toxicity; synthesis scalability and standardization remain challenging |
GOx: Glucose oxidase; MOFs: Metal-organic frameworks; ROS: Reactive oxygen species; SOD: Superoxide dismutase
This selective cytotoxicity, combined with the potential to engineer surface functionalities for targeted delivery, positions NZs as powerful tools for precision oncology [2,4,14].
Beyond oncology, NZs show promise in neurodegenerative diseases, although more research is needed to confirm their ability to cross the blood-brain barrier. In Alzheimer's and Parkinson's disease, elevated ROS leads to neuronal damage. CeO2NZs, through their capacity to scavenge superoxide and H2O2 through Ce3+/Ce4+ redox cycling, offer neuroprotective benefits by maintaining redox homeostasis and reducing neuroinflammation [15].
Infectious diseases and wound healing also constitute a vital application domain for NZs. CuO and other transition metal-based NZs have emerged as potent antibacterial agents, primarily through their ability to generate ROS and disrupt microbial membranes. These NZs do not rely on traditional antibiotic pathways, thereby reducing risk of resistance. They can be embedded in hydrogels or device coatings to create antimicrobial environments [5,16].
NZs have also revolutionized biosensing and diagnostics. Their intrinsic catalytic activity enables signal amplification, allowing for the detection of biomarkers at ultra-low concentrations. Au NZs, with their excellent oxidase-like activity and ease of functionalization, are extensively used in colorimetric and electrochemical biosensors for the rapid detection of glucose, cholesterol, pathogens, and tumor markers in clinical samples. Their adaptability and robustness make them promising candidates for point-of-care and wearable biosensing technologies [3,17].
In addition to therapy and diagnostics, NZs play a pivotal role in bioimaging and theranostics. Lanthanide-doped and europium-based NZs possess unique luminescent properties that enable high-resolution imaging. When integrated with magnetic or catalytic functionalities, these NZs can simultaneously facilitate imaging and treatment, allowing for real-time monitoring of therapeutic responses. For instance, gadolinium-doped NZs support both MRI imaging and therapeutic modulation [11,18].
2.2. Endocrine nanozymology: a new frontier in hormonal disease management
As shown, NZs offer innovative and highly adaptable solutions across multiple biomedical domains. Their enzyme-mimetic activities, combined with superior stability, cost-efficiency, and functional tunability, address long-standing limitations of natural enzymes and unlock new possibilities in diagnostics, imaging, and therapeutics. Of particular interest is the growing body of evidence pointing to the applicability of NZs in the context of metabolic and endocrine disorders. In recent years, an increasing number of studies have begun to explore the role of NZs in the diagnosis and management of diabetes mellitus and other endocrine dysfunctions. Despite this emerging potential, NZs remain underutilized in endocrinology, due not only to the historical focus of nanomedicine on cancer, but more importantly to the inherent complexity of hormonal signaling. Endocrine disorders, by their very nature, are systemic diseases that affect multiple organs through hormonal dysregulation, a distinctive feature that sets them apart from other medical disciplines. This systemic reach, combined with the dynamic and heterogeneous nature of endocrine networks, presents unique challenges for diagnosis and treatment. These factors justify the emergence of endocrine nanozymology as a distinct interdisciplinary field, integrating nanotechnology and enzymology to address the systemic complexity of endocrine dysfunctions.
Unlike previous reviews that primarily focus on oncology or general biosensing applications, this work provides a comprehensive and integrative overview of NZ applications specifically in endocrine disorders, including underexplored areas such as circadian rhythm regulation and hormone-responsive theranostic platforms. By providing an integrated perspective on their catalytic properties and biomedical utility, the following sections aim to highlight not only the present achievements of NZ research but also its promising future in the diagnosis, monitoring, and treatment of endocrine-related diseases. While the core applications of this research focus on conditions such as diabetes, atherosclerosis, and obesity, emerging areas of interest include thyroid, adrenal, and chromaffin tumors. Notably, many of these endocrine disorders disproportionately affect women, emphasizing the potential of nanozyme-based innovations to help address gender-related disparities in diagnosis and care.
3. Translational hurdles of nanozymes
Despite their promise, the transition from promising laboratory findings to clinically viable solutions remains hindered by several unresolved scientific and regulatory challenges.
Toxicity and biocompatibility. NZs can induce oxidative stress, inflammation, and cytotoxicity due to their high surface reactivity and catalytic activity. For example, iron oxide and copper-based nanozymes generate ROS, which can damage cellular components [19]. The shape, charge, and surface chemistry of NZs influence their interaction with biological systems, potentially triggering immune responses or membrane disruption [20]. Surface modifications, such as PEGylation or biomimetic coatings, have been shown to reduce toxicity and improve circulation times [21].
Biodistribution and clearance. NZs often accumulate in non-target organs like the liver, spleen, and kidneys due to uptake by the reticuloendothelial system, raising concerns about long-term safety [21]. Their clearance is influenced by size, surface charge, and protein corona formation. Inter-individual variability in metabolism and immune response further complicates pharmacokinetics, necessitating personalized design strategies [22].
Synthesis, scalability, and reproducibility. While NZs can be synthesized via various chemical and physical methods, scaling up production while maintaining consistent catalytic activity and morphology remains a challenge [20]. Batch-to-batch variability affects therapeutic efficacy and safety. Good Manufacturing Practice (GMP) compliance requires stringent control over particle size, shape, and surface functionalization, which is not yet standardized for NZs [21].
Regulatory ambiguity. NZs occupy a regulatory gray zone, often falling between drugs, biologics, and devices. Agencies like the FDA and EMA lack specific guidelines for NZ evaluation, delaying clinical translation [19]. The absence of harmonized international standards complicates toxicity testing, quality control, and approval pathways [23].
Mechanistic uncertainty and predictive modeling. Unlike natural enzymes, NZs may exhibit multi-modal or context-dependent catalytic behaviors. Their activity can be influenced by environmental factors such as pH, temperature, and macromolecular crowding [22]. This complexity hampers mechanistic understanding and predictive modeling. Emerging tools like machine learning and systems toxicology are being explored to forecast nanozyme behavior and optimize design [24].
Environmental and long-term safety. Concerns about environmental persistence and bioaccumulation of NZs are growing. Their long-term effects on ecosystems and human health remain poorly understood. Studies suggest that chronic exposure could lead to epigenetic changes, lysosomal destabilization, and immunogenicity [23]. Comprehensive life-cycle assessments and environmental impact studies are urgently needed to ensure biosafety [25].
4. Diabetes Mellitus, insulin resistance, obesity, and inflammation
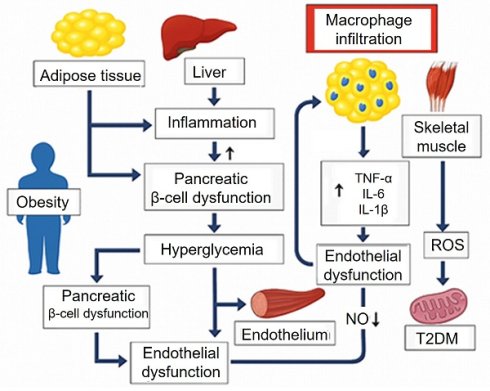
Diabetes mellitus represents a complex interplay of metabolic and endocrine disturbances that often coexist and exacerbate other endocrine disorders. These conditions are interlinked through common mechanisms such as insulin resistance, dysregulation of hormonal axes, chronic inflammation, and metabolic disturbances, which not only affect endocrine function but also contribute to the development of atherosclerosis, impaired wound healing, and other vascular complications. The intricate relationships between these diseases (Figure 1) underscore the need for a unified approach to treatment that addresses the shared pathological mechanisms and their systemic effects.
One of the central features of type 2 diabetes mellitus (T2DM) is insulin resistance, where tissues such as skeletal muscle, liver, and adipose tissue become less responsive to insulin. This leads to elevated blood glucose levels (hyperglycemia) and dysregulated lipid metabolism, both of which contribute to the development of atherosclerosis, a process characterized by the thickening and hardening of arterial walls due to lipid deposition and inflammation [26]. Insulin resistance also promotes endothelial dysfunction, an early event in atherosclerosis, where the ability of blood vessels to dilate and contract properly is impaired. This is compounded by increased levels of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which promote the recruitment of immune cells to the vessel walls and increase vascular permeability, further accelerating the atherosclerotic process [27].
Obesity, often a precursor to insulin resistance, exacerbates this process. Visceral fat in particular releases pro-inflammatory cytokines and adipokines, such as adiponectin and leptin, which influence insulin sensitivity and promote inflammatory pathways in the vasculature, accelerating the progression of atherosclerosis [28]. Additionally, hyperglycemia in diabetes increases the accumulation of advanced glycation end-products, leading to further endothelial injury, oxidative stress, and inflammation.
Impaired wound healing is a hallmark of diabetes mellitus, particularly in patients with poorly controlled blood glucose levels. High glucose levels disrupt the normal function of keratinocytes and fibroblasts, impair collagen deposition, and reduce angiogenesis, all of which are essential for effective wound healing. Moreover, chronic inflammation and increased oxidative stress in diabetes exacerbate tissue damage and delay the repair process. In addition to diabetes, hypothyroidism contributes to poor wound healing by impairing collagen synthesis and reducing fibroblast activity [29].
The interlinked mechanisms reveal a shared pathophysiological foundation involving insulin resistance, chronic inflammation, endothelial dysfunction, and metabolic dysregulation. These conditions often coexist and exacerbate one another. Understanding both the shared and distinct pathways underlying these disorders enables the development of more precise and effective therapeutic strategies. The NZ approaches should not only target the specific endocrine dysfunctions but also address the systemic metabolic and vascular disruptions they provoke [30].
Pathophysiological mechanisms linking insulin resistance, obesity, type 2 diabetes mellitus, and atherosclerosis. Obesity and insulin resistance promote chronic inflammation, dyslipidemia, and endothelial dysfunction. These processes impair glucose metabolism, damage vascular integrity, and accelerate atherogenesis, contributing to the development and progression of type 2 diabetes mellitus and atherosclerosis. AGEs: Advanced Glycation End-products; IL: interleukin; NF-kB: Nuclear Factor kappa-light-chain-enhancer of activated B cells; NO: Nitric Oxide; ROS: Reactive Oxygen Species; T2DM: Type 2 Diabetes Mellitus; TNF-α: Tumor Necrosis Factor-alpha.
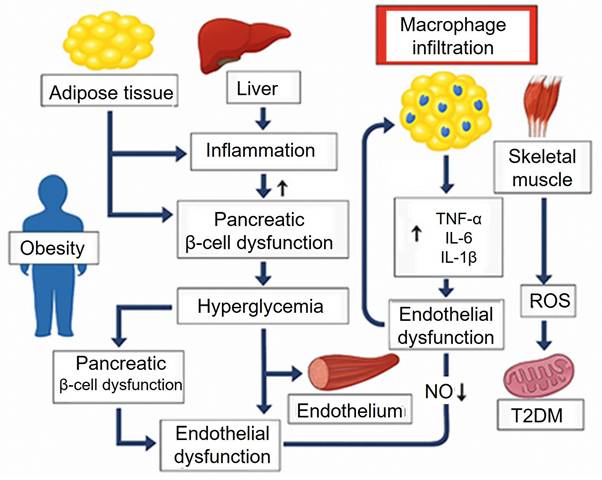
Nanozyme-based colorimetric biosensors for glucose detection and diabetes management. Schematic overview of nanozyme materials—classified as organic or organometallic—and their associated enzyme-mimicking activities (laccase, peroxidase, catalase, oxidase), detection methods (fluorescence, colorimetry, NIR spectroscopy), and color correction strategies using deep learning and color space model. Reproduced from Jeon et al. [35]. This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
5. Nanozymes in Diabetes Mellitus: diagnostic, therapeutic, and nanotheranostic applications
5.1. Diagnostic applications of nanozymes
Glucose detection in blood, urine, and saliva. Portable and efficient devices
One of the primary diagnostic uses of NZs in diabetes management is the detection of glucose levels in biological fluids such as blood, urine, or saliva. For instance, Lee et al. developed a glucose oxidase-conjugated graphene oxide/MnO2 nanozyme, which exhibited horseradish peroxidase-mimic activity to catalyze the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2, allowing direct detection of glucose in whole blood. This platform demonstrated a wide linear quantification range from 25 mg/dL to 300 mg/dL, providing a method for glucose monitoring without the need for blood sample pretreatment [31]. Similarly, Karim et al. developed a silver nanoparticle-embedded cotton fabric-based nanozyme sensor for the colorimetric detection of glucose in urine. This system was advantageous due to the high surface area of cotton fibers that facilitated the adsorption and detection of glucose molecules, allowing for rapid glucose measurements in urine [32]. Also, Naveen Prasad et al. introduced a copper nanozyme for non-invasive glucose detection in human urine. The copper nanozyme demonstrated the ability to catalyze the oxidation of TMB to its double oxidation diimine product, rather than the charge-transfer complex, providing a glucose measurement with minimal sample processing [33]. Roy et al. evaluated biocompatible nano-hybrids composed of Mn3O4 nanoparticles for the non-invasive detection of glucose in human saliva [34].
NZ-based colorimetric biosensors for noninvasive glucose monitoring have been the subject of extensive scientific investigation. As recently reviewed by Jeon et al. [35], other biofluids analyzed included sweat, tears, and interstitial fluids. The primary detection methods employed were optical and electrochemical techniques. Among the nanomaterials used, AuNZs were the most common, although a diverse range of other NZs have also been explored, including CuInS₂, SiO₂, ZnO, and CuO [35]. Yi et al. reported a smartphone-based, paper-based analytical device utilizing an acetylene black-hemin NZ complex for glucose detection in blood. Compared with graphene oxide-supported hemin, acetylene black-hemin exhibited superior signal response on paper. The device provided a linear detection range from 3.6 mg/dL to 540 mg/dL, demonstrating potential for use in diabetes diagnostics [36]. Similarly, Khachornsakkul et al. developed a microfluidic paper-based analytical device using gold and silver nanoparticles as peroxidase-like NZs and a colorimetric probe to monitor glucose levels using a smartphone application, achieving clinically relevant glucose detection in 20 minutes [37].
