13.3
Impact Factor
Theranostics 2026; 16(4):1658-1670. doi:10.7150/thno.112012 This issue Cite
Research Paper
Intra-arterial peptide receptor radionuclide therapy (IA-PRRT) in patients with SSTR-expressing neuroendocrine neoplasms: short- and long-term safety and efficacy for up to 13 years
1. Department of Diagnostic Radiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 119074, Singapore.
2. Theranostics Center of Excellence, Yong Loo Lin School of Medicine, National University of Singapore, 11 Biopolis Way, Helios, Singapore 138667, Singapore.
3. Clinical Imaging Research Centre, Centre for Translational Medicine, National University of Singapore, Singapore 117599, Singapore.
4. Nanomedicine Translational Research Program, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Singapore.
5. Department of Diagnostic & Interventional Radiology, Philipps-University Marburg, Germany
6. Department of Diagnostic and Interventional Radiology and Neuroradiology, Central Hospital Bad Berka, Germany.
7. Curanosticum Wiesbaden-Frankfurt, Department of Molecular Radiotherapy, ICPO Center of Excellence, DKD HELIOS Klinik, Wiesbaden, Germany.
8. THERANOSTICS Center for Molecular Radiotherapy and Molecular Imaging, ENETS Center of Excellence, Zentralklinik Bad Berka, Bad Berka, 99437, Germany.
Received 2025-2-12; Accepted 2025-9-18; Published 2026-1-1
Abstract
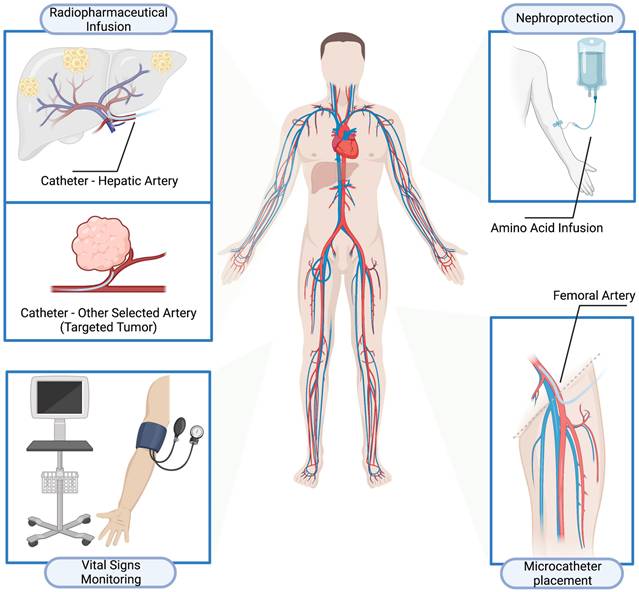
Intravenous peptide receptor radionuclide therapy (IV-PRRT) has established its role in the treatment algorithm of somatostatin receptor (SSTR)-expressing neuroendocrine neoplasms (NENs). This study aims to evaluate the safety and efficacy of intra-arterial PRRT (IA-PRRT) in patients with SSTR-expressing NENs.
Methods: The radiopharmaceutical was injected by a dedicated radionuclide infusion set via an intra-arterial catheter entering the femoral artery access site, with a microcatheter placed in the common hepatic artery or other selected artery via a standard access using the common femoral artery. Morphologic and molecular responses were evaluated in accordance with RECIST 1.1 and the EORTC criteria with 68Ga-SSTR PET/CT. Kaplan-Meier survival analysis was performed to calculate median progression-free survival (PFS) and overall survival (OS). Short- and long-term toxicities were documented in accordance with the CTCAE, version 5.0.
Results: 52 patients with SSTR-expressing NENs treated with intra-arterial PRRT with 177Lu- or 90Y-DOTATOC/DOTATATE from February 1999 to January 2019 were reviewed. The median follow-up time was 94.4 mo. Safety analysis demonstrated anemia (grade 1, n=4), leukocytopenia (grade 1, n=3; grade 2, n=1; grade 3, n=1), thrombocytopenia (grade 1, n=11) following IA-PRRT compared to baseline. No severe nephrotoxicity or liver dysfunction was observed after IA-PRRT. According to RECIST 1.1, the disease control rate at 3-6 mo after IA-PRRT was 89.4%, and the best objective response rate was 36.2%. For the entire cohort received IA-PRRT (n=52), the median PFS and OS were 29.9 and 68.9 months, respectively. In the subgroup of patients with neuroendocrine liver metastases receiving liver directed IA-PRRT, the median PFS and OS for patients with hepatic only tumor with or without lymph node metastases were significantly longer than those with extrahepatic-tumor (PFS, 35.9 mo vs. 21.6 mo, p=0.0128; OS, 80.1 mo vs. 50.5 mo, p=0.0470).
Conclusions: Intra-arterial PRRT is well-tolerated, safe and effective in patients with SSTR-expressing neuroendocrine neoplasms. The median OS and PFS appear promising, particularly in patients with hepatic tumor burden. No additional severe hematotoxicity, nephrotoxicity or hepatotoxicity was observed after IA-PRRT and during long-term follow-up. In particular, this procedure can be considered in patients with neuroendocrine liver metastases only or liver metastases mainly. Prospective studies are warranted to verify these results.