Achievements and challenges. As shown, recent progress in NZs has greatly advanced colorimetric-based glucose biosensing. Their use enhances the practicality of simple, visual glucose detection, particularly when combined with digital tools like smartphone-based analysis. The reported studies, nevertheless, stand only at the in vitro diagnostic level, highlighting both the promise and the current limitations of this field. However, these cases demonstrate how NZs have been adapted to various biological fluids (blood, urine, saliva, sweat, and tears), each with tailored design strategies. For instance, graphene oxide/MnO₂ NZs enable direct blood glucose detection without pretreatment, while silver nanoparticle-embedded cotton fabrics enhance surface interaction for rapid urine analysis. Copper NZs improve signal clarity by producing stable diimine products, and Mn₃O₄ hybrids offer biocompatibility for saliva-based sensing. Smartphone-integrated platforms using acetylene black-hemin or gold/silver NZs in paper-based and microfluidic formats provide fast, portable, and user-friendly diagnostics. Despite these advances, several challenges remain. Most NZs primarily mimic peroxidase activity, limiting the range of catalytic functions and detectable analytes. The accuracy of colorimetric detection is also affected by environmental conditions and variations in smartphone camera performance, reducing reliability and reproducibility. Moreover, potential toxicity, especially from metal-based NZs, raises safety concerns for wearable or implantable applications, necessitating further toxicological studies. Machine learning offers promising solutions by enabling smarter nanozyme design and more accurate image analysis. Nonetheless, limitations in catalytic activity, fabrication complexity, and safety still hinder clinical and commercial adoption. Continued interdisciplinary research is essential to overcome these barriers and fully realize the potential of nanozyme-based biosensors.
5.2. Therapeutic and theragnostic potential of nanozymes in Diabetes Mellitus
The application of NZs in the treatment of diabetes mellitus is based on their ability to modulate oxidative stress, inflammation, insulin resistance, and hyperglycemia, which are critical contributors to the pathogenesis of diabetes.
Therapeutic applications improving insulin resistance
Wang et al. [38] demonstrated the therapeutic potential of Au@Pt NZs, which were found to enhance glucose tolerance and reduce triglyceride levels in high-fat diet-induced diabetic mice. Their multi-omics analysis revealed that Au@Pt NZs treatment modulated critical metabolic pathways such as glycolysis, pyruvate metabolism, PPAR signaling, and insulin signaling. The findings suggest that Au@Pt NZs can target both the liver and the gut microbiome [38]. Similarly, Song et al. [39] explored the use of a single-atom Ce-N4-C-(OH)2 NZs that promoted glucose absorption in lysosomes, leading to increased reactive ROS production in human hepatic HepG2 cells and initiating a cascade reaction involving SOD-like, oxidase-like, catalase-like, and peroxidase-like activities. This significantly improved insulin sensitivity and glucose tolerance in diabetic mice without significant toxicity [39]. In another study, Zhou et al. [40] used Fe3O4 NZs to activate adenosine monophosphate-activated protein kinase (AMPK), an essential regulator of glucose metabolism, within lysosomes. By enhancing glucose uptake and insulin sensitivity, Fe3O4 NZs improved the metabolic profile in genetically and diet-induced diabetic models. This emphasizes the organelle-specific properties of Fe3O4 NZs in managing hyperglycemia and insulin resistance [40]. Lin et al. [41] introduced a single-atom Ce-N-C NZ that improved insulin resistance and promoted glucose metabolism in both cell cultures and mouse models of type 2 diabetes. All these studies were conducted at the in vivo level in diabetic mice but have not yet progressed to human clinical studies.
Therapeutic applications targeting oxidative stress
Zhang et al. [42] targeted the liver using a biodegradable silica nanoshell embedded with platinum NZs (Pt-SiO2) acting as a ROS scavenger, mitigating oxidative stress and restoring glucose consumption in the liver. The Pt-SiO2 NZ significantly improved glucose tolerance, insulin resistance, and lipid accumulation in diabetic mice. Moreover, Li et al. [43] demonstrated the efficacy of CeO2 NZs in improving skeletal muscle function in gestational diabetes mellitus offspring. By attenuating mitochondrial oxidative stress and restoring mitochondrial activity, CeO2 NZs enhanced insulin sensitivity and skeletal muscle motility, suggesting a potential therapeutic approach for mitigating the long-term effects of gestational diabetes on offspring.
Therapeutic applications modulating inflammation
Shen et al. [44] designed AuCePt porous hollow cascade NZs for targeted delivery of disulfiram, a drug known to reduce hepatic insulin resistance. The multifunctional AuCePt NZs exhibited SOD and catalase-like activities, enabling them to scavenge ROS and improve glucose uptake in insulin-resistant hepatocytes. The targeted drug delivery system demonstrated therapeutic effects in diabetic mice [44].
Therapeutic applications regulating hyperglycemia and glucose homeostasis
Therapeutic strategies may additionally focus on direct regulation of glucose levels. For example, Ce-N₄-C NZs improved glucose tolerance through lysosomal modulation [39]. Kim and Kim [45] developed a glucose-responsive protein delivery system model enabling glucose-responsive protein release in vitro, offering potential for closed-loop insulin delivery in diabetes management.
Theranostic approaches
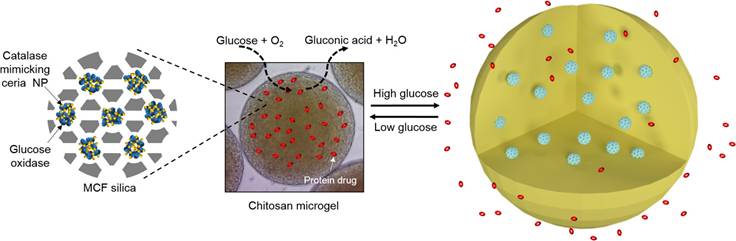
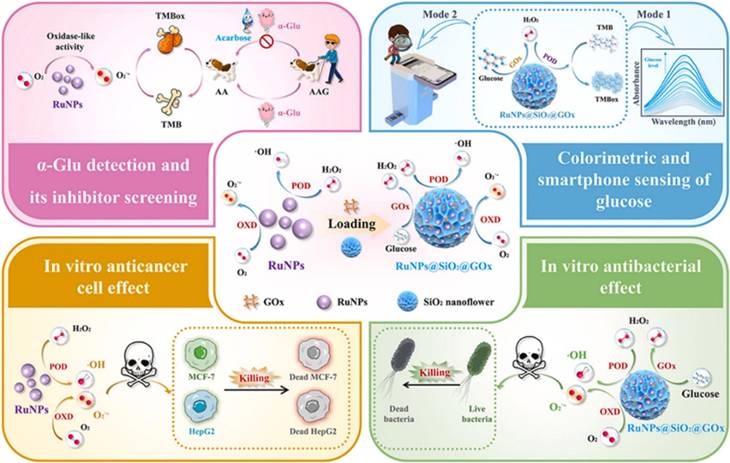
In addition to therapeutic applications described above, NZs have gained significant attention for their theragnostic potential, combining diagnostic and therapeutic functions in a single platform. As previously mentioned, Kim and Kim [45] developed a glucose-responsive protein delivery system utilizing chitosan microgels integrated with enzyme-mimicking inorganic nanoparticles. These pH-sensitive microgels were fabricated via electrospray and embedded with mesocellular foam (MCF) of large-pore mesoporous silica. The MCF was loaded with CeO2 NZs, which exhibit catalase-like activity, and glucose oxidase (GOx). In hyperglycemic conditions, GOx catalyzes glucose oxidation, producing H₂O₂. CeO2 decomposes the H₂O₂, regenerating oxygen and protecting GOx from denaturation. This enzymatic activity lowers the local pH, causing the chitosan microgels to swell and release encapsulated proteins, such as bovine serum albumin and insulin. The system demonstrated basal release under normoglycemic conditions and enhanced release under hyperglycemic conditions, offering potential for closed-loop insulin delivery in diabetes management (Figure 3). In a recent study, Wang et al. [46] synthesized ruthenium NZ (RuNZs) with both oxidase and peroxidase activities through a solvothermal method. These RuNZs were employed in a multifunctional nanoplatform for in vitro disease marker detection, cancer cell elimination, and antibacterial applications. Leveraging their oxidase (GOx) activity, the researchers developed a colorimetric biosensor to measure α-glucosidase activity and screen potential inhibitors. Additionally, a RuNZs@SiO₂@GOx nanoreactor was engineered, integrating biological enzymes with NZs, to create a colorimetric sensing platform for rapid blood glucose detection. The multifunctional RuNZs also demonstrated efficacy in anticancer and antibacterial activities, broadening the potential applications of NZs in theranostics (Figure 4).
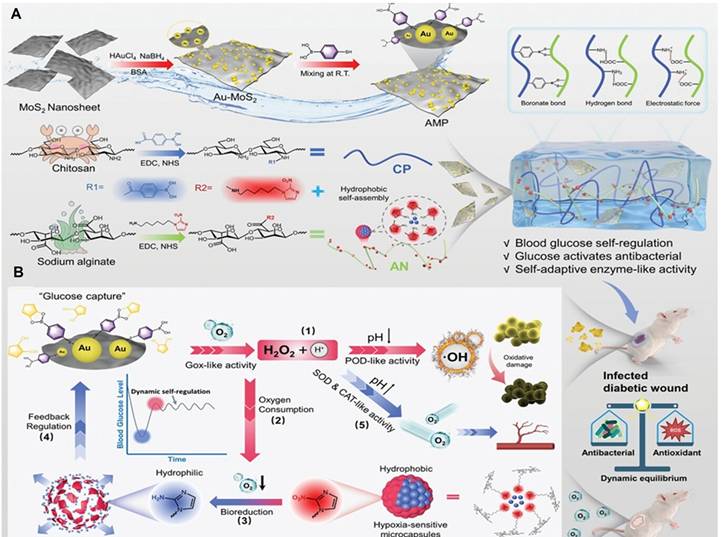
Yang et al. [47] used a glucose-activated self-switching enzyme-like activity to program a hydrogel for regulating insulin release in response to blood glucose fluctuations, allowing for both blood glucose management and wound healing. The hydrogel was composed of Au and MoS2 NZs, and insulin was loaded into hypoxia-sensitive microcapsules. In hyperglycemia, glucose generated ROS for antibacterial activity and triggered insulin for blood glucose regulation. In a normoglycemia environment, the enzyme-like activity supplied oxygen, inhibiting further insulin release and facilitating wound recovery in mice through blood glucose regulation and an improved wound microenvironment (Figure 5).
Glucose-responsive chitosan microgels for self-regulated insulin delivery. A glucose-responsive insulin delivery system was developed using pH-sensitive chitosan microgels loaded with mesoporous silica and ceria nanoparticles. Under hyperglycemic conditions, enzymatic reactions lower the pH, causing microgel swelling and controlled insulin release, with ceria nanoparticles protecting the enzymatic activity by decomposing H2O2. Reprinted with permission from Kim et al. [45]. Copyright © 2017, American Chemical Society.Final del formulario
Multifunctional applications of Ruthenium nanozymes (RuNz). RuNZs were used for disease marker detection, cancer cell elimination, and antibacterial applications. A colorimetric biosensor for α-glucosidase (GOx) activity was designed. Additionally, a RuNPs@SiO2@GOx-based nanoreactor was developed for blood glucose detection using a smartphone-integrated platform. The multifunctional RuNZs also showed potential in anticancer and antibacterial applications. Reprinted with permission from Wang et al. [46]. Copyright © 2024, Elsevier B.V.
Construction (A) and tissue regeneration-promoting effects (B) of a glucose-activated programmed hydrogel for infected diabetic wound healing. The hydrogel combines phenylboronic-acid-modified chitosan, nitroimidazole-modified alginate, and Au-MoS₂ nanozymes. Under hyperglycemia, it generates ROS to kill bacteria and triggers insulin release in hypoxic conditions. In normoglycemia, it releases oxygen to relieve hypoxia. This example of a dynamic system enables feedback-controlled glucose regulation and promotes orderly wound healing. Reprinted with permission from Yang et al. [47]. Copyright © 2025, Wiley.
Nanozymes in diabetic wound healing
Among the different therapeutic aspects of diabetes, the field of diabetic wound healing, particularly concerning diabetic foot ulcers (DFUs), has garnered significant attention and scientific output. A recent review has compiled and analyzed key studies related to DFUs, highlighting the prominence of this area in diabetes research [48]. Therefore, we include here only recent works focusing on diabetic wound healing. DFUs are chronic, non-healing wounds resulting from diabetic neuropathy, vascular disease, and bacterial infections. The complexity of DFUs arises from a multifaceted microenvironment characterized by recurrent infections, excessive oxidative stress, persistent inflammation, and ischemia-hypoxia, all secondary to hyperglycemia [49]. Current clinical treatments often yield suboptimal outcomes, and the development of new treatments is a priority to improve the quality of life for diabetic patients [50]. Compared to natural enzymes, NZs offer more stable catalytic activity, lower production costs, and greater maneuverability. Notably, many NZ exhibit multienzyme activities, enabling them to catalyze multiple enzyme-like reactions simultaneously throughout the DFU recovery process. Additionally, their favorable photothermal and acoustic properties can be exploited to further enhance therapeutic effects.
The recent studies on NZs for wound healing are summarized in Table 2. NZs have been integrated into various hydrogel and microneedle systems to enhance wound healing through multiple mechanisms. These include scavenging of ROS, generating oxygen, modulating immune responses, and providing antibacterial effects. NZs are often combined with other therapeutic agents, such as growth factors and antimicrobial peptides, to create multifunctional wound dressings that address the complex microenvironment of diabetic wounds. The studies demonstrate that NZ-based treatments can accelerate wound closure, reduce inflammation, promote angiogenesis, and improve collagen deposition, leading to enhanced healing outcomes. For example, Wei et al. [51] reported a selenium-based Janus liposozyme that promoted macrophage polarization and tissue regeneration in a large-animal model (mini pigs), providing rare evidence of NZs efficacy beyond rodent studies. Similarly, MnO₂-loaded hydrogels [52] not only scavenged ROS and generated oxygen and nitric oxide but also reduced neutrophil infiltration, modulated cytokine expression, and promoted neovascularization, illustrating the multi-targeted potential of NZ therapies in diabetic wounds.
The scientific literature reveals that different targeting strategies have been employed in NZ-based therapies to promote diabetic wound healing, including modulation of oxidative stress, inflammation, angiogenesis, and antibacterial activity. Targeting oxidative stress has been a major approach in NZ-based wound healing. Several NZ-based hydrogels or composite systems scavenge ROS and generate oxygen to restore redox balance in DFUs. Examples include Pt-SiO₂, CeO₂, and MnO₂ NZ, which demonstrated reduced oxidative injury, improved mitochondrial activity, and enhanced angiogenesis [60,67,68,75,89]. Additional systems such as Cu-based NZs assembled with polyphenol ligands [80] and Zn-based polymetallic constructs [78] further contributed to ROS scavenging and collagen regeneration. These systems collectively reduce oxidative injury, restore cellular metabolism, and accelerate wound closure. All of these studies have been conducted in vitro and in animal models, primarily in diabetic rats and mice (Table 2), except the study by Wei et al. [51], which also employed mini pigs. To date, no clinical studies have been reported.
Inflammation modulation represents another critical strategy. NZ therapies attenuate inflammatory cascades by lowering pro-inflammatory cytokines (e.g., IL-1β, IL-6, TNF-α) and promoting macrophage polarization toward a regenerative phenotype. Representative systems include MnO₂-based hydrogels and Cu/Zr MOFs [52,53,63,72]. PtCuTe nanosheets [65], Pt hydrogels [66], and CuCeO₂ NZs [85] also demonstrated NF-κB inhibition and Nrf2 activation, enhancing granulation tissue formation and immune homeostasis. These anti-inflammatory effects are crucial for transitioning wounds from the inflammatory to the proliferative phase.
Promotion of angiogenesis and tissue regeneration is also essential in DFU recovery. Certain NZs directly stimulate angiogenesis and collagen deposition, thereby accelerating wound closure. Examples include Prussian blue NZ microneedles with VEGF loading, FeS/Au hybrids upregulating HIF-1 and VEGF, and Zn-based polymetallic constructs that enhanced collagen regeneration [60,73,78]. Other systems, such as Cu-ZnO hydrogels [76], Pt-based hydrogels [61,99], and Au-Pt NZs [83] supported endothelial cell function and osteogenic differentiation.
Finally, antibacterial strategies play a key role in addressing DFUs, which are prone to recurrent infections. NZs such as Au/MoS₂, Au-Pt hydrogels, and aptamer-functionalized glucose oxidase NZ demonstrated strong antibacterial efficacy in wound models, either through ROS generation or pathogen-triggered activation [54,56,58]. Additional antibacterial systems include Fe₂(MoO₄)₃ NZs [59], Cu-MOFs [102], and FeS aerogels [103], which were effective against biofilms and resistant pathogens.
Overview of Nanozyme Compositions, Enzymatic Activities, and Biological Effects in Diabetic Wound Healing.
| Nanozyme | Base | Nanozyme activity | Biological effects | Animal model | Ref. |
|---|---|---|---|---|---|
| Mn CoO MOFs | Hydrogel | Catalase | Macrophage polarization, epithelialization, collagen deposition, angiogenesis. | Diabetic rats | 53 |
| Au MoS2 | Hydrogel | Glucose oxidase Peroxidase | Anti-inflammatory, antibacterial | Diabetic rats | 54 |
| Au Porphyrin MOFs | ROS generation, photothermal. | Upregulation of cell proliferation factors, antibacterial. | Diabetic rats | 55 | |
| MnO2 | Hydrogel Pravastatin | ROS-scavenging, O2 generation | Decreased neutrophil infiltration. Macrophage polarization. Reduction of IL-1β, IL-6, TNF-α, CXCL-1. Increase of IL-4 and IL-10, TGF-β Neovascularization. Collagen deposition. Antibacterial | Diabetic rats and mice | 52 |
| Au-Pt | Hydrogel | Glucose oxidase, Catalase | Reduction of ROS damage. Antibacterial | Diabetic rats | 56 |
| Fe3O4 | Glucose oxidase | (pH-switchable) Glucose oxidase Catalase Peroxidase | Proliferation and remodeling | Diabetic mice | 57 |
| Aptamer-functionalized NZ | Glucose oxidase Hyaluronic acid | Antibacterial (activated by bacteria-secreted hyaluronidase) | Antibacterial | Diabetic mice | 58 |
| Fe2(MoO4)3 | Glucose oxidase | Catalase | Reduction of ROS damage Antibacterial | Diabetic mice | 59 |
| Prussian blue NZ | Microneedle Vascular Endothelial Growth Factor, Polymixin | Antioxidant Antibacterial | Proangiogenesis Antibacterial | Diabetic mice | 60 |
| Pt | Hydrogel | Glucose oxidase NADH oxidase Peroxidase Catalase SOD | Anti-inflammatory Proliferation Antibacterial | Diabetic rats | 61 |
| Au-Cu2 MoS4 | Microneedle | Glucose oxidase Catalase | Antibacterial | Diabetic mice | 62 |
| Cu Zr MOFs | Hydrogel | ROS scavenging | Anti-inflammatory Proliferation | Diabetic mice and rats | 63 |
| MnO2 | Fiber-based compartmentalization | Peroxidase, catalase | Anti-inflammatory Proliferation | Diabetic mice | 64 |
| PtCuTe | - | ROS scavenging Antibacterial | Vascular formation, macrophage polarization, fibroblast mobility, regeneration of highly vascularized skin. Antibacterial. | Diabetic mice | 65 |
| Pt | hydrogel | Glutathione reductase Catalase Peroxidase | Anti-inflammatory Angiogenesis | Diabetic mice | 66 |
| Cu | 2,5-dimercaptoterephthalic acid | Catalase | Cell proliferation, migration, angiogenesis, antibacterial | Diabetic mice | 67 |
| MnO2 | Bioactive glasses, cryogel | ROS scavenging Peroxidase | Cell proliferation, ant-inflammatory, collagen III deposition, angiogenesis, antibacterial | Diabetic rats | 68 |
| Au, CuO | Hydrogel | SOD, catalase. glucose oxidase, peroxidase, nitric oxide synthase | Lower glucose, angiogenesis, antibacterial | Diabetic rats | 69 |
| Fe2C | Glucose oxidase. Microneedle | Antibacterial | Antibacterial | Diabetic mice | 70 |
| Fe, Au | - | Glucose oxidase, Peroxidase Antibacterial | Lower glucose, Antibacterial | Diabetic mice | 71 |
| CoO | Hydrogel | Catalase | Anti-inflammatory, increased re-epithelialization, collagen deposition, functional blood vessel growth | Diabetic rats | 72 |
| Se | Janus liposozyme | ROS scavenging | Macrophage polarization, skin wound repair. Tissue regeneration in mini pigs. Antibacterial | Diabetic mice and mini pigs | 51 |
| FeS Au | H2S | Glucose oxidase Peroxidase | Upregulation of HIF-1, VEGF, and angiogenesis factors. Protection of endothelial cells. Angiogenesis, collagen deposition Antibacterial. | Diabetic rats | 73 |
| MOFs | Zr Natural SOD | ROS scavenging | Inhibition of fibroblast senescence, ferroptosis, and STING signaling pathway activation in macrophages. | Diabetic mice | 74 |
| CeO2 | Glucose oxidase | Peroxidase Catalase | Angiogenesis, anti-inflammatory, antibacterial. | Diabetic mice | 75 |
| Cu ZnO | Hydrogel Rhein | SOD Antibacterial | Vascular regeneration, collagen deposition, anti-inflammatory, antibacterial. | Diabetic rats | 76 |
| Ru | Hydrogel | Nitric oxide and ROS scavenging | Bone macrophage polarization, improved bone marrow-derived mesenchymal stem cells, and endothelial cells | Diabetic mice | 77 |
| Zn-based polymetallic oxonate | Aldehyde and methacrylic anhydride-modified hyaluronic acid hydrogel, chitosan nanoparticles, glucose oxidase | Catalase, SOD | macrophage polarization through MAPK/IL-17, lower pro-inflammatory cytokines, higher anti-inflammatory mediators, angiogenesis, collagen regeneration. Antibacterial. | Diabetic rats | 78 |
| Os | Hydrogel Glucose oxidase | Catalase, hydroxyl free radicals' generation | Lower bone inflammation, lower glucose levels, antibacterial | Diabetic mice | 79 |
| Cu | Polyphenol ligands | ROS scavenging | Re-epithelialization, collagen deposition, angiogenesis, and immunoregulation | Diabetic rats | 80 |
| Fe | Hydrogel | Catalase | Epidermis formation and collagen deposition | Diabetic mice | 81 |
| Prussian blue NZ | Zr | ROS scavenging | Macrophage polarization, activation of vascular endothelial cells. | Diabetic rats | 82 |
| Au@Pt | Hydrogel | Glucose Oxidase Catalase | Bone marrow mesenchymal stem cells protection. Osteogenic differentiation. Anti-inflammatory. Angiogenesis. Improved bone regeneration. | Diabetic rats | 83 |
| Zn | C-dots | ROS scavenging | Anti-inflammatory, angiogenesis, collagen deposition, tissue remodeling. Antibacterial. | Diabetic rats | 84 |
| Cu CeO2 | Hydrogel JSH-23 | ROS scavenging | Improved Nrf2 transcriptional activity of macrophages. | Diabetic mice | 85 |
| Au Prussian blue | Not specified | Peroxidase | Upregulation of VEGF and CD31. Angiogenesis. Antibacterial | Diabetic rats | 86 |
| MnO₂ Au MOFs | Hydrogel mSiO2 Acidic fibroblast growth factor | Catalase SOD | Cell proliferation, migration, angiogenesis macrophage polarization | Diabetic mice and rats | 87 |
| Au Pt | Hydrogel DNA aptamer | Glucose oxidase ROS scavenging | Anti-inflammatory Antibacterial | Diabetic mice | 88 |
| Cu Se | Hydrogel Glucose oxidase | Peroxidase | Production of mature collagen. Migration and proliferation of endothelial cells Antibacterial | Diabetic rats | 89 |
| MnO2 | Hydrogel | ROS scavenging | Fibroblast proliferation Angiogenesis. Antibacterial | Diabetic mice and rats | 90 |
| Fe3O4 | Glucose oxidase l-arginine (l-Arg) | Catalase NO release | Antiinflammatory Antibacterial | Diabetic mice | 91 |
| Cu | Lysyl oxidase (catalytic domain) | Lysyl oxidase | Bone collagen synthesis and biomineralization. | Diabetic mice | 92 |
| Fe Cu | Tannic acid | Peroxidase | Vascular migration and regeneration Antibacterial | Diabetic mice | 93 |
| Fe3O4 | MXene microneedle | Catalase SOD | ROS upregulation, lipid peroxidation. | Diabetic rats | 94 |
| CuP | Hydrogel | Peroxidase catalase | Angiogenesis. Antibacterial | Diabetic mice | 95 |
| Mo Fe/Cu | Hydrogel Glucose oxidase | Peroxidase Catalase SOD | Angiogenesis. Antibacterial | Diabetic mice | 96 |
| Ni MOFs | - | ROS scavenging | Cell migration, angiogenesis, TGF-β1 activation, macrophage polarization | Diabetic mice | 97 |
| Au Pd | Hydrogel | Glucose oxidase Catalase SOD | NF-κB inhibition, macrophage polarization, cell migration, and vascularization. | Diabetic mice | 98 |
| Pt | Hydrogel | Catalase | Granulation tissue formation, angiogenesis, type III collagen deposition. Antibacterial | Diabetic mice | 99 |
| Cu | - | ROS scavenging | Antiinflammatory, collagen synthesis, angiogenesis | Diabetic mice | 100 |
| Cu, O, Zn, MOFs | Hydrogel | Catalase | Macrophage polarization, cell migration, angiogenesis, collagen deposition | Diabetic rats | 101 |
| Cu MOFs | Hydrogel Glucose oxidase | Catalase | Angiogenesis, collagen deposition. Antibacterial | Diabetic rats | 102 |
| FeS | Aerogel Glucose oxidase | Glucose oxidase | Antibacterial. | Diabetic mice | 103 |
CD31: cluster of differentiation 31; CXCL-1: chemokine (C-X-C motif) ligand 1; HIF-1: Hypoxia-Inducible Factor 1; IL: interleukin; MAPK: Mitogen-Activated Protein Kinase; NADH: Nicotinamide Adenine Dinucleotide (Reduced form); NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NO: nitric oxide; Nrf2: nuclear factor erythroid 2-related factor 2; ROS: reactive oxygen species; SOD: superoxide dismutase; STING: stimulator of interferon genes; TGF-β1: transforming growth factor beta 1; TNF: tumor necrosis factor; VEGF: vascular endothelial growth factor.
6. Potential role of nanozymes in atherosclerosis and obesity
6.1. Interlinking metabolic disorders: diabetes, atherosclerosis, and obesity
The global surge in metabolic disorders has underscored the critical interrelationship between diabetes mellitus, atherosclerosis, and obesity, often referred to as the metabolic triad. Obesity is a major risk factor for both T2DM and atherosclerosis due to its strong association with chronic low-grade inflammation, insulin resistance, and dyslipidemia. Excess adipose tissue secretes pro-inflammatory adipokines that not only impair glucose metabolism but also contribute to endothelial dysfunction and oxidative stress, setting the stage for vascular complications [104]. T2DM exacerbates atherosclerotic processes by promoting glycation of lipoproteins, enhancing oxidative stress, and activating inflammatory pathways that destabilize atherosclerotic plaques. Hyperglycemia further induces endothelial damage, accelerates foam cell formation, and enhances monocyte adhesion. Meanwhile, atherosclerosis can impair insulin delivery and exacerbate diabetic complications by reducing vascular perfusion [105]. Given their overlapping pathophysiological features, therapeutic strategies targeting these shared mechanisms hold promise for simultaneously addressing obesity, T2DM, and atherosclerosis. In this context, NZs have emerged as multifunctional platforms capable of modulating oxidative and inflammatory pathways, offering novel therapeutic opportunities across these interconnected conditions.
6.2. Nanozymes in atherosclerosis therapy
Atherosclerosis, a chronic inflammatory disease characterized by plaque formation and lipid accumulation in arterial walls, has shown promising therapeutic responses to nanozyme-based interventions. NZs can address several pathological hallmarks of atherosclerosis, including oxidative stress, inflammation, and foam cell formation. Chen et al. [106] designed a biomimetic nanozyme platform by coating cerium-doped supra-carbon dots with macrophage membranes to achieve ROS-responsive theragnostic for atherosclerosis. These nanoparticles demonstrated dual-mode imaging capabilities (fluorescence and photoacoustic) and cascade enzymatic activity activated by the plaque's oxidative microenvironment. Functionally, the NZ platform enabled precise imaging and reduced ROS levels, suppressed M1 macrophage infiltration, and inhibited foam cell formation, effectively regulating the plaque microenvironment and slowing atherogenesis.
Another multifunctional approach involved Prussian blue-based NZs encapsulated with bovine serum albumin and curcumin. Xu et al. [107] demonstrated ROS scavenging and reduced pro-inflammatory cytokines such as TNF-α and IL-1β in foam cells. Additionally, it promoted cholesterol efflux by upregulating ABCA1 (ATP-binding cassette transporter A1) and ABCG1 (ATP-binding cassette transporter G1) in foam cells, ultimately decreasing lipid burden and metalloproteinase activity within plaques.
Expanding on this, Wang et al. [108] developed CS-Lip/PB@Rap, a chondroitin sulfate-modified Prussian blue nanozyme loaded with rapamycin. This system exhibited dual-targeting capabilities via CD44 receptors on inflammatory macrophages and vascular smooth muscle cells. Its enzyme-like properties allowed it to restore redox homeostasis and disrupt inflammatory crosstalk by suppressing NF-κB-mediated cytokine feedback loops. The nanozyme also attenuated vascular smooth muscle cells' phenotype switching and ox-LDL formation, both crucial in plaque destabilization.
Chen et al. [109] further contributed to the field with CLIKKPF-peptide-functionalized carbon-dot NZs, which provided real-time monitoring of plaque progression and showed significant ROS-scavenging and anti-inflammatory effects. Their specific binding to phosphatidylserine on foam cell membranes enhanced selective plaque targeting, optimizing both diagnostic precision and therapeutic outcomes.
Key takeaways
- Nanozyme types: CeO₂, Prussian blue, and manganese-based.
- Mechanisms: Mimic antioxidant enzymes (SOD, CAT, glutathione peroxidase) to reduce ROS, stabilize plaques, and regulate macrophage polarization.
- Translational status: Preclinical models show strong potential for anti-inflammatory and plaque-stabilizing therapies; still in early-stage development.
6.3. Nanozymes in obesity and metabolic dysfunction
As a multifactorial metabolic disorder characterized by chronic inflammation, oxidative stress, and lipid dysregulation, obesity presents complex therapeutic challenges. Antioxidant and immunomodulatory properties of NZs offer a novel paradigm for addressing obesity-related comorbidities.
Parra-Robert et al. [110] developed mesoporous silica-coated cerium oxide (CeO₂) NZs that significantly improved lipid profiles and reduced systemic inflammation in obese Zucker rats. These NZs modulated hepatic and adipose gene expression and downregulated the PI3K/mTOR/AKT signaling pathway, contributing to long-term metabolic improvements.
In a combined mouse model of non-alcoholic steatohepatitis and alcohol-associated liver disease, Gopal et al. [111] reported that nanoformulated SOD1 ameliorated liver damage and systemic inflammation. Notably, the NZs reduced CYP2E1 (Cytochrome P450 2E1) expression in visceral adipose tissue and increased SOD1 activity in the liver, curbing oxidative damage and metabolic stress.
Macrophage polarization plays a pivotal role in obesity-related inflammation. Duan et al. [112] identified CD146 as a key regulator of pro-inflammatory macrophage activation in obese adipose tissue. Targeting the CD146/Gp130 complex with antibodies in obese mice modulated JNK and STAT3 signaling pathways, shifting macrophage polarization toward an anti-inflammatory phenotype and improving insulin sensitivity.
Ding et al. [113] introduced aptamer-modified gold nanoclusters as targeted NZs for ROS scavenging in white adipocytes in vitro. These nanoclusters exhibited high SOD- and catalase-mimetic activity and strong adipocyte-specific targeting, effectively reducing oxidative stress with minimal toxicity, offering a tailored approach for managing obesity-induced oxidative burden.
Lastly, Natarajan et al. [114] demonstrated that copper/zinc SOD NZs protected against ethanol-induced hepatic steatosis and adipose inflammation in mice. The NZ therapy modulated lipogenic transcription factors such as SREBP-1c and enhanced AMPK phosphorylation, while increasing anti-inflammatory markers in adipose tissue, showcasing their dual-organ therapeutic potential.
Key takeaways
- Nanozyme types: CeO₂, iron oxide, and carbon-based.
- Mechanisms: Regulate oxidative stress, modulate mitochondrial function, and improve insulin sensitivity.
- Translational status: mostly animal model studies; translational pipeline still emerging.
6.4. Achievements and challenges
As shown, a transformative class of therapeutic agents capable of mimicking natural enzymatic activities with enhanced stability, tunability, and multifunctionality has been satisfactorily evaluated in different models of disease. Their application in addressing chronic metabolic alterations in atherosclerosis and obesity has shown substantial promise, particularly through their ability to modulate oxidative stress, regulate inflammatory pathways, and target pathological microenvironments with high specificity. Globally, for atherosclerosis, NZs, such as Prussian blue derivatives, CeO2-based nanostructures, and functionalized carbon dots have demonstrated robust ROS-scavenging and anti-inflammatory properties, enabling suppression of foam cell formation, stabilization of plaques, and even real-time imaging of lesion sites through integrated theragnostic modalities. Similarly, in the context of obesity, NZs, like CeO₂, nanoformulated SOD1, and aptamer-conjugated gold nanoclusters have effectively improved adipose tissue oxidative status, reduced pro-inflammatory macrophage infiltration, and ameliorated hepatic and systemic metabolic dysregulation.
7. Potential role of nanozymes in other endocrine disorders
The application of NZs in biomedical diagnostics and therapeutics has been comparatively underexplored in endocrine disorders beyond diabetes and obesity. The primary advantages explored thus far include their seamless integration into point-of-care diagnostic platforms. In recent years, NZs have demonstrated significant potential across a spectrum of endocrine disorders, particularly in enhancing the sensitivity and specificity of biomarker detection. Nevertheless, early studies reveal considerable potential, especially in terms of diagnostic precision, therapeutic targeting, and integration into point-of-care platforms.
7.1. Nanozymes in Cushing's syndrome and autonomous hypercortisolism
Adrenal hyperfunction, particularly in Cushing's syndrome, whether adrenocorticotropic hormone (ACTH, a pituitary hormone that stimulates cortisol secretion) dependent or independent, may affect glucose metabolism and induce cardiovascular diseases. Specifically, Cushing's syndrome and the less severe autonomous cortisol secretion phenotype are characterized by central obesity, hypertension, insulin resistance, hyperglycemia, and an altered coagulability, all of which accelerate the development of atherosclerosis [115]. Increased deposition of visceral fat worsens the inflammatory milieu in the vasculature, insulin resistance, and endothelial dysfunction [116,117]. Moreover, Cushing's syndrome interferes with wound healing through the excess of cortisol that suppresses immune function, reduces fibroblast proliferation, and delays the inflammatory phase of wound healing. The associated chronic tissue inflammation and hyperglycemia may also inhibit tissue regeneration mechanisms [115]. Addison's disease (hypocortisolism), on the other hand, results in adrenal insufficiency, leading to metabolic disturbances, including hypoglycemia and electrolyte imbalance [118]. Diagnosis of these entities poses diagnostic challenges due to fluctuating hormone levels and the limited sensitivity of conventional assays. Recent studies have demonstrated how NZs can transform the detection landscape for these conditions. Du et al. [119] developed an ultrasensitive electrochemical immunosensor for cortisol detection using gold single-atom NZs. These NZs, in combination with horseradish peroxidase-labeled antibodies, achieved a highly sensitive detection platform through an Au-S bond-mediated conjugation, offering improved detection limits and signal amplification in hormone assays. Similarly, Yang et al. used a label-free electrochemical immunosensor with a metal-organic framework (SnS2/NiCo MOFs) for the sensitive detection of cortisol [120]. Silver nanoclusters have also been used to achieve low limits of detection in cortisol measurements [121].
Key takeaways
- Nanozyme types: Au, MOF-based NZ (SnS₂/NiCo), and Ag nanoclusters.
- Mechanisms: Enhanced electrochemical and photoelectrochemical signal amplification for cortisol detection, enabling highly sensitive and rapid assays.
- Translational status: Proof-of-concept diagnostic studies only; no therapeutic applications yet, but strong potential for point-of-care testing in endocrine practice.
7.2. Nanozymes in thyroid disorders
Thyroid diseases may have some metabolic effects that overlap with diabetes and obesity. In hypothyroidism, there is a reduction in basal metabolic rate, leading to weight gain and an increase in serum cholesterol levels, both of which contribute to atherosclerotic cardiovascular disease. On the other hand, hyperthyroidism results in increased metabolism, but this can lead to increased cardiac output, arrhythmias, and, paradoxically, cardiovascular issues such as left ventricular hypertrophy [122]. Furthermore, thyroid hormones play an important role in regulating lipid metabolism, and thyroid dysfunction can exacerbate the lipid disturbances seen in both diabetes and obesity, further increasing the risk of atherosclerosis and cardiovascular morbidity.
One promising strategy involves the development of NZ-based colorimetric sensors for the detection of antithyroid drugs. Zhang et al. [123] designed a system based on iron single atoms encapsulated in nitrogen and phosphorus co-doped carbon nanosheets, which exhibited enhanced peroxidase-like activity. This catalytic activity enabled the oxidation of TMB into a visually detectable blue product (oxTMB). Notably, methimazole, a commonly used antithyroid drug, acted as a peroxidase inhibitor in this system, effectively reducing oxTMB and causing the blue color to fade. Leveraging this mechanism, the authors established a visual, dual-functional sensor capable of detecting both methimazole and H2O2 with linear ranges of 5-50 mM and 3-50 mM, respectively, and a limit of detection for methimazole of 1.57 mM. This approach underscores the potential of NZs for rapid, accessible diagnostics in thyroid disorders.
In an elegant study, Wu et al. [124] explored the role of the NZ coordination environment in mimicking the natural inhibition patterns of thyroid peroxidase, the enzyme targeted by antithyroid drugs. They developed NZs with atomically dispersed CuN₄ catalytic sites, modified with adjacent hydroxyl groups (CuNC-OH), which mimicked the enzymatic pocket of thyroid peroxidase. This biomimetic configuration allowed for highly specific interactions with antithyroid drugs, offering a new platform for the high-throughput screening of candidate compounds. Importantly, this system provided a significant cost advantage over traditional enzyme-based kits, positioning nanozyme-assisted drug discovery as a scalable alternative in endocrine pharmacology.
Moving from diagnostics to therapy, Wang et al. [125] introduced a multifunctional nanozyme-based platform for the treatment of thyroid cancer. Their design incorporated platinum nanoparticles within hollow polydopamine carriers, which were loaded with the sonosensitizer Chlorin e6 and the chemotherapeutic agent lenvatinib. This nanoplatform was engineered to target galectin-3 receptors, which are overexpressed in thyroid cancer cells, facilitating receptor-mediated endocytosis. Upon ultrasound irradiation, the Chlorin e6 generated ROS, inducing oxidative stress and apoptosis. Simultaneously, the embedded platinum nanoparticles catalyzed the decomposition of H2O2 into oxygen, thereby alleviating tumor hypoxia and enhancing the therapeutic effect. Transcriptomic analyses further confirmed the efficacy of the treatment in promoting cancer cell apoptosis. This approach illustrates the therapeutic versatility of NZs when combined with targeted delivery and multimodal treatment strategies.
Key takeaways
- Nanozyme types: Au and CeO2.
- Mechanisms: Catalytic antioxidant activity reduces oxidative thyroid damage and supports hormone balance.
- Translational status: Proof-of-concept studies in thyroiditis and related dysfunctions; no clinical trials yet.
7.3. Nanozymes in chromaffin-secreting tumors
Chromaffin tumors, including pheochromocytoma and paragangliomas, are rare neuroendocrine neoplasms that arise from chromaffin cells of the adrenal medulla or extra-adrenal paraganglia. These tumors are characterized by excessive secretion of catecholamines, primarily epinephrine and norepinephrine, which leads to episodic hypertension, tachycardia, headaches, and metabolic disturbances. Their pathobiology involves genetic mutations (e.g., SDHx, RET, VHL) that affect mitochondrial function, redox balance, and cellular proliferation. The fluctuating nature of hormone secretion and the low abundance of circulating tumor cells (CTCs) pose diagnostic challenges due to fluctuating hormone levels and the limited sensitivity of conventional assays. NZs, with their enzyme-mimetic activity and signal amplification capabilities, offer promising solutions for improving the detection and monitoring of these tumors. Recently, Liu et al. [126] engineered a cascaded NZ-based microfluidic platform that integrates Au@CuMOF NZs with glucose meters and smartphone applications for the rapid detection CTCs associated with pheochromocytoma. This dual-enzyme mimetic system capitalized on glucose oxidase- and peroxidase-like activity to enable dual-mode (colorimetric and portable glucose meter-based) detection.
Several groups have harnessed laccase-like activity to detect catecholamines such as epinephrine and norepinephrine. Zhu et al. [127] synthesized nitrogen-doped carbon Co/CoOx NZs with strong laccase mimetic function, offering a simple and effective colorimetric platform for epinephrine detection. The system demonstrated a linear detection range suited for clinical diagnosis. Similarly, Kulandaivel et al. [128] designed a bioinspired copper-based coordination polymer capable of detecting both epinephrine and norepinephrine in complex biological matrices such as artificial urine, providing a strategy for monitoring treatment response in pheochromocytoma. The electrochemical and visual detection of pheochromocytoma-related CTCs has also seen advancement through a dual-mode platform developed by Liu et al. [129]. This approach utilized a covalent organic framework-supported platinum NZ to amplify signals and enable highly sensitive cell detection, demonstrating the diagnostic relevance of NZs in low-abundance biomarker analysis. Huang et al. [130] further developed a layered double hydroxide-based NZ mimicking laccase activity for chromogenic detection of catecholamine biomarkers. This system, which used CuCoFe-based LDHzymes, allowed smartphone-assisted detection, reinforcing the suitability of NZs for portable diagnostics.
Key takeaways
- Nanozyme types: Iron oxide and hybrid nanozymes.
- Mechanisms: Exploit ROS generation and redox modulation for selective tumor suppression.
- Translational status: Limited to preliminary experimental data; requires further validation before clinical use.
7.4. Nanozymes in other endocrine-related disorders
Reproductive axis. NZs have also been utilized to improve the detection of other key hormones, most notably human chorionic gonadotropin (hCG). Palladium-, platinum-, and ruthenium-based NZ have demonstrated excellent peroxidase-like activity. Wang et al. synthesized Pd@Pt-Ru dendritic NZs for ultrasensitive lateral flow immunoassays, enabling a 250-fold improvement in hCG detection sensitivity over conventional gold nanoparticles [131]. Similarly, Cao et al. [132] introduced a “popcorn-like” Pd@Pt nanozyme, produced via green synthesis using Cornus officinalis extracts. This construct also significantly lowered the hCG detection threshold in lateral flow immunoassays. Yang et al. [133,134] reported the development of immunochromatographic test strips incorporating Au/Fe3O4 NZ and β-FeOOH nanorods. These platforms exhibited enhanced catalytic activity and improved signal amplification for hCG detection, with detection limits far surpassing traditional methods. Expanding beyond diagnostics, NZs are also being explored as therapeutic tools. Sun et al. [135] introduced a macrophage membrane-coated MnO2 nanosheet with nanozyme-like estrogen scavenging capabilities for the treatment of endometriosis. This therapeutic NZs inhibited estradiol-driven proliferation and inflammation in vitro and in vivo, presenting a promising nanomedical approach for hormone-dependent endometriosis.
Circadian rhythm. As mentioned, NZs have been employed in the detection of cortisol, the major potential source of circadian rhythm disruption. Interestingly, for evaluating the circadian rhythm, Wang et al. [136] developed a fluorescence-based sensor for melatonin quantification by integrating carbon dots with platinum/ruthenium NZs. The system enabled smartphone-compatible ratiometric sensing, with high sensitivity across physiological melatonin concentrations, highlighting the role of NZs in sleep and endocrine rhythm research.
Gastrointestinal disorders. In the gastrointestinal domain, Zheng et al. [137] proposed a dual-mode magnetic lateral flow assay using Fe3O4@Pt NZ for the detection of gastrin-17. The platform exhibited good sensitivity and specificity within clinically relevant ranges, enabling rapid, quantitative hormone testing suitable for conditions such as gastritis and gastric cancer screening.
7.5. Achievements and challenges
NZs have demonstrated considerable promise in the detection of hormones beyond those involved in metabolic regulation, such as hCG, melatonin, and gastrin, which are essential for reproductive and gastrointestinal health. The key advantages of using NZs in these contexts include their high catalytic efficiency, robustness under diverse environmental conditions, and compatibility with point-of-care diagnostic formats, allowing for rapid, low-cost, and potentially field-deployable hormone assays. Furthermore, their tunable surface properties enable selective detection, while their enzyme-mimetic activity supports signal amplification, enhancing sensitivity in low-abundance hormone detection. However, despite these benefits, the application of NZs in this area remains limited. The number of studies focusing on NZ-based detection of these specific hormones is still relatively small, which restricts the generalizability of current findings and the development of standardized protocols. Additionally, challenges remain regarding the specificity of NZ-hormone interactions in complex biological matrices, as well as the potential for interference by structurally similar molecules [138,139]. Continued research and validation in clinical settings are necessary to fully realize the potential of NZs in hormone diagnostics and endocrine therapy.
Summary of nanozyme-based therapeutic strategies for non-diabetic endocrine disorders.
| Target | Nanozyme Type | Function | Detection Method | In vitro/in vivo | Key Outcome | Ref. |
|---|---|---|---|---|---|---|
| Adrenal/Cortisol | Au | HRP-like, signal amplification | Electrochemical | In vitro diagnostic | Ultra-sensitive cortisol detection | 119 |
| Adrenal/Cortisol | AuNPs@SnS2/NiCo MOFs | electrochemical | In vitro diagnostic | Ultra-sensitive cortisol detection | 120 | |
| Adrenal/Cortisol | Ag, Cd, Au | photoelectrochemical | In vitro diagnostic | Ultra-sensitive cortisol detection | 121 | |
| Methimazole / thyroid drug | Fe | peroxidase | Colorimetric | In vitro diagnostic | Linear range of 5-50 mM | 123 |
| Thyroid | Cu | peroxidase | - | In vitro diagnostic | drug discovery | 124 |
| Thyroid cancer | Pt | ROS generation | - | In vivo (mice) | Cancer cell apoptosis | 125 |
| Adrenal/Catecholamines | Au@CuMOF | GOx- and peroxidase-like | POC microfluidic + PGM | In vitro diagnostic | Multiplex CTC detection | 126 |
| Adrenal/Catecholamines | N-doped Co/CoOx | Laccase-like | Colorimetric | In vitro diagnostic | Sensitive epinephrine detection | 127 |
| Adrenal/Catecholamines | Cu-CP | Laccase-like | Fluorescence + Colorimetric | In vitro diagnostic | Sequential dual biomarker detection | 128 |
| Adrenal/Catecholamines | COF@Pt | Peroxidase mimic | Electrochemical +colorimetric | In vitro diagnostic | High sensitivity | 129 |
| Adrenal/Catecholamines | CuCoFe-LDH | Laccase mimic | Smartphone colorimetric | In vitro diagnostic | Visual detection | 130 |
| hCG | Pd@Pt-Ru | Peroxidase-like | LFIA | High sensitivity | 131 | |
| hCG | CO-Pd@Pt | Peroxidase-like | LFIA | In vitro diagnostic | High sensitivity | 132 |
| hCG | Au/Fe3O4 | Peroxidase-like | ICT strip | In vitro diagnostic | High sensitivity | 133 |
| hCG | β-FeOOH | Peroxidase mimic | ICT strip | In vitro diagnostic | High sensitivity | 134 |
| Estradiol (Endometriosis) | MnO2 | Estrogen scavenging | - | In vivo (mice) | Therapeutic. Suppressed lesion growth | 135 |
| Cyrcardian/Melatonin | PtRu | Peroxidase-like | Fluorescence (ratiometric) | In vitro diagnostic | Smartphone sensing, LOD = 23 nM | 136 |
| Gastrointestinal / Gastrin | Fe3O4@Pt | Dual-mode (magnetic & peroxidase) | Magnetic LFIA | In vitro diagnostic | LOD = 3 pg/mL | 137 |
CTC: circulating tumor cells; hCG: human chorionic gonadotropin; ICT: immunochromatographic test; LFIA: lateral flow immunoassay; LOD: limit of detection; PGM: personal glucose meter; POC: point-of-care; ROS: reactive oxygen species.
Intersection of Nanozyme Structures and Activities in Diabetes and Endocrine Disorders. SOD: Superoxide dismutase.
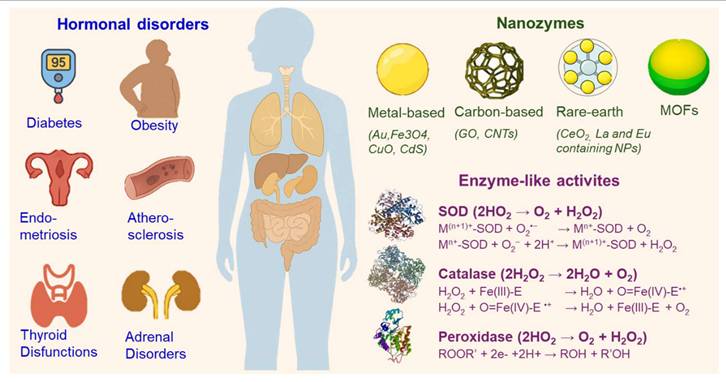
8. Mechanistic insights, comparative benefits, translational gaps, and regulatory considerations
Schema 1 and Table 4 present an overview of NZ classes, highlighting their catalytic mechanisms, target organs, and applications in the treatment of endocrine and metabolic disorders. Catalytic activities directly influence biological processes central to endocrine disorders. These catalytic mechanisms are not merely biochemical reactions but are tightly coupled with disease-modifying pathways, offering a mechanistic rationale for the therapeutic and diagnostic use of NZs in endocrinology. Their SOD-like activity, for instance, mitigates oxidative stress in insulin-sensitive tissues such as the liver and skeletal muscle, thereby restoring mitochondrial function and improving insulin signaling. This mechanism is particularly relevant in type 2 diabetes and obesity, where chronic ROS accumulation impairs glucose uptake and lipid metabolism. Catalase-mimetic nanozymes further detoxify H2O2, promoting tissue regeneration and angiogenesis, which are key processes in diabetic wound healing and vascular repair. Peroxidase-like NZs, such as those based on iron oxide or Prussian blue, modulate inflammatory cascades by reducing NF-κB activation and cytokine release, which is critical in atherosclerosis and metabolic inflammation. Oxidase-mimetic NZs contribute to glucose-responsive systems by catalyzing glucose oxidation, enabling smart drug delivery platforms that release insulin or therapeutic proteins in hyperglycemic conditions. In neuroendocrine tumors, laccase-mimetic NZs facilitate hormone detection through selective oxidation, enhancing diagnostic sensitivity.
Compared to conventional endocrine therapeutics, such as hormone replacement therapies, biologics, or small molecule antioxidants, NZs offer distinct advantages, which are often interlinked in endocrine pathophysiology. For example, NZs with SOD-like activity reduce mitochondrial ROS in insulin-resistant tissues, restoring redox balance and enhancing insulin receptor sensitivity. Catalase-mimetic NZs further detoxify H2O2, promoting cellular survival and tissue regeneration, particularly relevant in diabetic wound healing and thyroid dysfunction. Beyond redox modulation, NZs can influence hormone biosynthesis and receptor dynamics. CeO₂ and Fe₃O₄ NZs have been shown to activate AMPK and PPARγ pathways, which regulate glucose and lipid metabolism and intersect with hormonal feedback loops. The structural tunability of NZs enables integration into smart delivery platforms (e.g., hydrogels, microneedles, mesoporous carriers) and theranostic systems that combine diagnostic and therapeutic capabilities. Through advanced surface engineering strategies, NZs also overcome biological barriers. PEGylation or ligand conjugation improves circulation time and facilitates tissue-specific accumulation, while macrophage membrane coating enables immune evasion and targeted delivery to inflamed or hormonally active tissues. Organelle-level targeting, such as lysosomal localization of Fe₃O₄ NZs, enhances intracellular signaling cascades, improving glucose uptake and insulin sensitivity. These mechanistic features position NZs as versatile agents capable of addressing the systemic complexity of endocrine disorders through catalytic precision and spatial control. The advantages of NZs also lie in their multi-functionality, engineering flexibility, and stability under physiological conditions, which surpass many traditional antioxidants and biologics. Their biomimetic and targeted delivery capabilities provide an innovative platform for intervening in complex disease networks characterized by oxidative stress and chronic inflammation. However, despite the growing body of evidence supporting the potential of NZs in endocrine and metabolic disorders, it is important to emphasize that the vast majority of these applications remain at the preclinical stage. Current findings are largely derived from in vitro experiments and small-animal studies, with no progression toward large-animal validation or human clinical trials. To date, no NZ-based platform targeting endocrine diseases has advanced beyond early translational research.
Several critical gaps must be addressed to facilitate clinical implementation. First, there is a need for comprehensive biosafety and toxicological profiling, particularly regarding the long-term biodistribution, clearance, and potential accumulation of metal-based nanozymes. Second, challenges in manufacturing and standardization persist, as reproducible large-scale synthesis with consistent catalytic performance remains underdeveloped. Third, the absence of a clear regulatory framework for multifunctional, enzyme-mimetic nanomaterials introduces uncertainty in approval pathways, slowing clinical translation. Fourth, most preclinical studies rely on simplified or reductionist models that inadequately replicate the complexity of human endocrine pathophysiology. Incorporation of advanced disease models, including patient-derived systems and organ-on-chip technologies, will be essential to bridge this gap. Finally, clinical integration strategies must be considered early, including how NZ-based sensors or therapeutics can complement existing diagnostic workflows and endocrine treatments. Integrating precision-targeting strategies, smart responsive designs, and combinatorial therapies with NZs may overcome current limitations and accelerate their application in metabolic disease management [140]. Continued interdisciplinary research and early-phase clinical studies will be crucial to fully harness the potential of NZs as next-generation therapeutics in atherosclerosis, obesity, and their interlinked complications [141-142].
Nanozyme classes, catalytic mechanisms, target organs, and disease applications in endocrine and metabolic disorders.
| Catalytic Mechanism | Nanozyme Class | Target Organs | Diseases |
|---|---|---|---|
| SOD-mimetic | CeO₂ | Liver, Skeletal Muscle | Diabetes, Obesity, Neurodegeneration, Wound Healing |
| AuCePt | Liver | Diabetes | |
| Au | Adipose Tissue | Obesity | |
| Cu/Zn | Liver, Adipose Tissue | Obesity, Alcoholic Liver Disease | |
| Nickel MOFs | Skin | Diabetic Wound Healing | |
| Peroxidase-mimetic | Fe₃O₄ | Liver | Diabetes |
| Prussian Blue | Vascular System | Atherosclerosis | |
| AuPt | Liver, Gut | Diabetes | |
| PtSiO₂ | Liver | Diabetes | |
| MnO₂ | Skin | Endometriosis, Wound Healing | |
| Fe | Blood | Thyroid Drug Monitoring | |
| Ru | Blood | Diabetes, Cancer | |
| Peroxidase-mimetic | Pd@Pt | Blood | hCG Detection |
| Au/Fe₃O₄ | Blood | hCG Detection | |
| Oxidase-mimetic | Au | Blood | Glucose Detection |
| CuO | Skin | Wound Healing | |
| RuN | Blood | Diabetes, Cancer | |
| Pt | Thyroid | Thyroid Cancer | |
| Pt-Ru | Blood | Melatonin Detection | |
| Catalase-mimetic | CeO₂ | Liver, Skeletal Muscle | Diabetes, Obesity, Neurodegeneration |
| AuCe | Liver | Diabetes | |
| Pt-SiO₂ | Liver | Diabetes | |
| CeO₂ | Blood | Diabetes | |
| Laccase-mimetic | CuCoFe | Blood, Urine | Pheochromocytoma |
| Co/CoOx | Blood | Pheochromocytoma | |
| Cu | Urine | Pheochromocytoma |
NZs pose regulatory challenges due to their hybrid nature, combining nanostructural properties with enzyme-like biochemical activity. Both the FDA and EMA classify products based on their primary mode of action and intended use. According to FDA guidance, NZs that exert their therapeutic effect through chemical action within or on the body are typically classified as drugs. If the effect is mechanical or physical, such as in diagnostic or structural applications, they may be regulated as medical devices [143]. In cases where both mechanisms are present, NZs may be designated as combination products, requiring coordinated review across regulatory centers. The EMA applies a similar approach, evaluating NZs under either medicinal product or medical device legislation, depending on their mechanism of action and composition. EMA guidance on nanotechnology-based medicinal products highlights the need for case-by-case assessment, especially for borderline technologies [144]. The lack of harmonized definitions and regulatory pathways for NZs complicates their clinical translation. The classification directly affects the design of preclinical studies, the required physicochemical characterization, and the scope of safety and efficacy evaluations [145].
Taken together, while NZs represent a highly versatile and innovative class of materials for endocrine medicine, their translation from laboratory to clinic will require coordinated efforts in toxicology, materials science, regulatory policy, and clinical trial design. Addressing these barriers will be crucial to realizing their full potential as next-generation diagnostic and therapeutic agents.
9. Conclusion and future perspectives
The convergence of nanotechnology and enzyme-mimetic systems has ushered in a transformative era for biomedical applications. NZs have demonstrated exceptional versatility across a wide range of diagnostic, therapeutic, and theragnostic domains. Their ability to modulate oxidative stress, inflammation, and metabolic dysfunctions has positioned them as potent tools in the management of diabetes mellitus, atherosclerosis, obesity, and other interlinked endocrine disorders. Moreover, their adaptability for incorporation into biosensing platforms, smart drug delivery systems, and regenerative medicine approaches underscores their role as next-generation biomedical agents.
In diabetes care, NZs have shown promise in non-invasive glucose monitoring and modulation of insulin resistance. Their incorporation into responsive hydrogels, microneedles, and smart therapeutics has further advanced the field of diabetic foot ulcer management. Likewise, their targeted capabilities have made significant strides in atherosclerosis by enabling plaque-specific imaging and immunomodulation. In obesity and other endocrine-related pathologies, including thyroid and adrenal disorders, NZs provide a highly adaptable and bioactive framework to intervene in complex metabolic cascades.
Despite these advancements, challenges remain. The long-term biosafety, biocompatibility, and immunogenicity of NZs in vivo require comprehensive evaluation. Issues such as large-scale production, reproducibility, regulatory approval, and targeted delivery mechanisms must be addressed before clinical translation can be fully realized. The potential toxicity of metal-based NZs and limitations in catalytic specificity also necessitate more refined design strategies.
Materials engineering
To move beyond proof-of-concept, NZs must evolve from static catalysts to programmable systems capable of responding to biochemical stimuli such as ROS levels, glucose fluctuations, or hormonal gradients. This shift demands architectures with spatial and temporal control, including organelle-targeted designs and artificial intelligence-guided synthesis strategies. These innovations will enable NZs to operate with greater precision and reduced off-target effects in endocrine tissues.
Targeting specific diseases
To maximize clinical relevance, NZs should be tailored to the molecular pathology of specific endocrine disorders. In thyroid cancer, they may remodel hypoxic microenvironments while delivering targeted therapies. In adrenal hyperfunction, cortisol-sensitive NZs could enable feedback-controlled drug release. In obesity and metabolic syndrome, NZs that modulate macrophage polarization and mitochondrial function may restore metabolic balance. These approaches require integration with hormonal feedback loops and tissue-specific signaling networks.
Looking ahead, the integration of NZs with machine learning, artificial intelligence (AI), and precision medicine offers a powerful route to optimize their design and application. Recently, AI-driven platforms such as AI-ZYMES and machine learning models are revolutionizing the rational design of NZ by predicting catalytic activity, guiding synthesis, and enabling multiplexed sensing [146-148]. These approaches facilitate the development of hybrid and stimulus-responsive NZ capable of performing multiplexed tasks within complex biological systems [149-151]. Future research should prioritize not only these advanced designs but also the advancement of clinical trials and interdisciplinary collaborative studies, which are essential to transition nanozyme-based technologies from bench to bedside.
Challenges in clinical translation
Clinical translation remains the most critical hurdle. Most NZs studied are confined to in vitro or small-animal models, which fail to capture the complexity of human endocrine physiology. Advanced platforms such as organ-on-chip systems and patient-derived organoids will be essential to simulate hormonal crosstalk and tissue-specific responses. Early-phase human trials should focus on biosafety, biodistribution, and pharmacokinetics, especially for metal-based NZs. Embedding NZs into wearable biosensors or diagnostic workflows may facilitate clinical adoption. Regulatory frameworks must evolve to accommodate their hybrid nature, requiring coordinated evaluation across drug and device categories.
To enable clinical translation, strategies for validation that may be helpful include conducting pilot human studies utilizing NZ-based biosensors for the monitoring of glucose or endocrine biomarkers; integrating NZ platforms into established diagnostic workflows to assess their compatibility, analytical performance, and added clinical value; and performing longitudinal safety evaluations in large-animal models to investigate biodistribution, clearance kinetics, and immunogenicity under physiologically relevant conditions. In summary, NZs stand at the frontier of personalized and systems-level medicine, offering dynamic and multifunctional solutions for the detection, monitoring, and treatment of endocrine and metabolic disorders. While current evidence is promising, further refinement in design, targeting, and validation is required to ensure robust clinical applicability. As the field matures, NZs are expected to redefine therapeutic paradigms and pave the way for smarter and more integrated healthcare interventions.
Acknowledgements
Generative AI (GPT-4o) was used for language assistance during writing, but not for content generation.
Funding
This work was supported by grants from Basic Research General Program Science program, Technology and Innovation Commission of Shenzhen Municipality (No. JCYJ20210324103407020); European Union through the Horizon Europe Framework (eprObes project, GA 101080219); Instituto de Salud Carlos III (PI24/00688), co-financed by FEDER, European Union, “A way of making Europe”; Agencia Estatal de Investigación (Project PID2022-138243OB-I00 funded by MICIU/AEI/10.13039/501100011033 and by FEDER, UE); Consolidated Research Group, Departament de Recerca i Universitats de la Generalitat de Catalunya (2021 SGR 00881); CIBERehd is financed by the Instituto de Salud Carlos III.
Author contributions
Huilun Lu, Zizhong Liu and Gregori Casals drafted the manuscript. Ya-chao Wang, Eudald Casals, Felicia A. Hanzu, Manuel Morales-Ruiz, and Muling Zeng contributed to the final version of the manuscript. All the authors revised and approved the final version of the manuscript.
Competing interests
The authors have declared that no competing interest exists.
References
1. Go L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577-83
2. Lin Y, Ren J, Qu X. Nano-gold as artificial enzymes: Hidden talents. Adv Mater. 2014;26(25):4200-17
3. Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater. 2010;22(19):2206-10
4. Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS. et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun. 2010;46(16):2736-38
5. Devaraji M, Thanikachalam PV, Elumalai K. The potential of copper oxide nanoparticles in nanomedicine: a comprehensive review. Biotechnol Notes. 2024;5:80-99
6. Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y. et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev. 2019;48(4):1004-76
7. Lim JV, Bee ST, Tin Sin L, Ratnam CT, Abdul Hamid ZA. A Review on the synthesis, properties, and utilities of uunctionalized uarbon uanoparticles for polymer nanocomposites. Polymers (Basel). 2021;13(20):3547
8. Ghasempour A, Dehghan H, Ataee M, Chen B, Zhao Z, Sedighi M. et al. Cadmium sulfide nanoparticles: Preparation, characterization, and biomedical applications. Molecules. 2023;28(9):3857
9. Zhu Z, Gu Z, Xu J, Jing Y, Li N, Miao X. et al. Recent advances in molybdenum disulfide-based nanozymes for sensing applications. J Anal Test. 2025;9:165-82
10. Zhang Q, O'Brien S, Grimm J. Biomedical applications of lanthanide nanomaterials, for imaging, sensing and therapy. Nanotheranostics. 2022;6(2):184-94
11. Alexander C, Guo Z, Glover PB, Faulkner S, Pikramenou Z. Luminescent lanthanides in biorelated applications: from molecules to nanoparticles and diagnostic probes to therapeutics. Chem Rev. 2025;125(4):2269-370
12. Liu Y, Tang Z. Multifunctional nanoparticle@MOF core-shell nanostructures. Adv Mater. 2013;25(40):5819-25
13. Manoharan D, Wang LC, Chen YC, Li WP, Yeh CS. Catalytic nanoparticles in biomedical applications: exploiting advanced nanozymes for therapeutics and diagnostics. Adv Healthc Mater. 2024;13(22):e2400746
14. Liang M, Yan X. Nanozymes: from new concepts, mechanisms, and standards to applications. Acc Chem Res. 2019;52(8):2190-200
15. Hanzha VV, Rozumna NM, Kravenska YV, Spivak MY, Lukyanetz EA. The effect of cerium dioxide nanoparticles on the viability of hippocampal neurons in Alzheimer's disease modeling. Front Cell Neurosci. 2023;17:1131168
16. Ge H, Wang M, Wei X, Chen XL, Wang X. Copper-based nanozymes: potential therapies for infectious wounds. Small. 2025;21(8):e2407195
17. Wei H, Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42(14):6060-93
18. Sozarukova MM, Kozlova TO, Beshkareva TS, Popov AL, Kolmanovich DD, Vinnik DA. et al. Gadolinium doping modulates the enzyme-like activity and radical-scavenging properties of CeO2 nanoparticles. Nanomaterials (Basel). 2024;14(9):769
19. Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 2022;5(6):1593-615
20. Tian Q, Li S, Tang Z, Zhang Z, Du D, Zhang X. et al. Nanozyme-enabled biomedical diagnosis: advances, trends, and challenges. Adv Healthc Mater. 2025;14(8):e2401630
21. Wang S, Wang L, Yan X, Jiang B. Cascade catalytic nanozymes: design, classification, and biomedical applications. ACS Appl Mater Interfaces. 2025;17(32):45354-81
22. Zhu Y, Liao Y, Zou J, Cheng J, Pan Y, Lin L. et al. Engineering single-atom nanozymes for catalytic biomedical applications. Small. 2023;19(30):e2300750
23. Lou-Franco J, Das B, Elliott C, Cao C. Gold nanozymes: from concept to biomedical applications. Nanomicro Lett. 2020;13(1):10
24. Kurup CP, Ahmed MU. Nanozymes towards personalized diagnostics: a recent progress in biosensing. Biosensors (Basel). 2023;13(4):461
25. Tagaras N, Song H, Sahar S, Tong W, Mao Z, Buerki-Thurnherr T. Safety Landscape of Therapeutic Nanozymes and Future Research Directions. Adv Sci (Weinh). 2024Dec;11(46):e2407816
26. Di Pino A, DeFronzo RA. Insulin Resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447-67
27. Sterpetti AV. Inflammatory Cytokines and atherosclerotic plaque progression. Therapeutic implications. Curr Atheroscler Rep. 2020;22(12):75
28. Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137:111315
29. Ekmektzoglou KA, Zografos GC. A concomitant review of the effects of diabetes mellitus and hypothyroidism in wound healing. World J Gastroenterol. 2006;12(17):2721-9
30. Varra FN, Varras M, Varra VK, Theodosis-Nobelos P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation mediating treatment options (review). Mol Med Rep. 2024;29:95
31. Lee PC, Li NS, Hsu YP, Peng C, Yang HW. Direct glucose detection in whole blood by colorimetric assay based on glucose oxidase-conjugated graphene oxide/MnO2 NZ. Analyst. 2019;144(9):3038-44
32. Karim MN, Anderson SR, Singh S, Ramanathan R, Bansal V. Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosens Bioelectron. 2018;110:8-15
33. Naveen Prasad S, Weerathunge P, Karim MN, Anderson S, Hashmi S, Mariathomas PD. et al. Non-invasive detection of glucose in human urine using a color-generating copper NanoZyme. Anal Bioanal Chem. 2021;413(5):1279-91
34. Roy L, Mondal S, Bhattacharyya N, Ghosh R, Banerjee A, Singh S. et al. A spectroscopy based prototype for the noninvasive detection of diabetes from human saliva using nanohybrids acting as nanozyme. Sci Rep. 2023;13(1):17306
35. Jeon HJ, Kim HS, Chung E, Lee DY. Nanozyme-based colorimetric biosensor with a systemic quantification algorithm for noninvasive glucose monitoring. Theranostics. 2022;12(14):6308-38
36. Yi X, Yuan Y, Qing M, Wang L, Li H, Bai L. Smartphone and paper-based device for glucose monitoring using acetylene black-hemin nanozyme as catalyst. Spectrochim Acta A Mol Biomol Spectrosc. 2023;296:122667
37. Khachornsakkul K, Rybicki FJ, Sonkusale S. Nanomaterials integrated with microfluidic paper-based analytical devices for enzyme-free glucose quantification. Talanta. 2023;260:124538
38. Wang Y, Zhang Q, Kan M, Chang F, He X, Cheng N. et al. Multi-omics analysis of Au@Pt nanozyme for the modulation of glucose and lipid metabolism. J Nanobiotechnology. 2024;22(1):524
39. Song G, Xu J, Zhong H, Zhang Q, Wang X, Lin Y. et al. Single-atom Ce-N4-C-(OH)2 nanozyme-catalyzed cascade reaction to alleviate hyperglycemia. Research (Wash DC). 2023;6:0095
40. Zhou Y, Liu C, Yu Y, Yin M, Sun J, Huang J. et al. An organelle-specific nanozyme for diabetes care in genetically or diet-induced models. Adv Mater. 2020;32(45):e2003708
41. Lin Y, Wang Y, Zhang Q, Gao R, Chang F, Li B. et al. Single-atom Ce-N-C nanozyme ameliorates type 2 diabetes mellitus by improving glucose metabolism disorders and reducing oxidative stress. Biomolecules. 2024;14(9):1193
42. Zhang Z, Zhou D, Luan X, Wang X, Zhu Z, Luo W. et al. Biodegradable Hollow Nanoscavengers Restore liver functions to reverse insulin resistance in type 2 diabetes. ACS Nano. 2023;17(10):9313-25
43. Li X, Zhu W, Liu R, Ding G, Huang H. Cerium oxide nanozymes improve skeletal muscle function in gestational diabetic offspring by attenuating mitochondrial oxidative stress. ACS Omega. 2024;9(20):21851-63
44. Shen H, Fu Y, Liu F, Zhang W, Yuan Y, Yang G. et al. AuCePt porous hollow cascade nanozymes targeted delivery of disulfiram for alleviating hepatic insulin resistance. J Nanobiotechnology. 2024;22(1):660
45. Kim MY, Kim J. Chitosan Microgels embedded with catalase nanozyme-loaded mesocellular silica foam for glucose-responsive drug delivery. ACS Biomater Sci Eng. 2017;3(4):572-8
46. Wang L, Zheng S, Lin X, Chen Y, Li J, Liu X. et al. A RuNPs-based multifunctional nanoplatform with excellent dual enzyme-mimic activities for diabetes diagnosis, cancer cell elimination, and in vitro antibacterial. Talanta. 2025;283:127121
47. Yang Y, Fang Q, Wang J, Li M, Li Z, Xu H. et al. Glucose-activated programmed hydrogel with self-switchable enzyme-like activity for infected diabetic wound self-adaptive treatment. Adv Mater. 2025;37(12):e2419158
48. Xiao X, Zhao F, DuBois DB, Liu Q, Zhang YL, Yao Q. et al. Nanozymes for the therapeutic treatment of diabetic foot ulcers. ACS Biomater Sci Eng. 2024;10(7):4195-226
49. Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic foot ulcers: a review. JAMA. 2023;330(1):62-75
50. Akkus G, Sert M. Diabetic foot ulcers: a devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J Diabetes. 2022;13(12):1106-21
51. Wei T, Pan T, Peng X, Zhang M, Guo R, Guo Y. et al. Janus liposozyme for the modulation of redox and immune homeostasis in infected diabetic wounds. Nat Nanotechnol. 2024;19(8):1178-89
52. Tu C, Lu H, Zhou T, Zhang W, Deng L, Cao W. et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286:121597
53. Li Z, Zhao Y, Huang H, Zhang C, Liu H, Wang Z. et al. A nanozyme-immobilized hydrogel with endogenous ROS-scavenging and oxygen generation abilities for significantly promoting oxidative diabetic wound healing. Adv Healthc Mater. 2022;11(22):e2201524
54. Li Y, Fu R, Duan Z, Zhu C, Fan D. Injectable hydrogel based on defect-rich multi-nanozymes for diabetic wound healing via an oxygen self-supplying cascade reaction. Small. 2022;18(18):e2200165
55. Zhao X, Chang L, Hu Y, Xu S, Liang Z, Ren X. et al. Preparation of photocatalytic and antibacterial MOF nanozyme used for infected diabetic wound healing. ACS Appl Mater Interfaces. 2022;14(16):18194-208
56. Zhang B, Lv Y, Yu C, Zhang W, Song S, Li Y. et al. Au-Pt nanozyme-based multifunctional hydrogel dressing for diabetic wound healing. Biomater Adv. 2022;137:212869
57. Du X, Jia B, Wang W, Zhang C, Liu X, Qu Y. et al. pH-switchable nanozyme cascade catalysis: a strategy for spatial-temporal modulation of pathological wound microenvironment to rescue stalled healing in diabetic ulcer. J Nanobiotechnology. 2022;20(1):12
58. Chen L, Xing S, Lei Y, Chen Q, Zou Z, Quan K. et al. A glucose-powered activatable nanozyme breaking pH and H2 O2 limitations for treating diabetic infections. Angew Chem Int Ed Engl. 2021;60(44):23534-39
59. Zhang Y, Li D, Xu Y, Niu Y. Application of a cascaded nanozyme in infected wound recovery of diabetic mice. ACS Biomater Sci Eng. 2022;8(4):1522-31
60. Guan G, Zhang Q, Jiang Z, Liu J, Wan J, Jin P. et al. Multifunctional silk fibroin methacryloyl microneedle for diabetic wound healing. Small. 2022;18(51):e2203064
61. Zhou Z, Mei X, Hu K, Ma M, Zhang Y. Nanohybrid double network hydrogels based on a platinum nanozyme composite for antimicrobial and diabetic wound healing. ACS Appl Mater Interfaces. 2023;15(14):17612-26
62. Shan J, Zhang X, Cheng Y, Song C, Chen G, Gu Z. et al. Glucose metabolism-inspired catalytic patches for NIR-II phototherapy of diabetic wound infection. Acta Biomater. 2023;157:200-9
63. Chao D, Dong Q, Yu Z, Qi D, Li M, Xu L. et al. Specific nanodrug for diabetic chronic wounds based on antioxidase-mimicking MOF-818 nanozymes. J Am Chem Soc. 2022;144(51):23438-47
64. Li G, Liu H, Yi J, Pu F, Ren J, Qu X. Integrating incompatible nanozyme-catalyzed reactions for diabetic wound healing. Small. 2023;19(10):e2206707
65. Guo Y, Ding S, Shang C, Zhang C, Li M, Zhang Q. et al. Multifunctional PtCuTe nanosheets with strong ROS scavenging and ROS-independent antibacterial properties promote diabetic wound healing. Adv Mater. 2024;36(8):e2306292
66. Zhang F, Kang Y, Feng L, Xi G, Chen W, Kong N. et al. Infected wound repair with an ultrasound-enhanced nanozyme hydrogel scaffold. Mater Horiz. 2023;10(12):5474-83
67. Huang W, Xu P, Fu X, Yang J, Jing W, Cai Y. et al. Functional molecule-mediated assembled copper nanozymes for diabetic wound healing. J Nanobiotechnology. 2023;21(1):294
68. Zhu S, Li M, Wang Z, Feng Q, Gao H, Li Q. et al. Bioactive glasses-based nanozymes composite macroporous cryogel with antioxidative, antibacterial, and pro-healing properties for diabetic iInfected wound repair. Adv Healthc Mater. 2023;12(29):e2302073
69. Shang L, Yu Y, Jiang Y, Liu X, Sui N, Yang D. et al. Ultrasound-augmented multienzyme-like nanozyme hydrogel spray for promoting diabetic wound healing. ACS Nano. 2023;17(16):15962-77
70. Sun C, Zhou X, Liu C, Deng S, Song Y, Yang J. et al. An integrated therapeutic and preventive nanozyme-based microneedle for biofilm-infected diabetic wound healing. Adv Healthc Mater. 2023;12(30):e2301474
71. Li S, Zhang Y, Jin H, Gao H, Liu S, Shi W. et al. Biomimetic dual-nanozymes with catalytic cascade reactions against diabetic wound infection. J Colloid Interface Sci. 2023;651:319-33
72. Zhao Y, Wang D, Qian T, Zhang J, Li Z, Gong Q. et al. Biomimetic nanozyme-decorated hydrogels with H2O2-activated oxygenation for modulating immune microenvironment in diabetic wound. ACS Nano. 2023;17(17):16854-69
73. Yin Y, Guo W, Chen Q, Tang Z, Liu Z, Lin R. et al. A single H2S-releasing nanozyme for comprehensive diabetic wound healing through multistep intervention. ACS Appl Mater Interfaces. 2025;17(12):18134-49
74. Li F, Mao Z, Du Y, Cui Y, Yang S, Huang K. et al. Mesoporous MOFs with ROS scavenging capacity for the alleviation of inflammation through inhibiting stimulator of interferon genes to promote diabetic wound healing. J Nanobiotechnology. 2024;22(1):246
75. Gong Y, Qi W, Lu W, Chang Q, Xie Y, Wang J. et al. Construction of CeO2/Ti3C2Tx heterojunction with antibacterial and antioxidant capabilities for diabetic wound healing. J Colloid Interface Sci. 2025;689:137247
76. Feng S, Peng X, Deng Y, Luo Y, Shi S, Wei X. et al. Biomimetic nanozyme-decorated smart hydrogel for promoting chronic refractory wound healing. ACS Appl Mater Interfaces. 2024;16(44):59862-79
77. Lin P, Qian Z, Liu S, Ye X, Xue P, Shao Y. et al. A single-cell RNA sequencing guided multienzymatic hydrogel design for self-regenerative repair in diabetic mandibular defects. Adv Mater. 2024;36(50):e2410962
78. Pu C, Wang Y, Xiang H, He J, Sun Q, Yong Y, Chen L, Jiang K, Yang H, Li Y. Zinc-based polyoxometalate nanozyme functionalized hydrogels for optimizing the hyperglycemic-immune microenvironment to promote diabetic wound regeneration. J Nanobiotechnology. 2024;22(1):611
79. He S, Lin M, Zheng Q, Liang B, He X, Zhang Y. et al. Glucose oxidase energized osmium with dual-active centers and triple enzyme activities for infected diabetic wound management. Adv Healthc Mater. 2024;13(16):e2303548
80. Chen Y, Yang X, Li K, Feng J, Liu X, Li Y. et al. Phenolic ligand-metal charge transfer induced copper nanozyme with reactive oxygen species-scavenging ability for chronic wound healing. ACS Nano. 2024;18(9):7024-36
81. Chen Y, Yuan B, Yang Z, Yan S, Ren K, Pi Q. et al. Catalase-like nanozyme-hybrid hydrogels utilizing endogenous ROS as an oxygen source to synergically regulate oxidative stress and hypoxia for enhanced diabetic wound healing. Biomacromolecules. 2025;26(3):1672-85
82. Chen S, Luo X, Ma R, Guo Z, Zhao J, Gao J. et al. Promotes M1-polarization and diabetic wound healing using Prussian blue nanozymes. Int Immunopharmacol. 2024;141:113009
83. Hui Y, Mao J, Rui M, Huang Y, Jiang X, Xu Y. et al. Hydrogel microsphere-encapsulated bimetallic nanozyme for promoting diabetic bone regeneration via glucose consumption and ROS scavenging. Adv Healthc Mater. 2024;13(32):e2402596
84. Dai S, Yao L, Liu L, Cui J, Su Z, Zhao A. et al. Carbon dots-supported Zn single atom nanozymes for the catalytic therapy of diabetic wounds. Acta Biomater. 2024;186:454-69
85. Zhu Z, Ding J, Qin M, Wang L, Jiang D, Zhao J. et al. Enhanced ·OH-scavenging activity of Cu-CeOx nanozyme via resurrecting macrophage Nrf2 transcriptional activity facilitates diabetic wound healing. Adv Healthc Mater. 2024;13(12):e2303229
86. Ren X, Hu Y, Sang Z, Li Y, Mei X, Chen Z. Preparation of Au-modified metal organic framework nanozyme with tunable catalytic activity used for diabetic wound healing. J Colloid Interface Sci. 2025;687:643-58
87. Gao S, He X, Liu H, Liu Y, Wang H, Zhou Z. et al. Multifunctional Bioactive Nanozyme Systems for Enhanced Diabetic Wound Healing. Adv Healthc Mater. 2025;14(8):e2401580
88. Lan F, Xin T, Zhang Y, Li A, Wan L, Du J. et al. Nanoconfinement-guided in situ co-deposition of single-atom cascade nanozymes combined with injectable sodium alginate hydrogels for enhanced diabetic wound healing. Int J Biol Macromol. 2025;304(Pt 2):140814
89. Jiang S, Xie D, Hu Z, Song H, Tang P, Jin Y. et al. Enhanced diabetic wound healing with injectable hydrogel containing self-assembling nanozymes. J Control Release. 2024;372:265-80
90. Luo Q, Yan C, Ma R, Dai L, Shu W, Asghar A. et al. E-PL/MnO2 NZ/gellan gum/hyaluronic acid-based multifunctional hydrogel to promote diabetic wound healing. Int J Biol Macromol. 2025;304(Pt 1):140777
91. Li G, Huang Y, Zhao L, Yang B, Guo J, Hu J. et al. Targeting and microenvironment-activated nanoreactor for diabetic chronic wound healing via multienzyme cascade reactions. ACS Appl Mater Interfaces. 2024;16(5):6315-26
92. Xu S, Zhang J, Che J, Sha Z, Zhang M, Zhang Z. et al. Lysyl oxidase nanozyme-loaded hydrogel for sustained release and promotion of diabetic bone defect regeneration. J Control Release. 2025;379:927-43
93. Qin X, Tian R, Wang B, Yang H, Chen J, Wang X. et al. Metal-phenolic nanocapsules with photothermal antibacterial and Ros scavenging ability for diabetic wound healing. Adv Healthc Mater. 2024;13(10):e2303604
94. You W, Cai Z, Xiao F, Zhao J, Wang G, Wang W. et al. Biomolecular microneedle initiates Fe3O4/MXene heterojunction-mediated nanozyme-like reactions and bacterial ferroptosis to repair diabetic wounds. Adv Sci (Weinh). 2025;12(11):e2417314
95. Feng Y, Su L, Zhang Z, Chen Y, Younis MR, Chen D. et al. pH-responsive wound dressing based on biodegradable CuP nanozymes for treating infected and diabetic wounds. ACS Appl Mater Interfaces. 2024;16(1):95-110
96. Li Q, Dong M, Han Q, Zhang Y, Yang D, Wei D. et al. Enhancing diabetic wound healing with a pH-responsive nanozyme hydrogel featuring multi-enzyme-like activities and oxygen self-supply. J Control Release. 2024;365:905-18
97. Liu J, Chen Z, Liu H, Qin S, Li M, Shi L. et al. Nickel-based metal-organic frameworks promote diabetic wound healing via scavenging reactive oxygen species and enhancing angiogenesis. Small. 2024;20(10):e2305076
98. Meng W, Chen X, Chen Y, Li M, Zhang L, Luo Q. et al. Self-Cascade of ROS/Glucose-Scavenging immunomodulatory hydrogels for programmed therapeutics of infected diabetic ulcers via Nrf2/NF-κB pathway. Small. 2025;21(7):e2411189
99. Sun X, Chen X, Wang S, Gu H, Bao H, Ning Z. et al. Oxygenous and biofilm-targeted nanosonosensitizer anchored with Pt nanozyme and antimicrobial peptide in the gelatin/sodium alginate hydrogel for infected diabetic wound healing. Int J Biol Macromol. 2025;293:139356
100. Hou B, Li C, Yang F, Deng W, Hu C, Liu C. et al. Ultrasmall antioxidant copper nanozyme to enhance stem cell microenvironment for promoting diabetic wound healing. Int J Nanomedicine. 2024;19:13563-78
101. Li Q, Xiao X, Yan T, Song D, Li L, Chen Z. et al. Heterojunction nanozyme hydrogels containing Cu-O-Zn bonds with strong charge transfer for accelerated diabetic wound healing. ACS Appl Mater Interfaces. 2024;16(50):68950-66
102. Chen S, Chen J, Wang X, Yang Z, Lan J, Wang L. et al. Glucose-activated self-cascade antibacterial and pro-angiogenesis nanozyme-functionalized chitosan-arginine thermosensitive hydrogel for chronic diabetic wounds healing. Carbohydr Polym. 2025;348(Pt B):122894
103. Shen B, Yan Z, Wang Y, Zhu L, Zhao Q, Jiang L. Nanozyme-chitosan-aerogel immobilized enzyme-driven biocatalytic cascade for therapeutic engineering of diabetic wounds. Carbohydr Polym. 2025;347:122690
104. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1-4
105. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835
106. Chen Q, Yang X, Yu Y, Duan X, Ni R, Song G. et al. Biomimetic cerium-assisted supra-carbon dots assembly for reactive oxygen species-activated atherosclerosis theranostic. Small. 2025;21(8):e2408980
107. Xu M, Ran D, Hu J, Mao J, Qiao D, Zhang Z. et al. Multifunctional Prussian blue nanozymes alleviate atherosclerosis through inhibiting the inflammation feedback loop. J Mater Chem B. 2025;13(4):1459-73
108. Wang C, He Y, Tang J, Mao J, Liang X, Xu M. et al. Chondroitin sulfate functionalized nanozymes inhibit the inflammation feedback loop for enhanced atherosclerosis therapy by regulating intercellular crosstalk. Int J Biol Macromol. 2024;282(Pt 3):136918
109. Chen Q, Duan X, Yu Y, Ni R, Song G, Yang X, et al Target functionalized carbon dot nanozymes with dual-model photoacoustic, fluorescence imaging for visual therapy in atherosclerosis. Adv Sci (Weinh). 2024; 11(6): e2307441.
110. Parra-Robert M, Zeng M, Shu Y, Fernández-Varo G, Perramón M, Desai D. et al. Mesoporous silica coated CeO2 nanozymes with combined lipid-lowering and antioxidant activity induce long-term improvement of the metabolic profile in obese Zucker rats. Nanoscale. 2021;13(18):8452-66
111. Gopal T, Kumar N, Perriotte-Olson C, Casey CA, Donohue TM Jr, Harris EN. et al. Nanoformulated SOD1 ameliorates the combined NASH and alcohol-associated liver disease partly via regulating CYP2E1 expression in adipose tissue and liver. Am J Physiol Gastrointest Liver Physiol. 2020;318(3):G428-G438
112. Duan H, Jing L, Xiang J, Ju C, Wu Z, Liu J. et al. CD146 associates with Gp130 to control a macrophage pro-inflammatory program that regulates the metabolic response to obesity. Adv Sci (Weinh). 2022;9(13):e2103719
113. Ding J, Du Y, Hu X, Zhao M, Li Y, Li L. et al. Aptamer-modified atomically precise gold nanoclusters as targeted nanozymes to scavenge reactive oxygen species in white adipocytes. Nanotechnology. 2023 34(36)
114. Natarajan G, Perriotte-Olson C, Casey CA, Donohue TM Jr, Talmon GA, Harris EN, et al Effect of nanoformulated copper/zinc superoxide dismutase on chronic ethanol-induced alterations in liver, adipose tissue. Alcohol. 2019; 79: 71-9.
115. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015;386(9996):913-27
116. Aranda G, Fernandez-Ruiz R, Palomo M, Romo M, Mora M, Halperin I. et al. Translational evidence of prothrombotic and inflammatory endothelial damage in Cushing syndrome after remission. Clin Endocrinol (Oxf). 2018;88(3):415-24
117. Vega-Beyhart A, Iruarrizaga M, Pané A, García-Eguren G, Giró O, Boswell L. et al. Endogenous cortisol excess confers a unique lipid signature and metabolic network. J Mol Med (Berl). 2021;99(8):1085-99
118. Dalin F, Nordling Eriksson G, Dahlqvist P, Hallgren Å, Wahlberg J, Ekwall O. et al. Clinical and immunological characteristics of autoimmune addison disease: a nationwide swedish multicenter study. J Clin Endocrinol Metab. 2017;102(2):379-89
119. Du Y, Guo M, Chen Y, Mo X, Cao J, Hu F. Ultrasensitive cortisol electrochemical immunosensor amplifying by Au single-atom nanozymes and HRP enzymes. Anal Chim Acta. 2024;1303:342462
120. Yang B, Li H, Nong C, Li X, Feng S. A novel electrochemical immunosensor based on SnS2/NiCo metal-organic frameworks loaded with gold nanoparticles for cortisol detection. Anal Biochem. 2023;669:115117
121. Luo D, Fu Q, Gao R, Su L, Su Y, Liu B. Signal-on photoelectrochemical immunoassay for salivary cortisol based on silver nanoclusters-triggered ion-exchange reaction with CdS quantum dots. Anal Bioanal Chem. 2022;414(9):3033-42
122. Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E. et al. Thyroid hormones, oxidative stress, and inflammation. Mediators Inflamm. 2016;2016:6757154
123. Zhang R, Mao YW, Li JQ, Ni LJ, Lin L, Wang AJ. et al. Fe single atoms encapsulated in N, P-codoped carbon nanosheets with enhanced peroxidase-like activity for colorimetric detection of methimazole. Spectrochim Acta A Mol Biomol Spectrosc. 2024;310:123934
124. Wu Y, Li J, Jiang W, Xu W, Zheng L, Wang C. et al. Second coordination sphere regulates nanozyme inhibition to assist early drug discovery. Nat Commun. 2025;16(1):3123
125. Wang D, Ma W, Zhang Y, Wang Y, Sun L, Jiang J. et al. A versatile nanoplatform carrying cascade Pt nanozymes remodeling tumor microenvironment for amplified sonodynamic/chemo therapy of thyroid cancer. Biomaterials. 2025;313:122778
126. Liu X, Fang Y, Chen X, Shi W, Wang X, He Z. et al. Cascaded nanozyme-based high-throughput microfluidic device integrating with glucometer and smartphone for point-of-care pheochromocytoma diagnosis. Biosens Bioelectron. 2024;251:116105
127. Zhu J, Cui Q, Long T, Wang Y, Wen W, Tian Z. et al. N-doped carbon Co/CoOx with laccase-like activity for detection of epinephrine. Mikrochim Acta. 2023;190(11):459
128. Kulandaivel S, Lin CH, Yeh YC. A bioinspired copper-based coordination polymer for the detection of pheochromocytoma biomarkers. Talanta. 2023;255:124206
129. Liu X, Wang F, Meng Y, Zhao L, Shi W, Wang X. et al. Electrochemical/visual microfluidic detection with a covalent organic framework supported platinum nanozyme-based device for early diagnosis of pheochromocytoma. Biosens Bioelectron. 2022;207:114208
130. Huang FW, Ma K, Ni XW, Qiao SL, Chen KZ. CuCoFe Layered double hydroxides as laccase mimicking nanozymes for colorimetric detection of pheochromocytoma biomarkers. Chem Commun (Camb). 2022;58(12):1982-85
131. Wang W, Cao Q, He J, Xie Y, Zhang Y, Yang L. et al. Palladium/platinum/ruthenium trimetallic dendritic nanozymes exhibiting enhanced peroxidase-like activity for signal amplification of lateral flow immunoassays. Nano Lett. 2024;24(27):8311-9
132. Cao Q, Liu Y, Yang L, Tan T, He J, Chen W, et al Popcorn-like bimetallic palladium/platinum exhibiting enhanced peroxidase-like activity for signal enhancement in lateral flow immunoassay. Anal Chim Acta. 2024; 1309: 342698.
133. Yang D, Wang L, Jia T, Lian T, Yang K, Li X. et al. Au/Fe3O4-based nanozymes with peroxidase-like activity integrated in immunochromatographic strips for highly-sensitive biomarker detection. Anal Methods. 2023;15(5):663-74
134. Yang D, Lei L, Yang K, Gao K, Jia T, Wang L. et al. An immunochromatography strip with peroxidase-mimicking ferric oxyhydroxide nanorods-mediated signal amplification and readout. Mikrochim Acta. 2022;189(2):58
135. Sun Q, Chen J, Yang M, Ding X, Zhang H, Huang Z. et al. Macrophage membrane-decorated MnO2 nanozyme catalyzed the scavenging of estradiol for endometriosis treatment. Colloids Surf B Biointerfaces. 2024;233:113633
136. Wang M, Luo X, Jiang M, Zhang L, Zhou Q, Wu C. et al. Ratio-fluorescence sensor based on carbon dots and PtRu/CN nanozyme for efficient detection of melatonin in tablet. Spectrochim Acta A Mol Biomol Spectrosc. 2024;321:124699
137. Zheng C, Jiang Q, Wang K, Li T, Zheng W, Cheng Y. et al. Nanozyme enhanced magnetic immunoassay for dual-mode detection of gastrin-17. Analyst. 2022;147(8):1678-87
138. Li Y, Liu J. Nanozyme's catching up: activity, specificity, reaction conditions and reaction types. Mater Horiz. 2021;8(2):336-50 ´
139. Wei G, Liu S, Peng Y-K, Wei H. On the specificity of nanozymes: A perspective. Chin J Chem. 2024;42(13):1515-22
140. Wang Y, He X, Huang K, Cheng N. Nanozyme as a rising star for metabolic disease management. J Nanobiotechnology. 2024;22(1):226
141. Zou Z, Bi X, Ma H, Chen L, Jin H, Gong S. et al. Recent advances in nanozymes for the treatment of atherosclerosis. Int J Nanomedicine. 2025;20:9447-72
142. Zhang R, Jiang B, Fan K, Gao L, Yan X. Designing nanozymes for in vivo applications. Nat Rev Bioeng. 2024;2:849-68
143. Food and Drug Administration. Classification of products as drugs and devices and additional product classification issues. FDA Guidance. 2017
144. European Medicines Agency. Nanotechnology-based medicinal products for human use - EU Horizon Scanning Report. EMA Report.
145. Ghorbani M, Izadi Z, Jafari S, Casals E, Rezaei F, Aliabadi A. et al. Preclinical studies conducted on nanozyme antioxidants: shortcomings and challenges based on US FDA regulations. Nanomedicine (Lond). 2021;16(13):1133-51
146. Yu Y, Zhang M, Fan K. Artificial intelligence-driven revolution in nanozyme design: from serendipity to rational engineering. Mater Horiz. 2025 Jun 26
147. Xuan W, Li X, Gao H, Zhang L, Hu J, Sun L. et al. Artificial intelligence driven platform for rapid catalytic performance assessment of nanozymes. Sci Rep. 2025;15(1):13305
148. Merlicek LP, Neumann J, Lear A, Degiorgi V, de Waal MM, Cotet TS. et al. AI.zymes: A modular platform for evolutionary enzyme design. Angew Chem Int Ed Engl. 2025;64(27):e202507031
149. Gao Y, Zhu Z, Chen Z, Guo M, Zhang Y, Wang L. et al. Machine learning in nanozymes: from design to application. Biomater Sci. 2024;12(9):2229-43
150. Park YS, Park BU, Jeon HJ. Advances in machine learning-enhanced nanozymes. Front Chem. 2024;12:1483986
151. Liu J, Chen X, Diao Q, Tang Z, Niu X. Machine-learning-assisted nanozyme-based sensor arrays: construction, empowerment, and applications. Biosensors (Basel). 2025;15(6):344
Author contact
![]() Corresponding author: Gregori Casals, Department of Biochemistry and Molecular Genetics, Hospital Clínic of Barcelona, Villarroel 170, 08036 Barcelona, Spain. +34 932275400 x 2667; casalscat.
Corresponding author: Gregori Casals, Department of Biochemistry and Molecular Genetics, Hospital Clínic of Barcelona, Villarroel 170, 08036 Barcelona, Spain. +34 932275400 x 2667; casalscat.
 Global reach, higher impact
Global reach, higher impact