Keywords: Peptide receptor radionuclide therapy (PRRT), somatostatin receptor (SSTR), intra-arterial PRRT (IA-PRRT), 177Lu, 90Y, neuroendocrine neoplasms (NENs)
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of neoplasms arising from diffuse neuroendocrine system cells ranging from indolent well-differentiated neuroendocrine tumors (NETs) to aggressive poorly-differentiated neuroendocrine carcinomas (NECs) [1, 2]. These tumors are characterized by high expression of somatostatin receptors (SSTRs), making them amenable to SSTR-directed imaging such as PET/CT using 68Ga-labeled somatostatin analogs and therapy with these analogs labeled with therapeutic radioisotopes such as β-emitters (e.g., 177Lu or 90Y) or α-emitters (e.g., 213Bi or 225Ac) for peptide receptor radionuclide therapy (PRRT) [3-10].
Over the past two decades, PRRT with β-emitters (177Lu or 90Y) labeled somatostatin analogs (DOTATATE or DOTATOC) has demonstrated remarkable success in the management of NETs [11-13]. The significant benefit in terms of progression-free survival and response rates of PRRT over cold somatostatin analog therapy demonstrated by the randomized, controlled NETTER-1 trial led to the approval of 177Lu-DOTATATE (Lutathera) by both the European Medicines Agency and the U.S. Food and Drug Administration for the treatment of SSTR-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in adults [7]. Quality of life is also significantly improved [14].
However, the overall tumor response rate comprising complete and partial remission after PRRT is still limited. A substantial portion of patients would remain stable disease after treatment. The objective response rate from NETTER-1 trial was 18% in NET patients after intravenous 177Lu-DOTATATE PRRT. Therefore, to maximize the full therapeutic potential of PRRT in NENs, more efforts are being investigated to further increase tumor dose delivery for highly effective tumor cell killing and more selective killing of targeted cancer cells while sparing surrounding healthy tissues.
The commonly used and currently established administration route is intravenous PRRT (IV-PRRT). As compared to IV-PRRT, selective intraarterial (IA) application of radiolabeled somatostatin analogs may provide intensifying therapy by delivering more concentrated doses to the tumor, particularly in patients with focal targeted primary tumor or liver metastases which is the most common site of GEP-NETs [15-17]. A study from Heidelberg demonstrated increased tumor uptake of 68Ga-DOTATOC, in both the primary tumor of 1.44- to 7.8-fold and liver metastases of 3.75-fold higher uptakes, after selective IA administration in comparison with IV injection in neuroendocrine neoplasms [18]. In addition to the increased tumor uptake, selective IA application of radiolabeled somatostatin analogs is also hypothesized to reduce the dose delivery to normal organs and thus further reduce the toxicity of the treatment [19-22]. However, data on IA-PRRT for the treatment of NENs are very limited. Dosimetric analyses of IA versus standard IV administration indicated that IA administration of 177Lu-DOTATATE resulted in higher concentration and absorbed dose in hepatic metastases in patients of GEPNETs as compared to IV-PRRT, and thus seemed to be a powerful tool to improve the efficacy of PRRT [23]. Limouris et al. [24] and Kratochwil et al. [25] reported promising therapeutic outcome with a high rate of tumor response in NET patients treated with arterial infusion of 177Lu-DOTA-TATE, and 90Y-/177Lu-DOTATOC, respectively. In the latter study, median time to progression was not reached within a mean follow-up period of 20 months [25].
The aim of this study was to assess the safety, covering both short-term toxicity and long-term toxicity, and efficacy, in terms of response rate and survival analysis, of IA-PRRT in patients with SSTR-expressing NENs.
Materials and Methods
Patients
All patients received 177Lu- or 90Y-DOTATOC/DOTATATE IA-PRRT under the compassionate use clause of the German Medicinal Products Act. This retrospective study was performed in accordance with German regulations (Federal Agency for Radiation Protection) concerning radiation safety and was approved by the local ethics committee (Bad Berka, Germany). All patients had undergone multiple lines of treatment, including surgery, long-acting somatostatin analogues, chemotherapy, previous IV PRRT, etc. IA-PRRT was done as part of a sequence of PRRT, i.e., with systemic IV-PRRT during previous or following treatment courses. Decision to treat the patients by IA-PRRT was taken by internal or external tumor boards. All patients were either progressive before IA-PRRT, as determined by morphological imaging (CT or MRI) or by 68Ga-SSTR PET/CT, or were severely symptomatic due to extensive tumor mass or functional syndromes. All patients signed a detailed written informed consent form before undergoing the treatment, as well as consenting to the use of their anonymized clinical data for scientific purposes.
IA-PRRT Treatment Regimen
The DOTA-conjugated somatostatin analogs DOTATOC and DOTATATE were labeled with 68Ga for SSTR PET imaging and either 177Lu or 90Y for IA-PRRT, in accordance with current good manufacturing practice (CGMP) regulations [26]. More details are shown in the supplementary material.
Pre-medication was pursued with intravenous glucocorticoid and antiemetic in adequate doses. The patient was transferred to the Angio suite. A microcatheter was placed in the common hepatic artery (for liver metastases) or other selected artery (targeted tumors at other sites, i.e., primary tumors) via a standard access using the common femoral artery, by an interventional radiologist. The standard amino acid infusion for nephroprotection commenced intravenously via a peripheral venous cannula about 30 min before the intraarterial injection of the therapeutic dose of radionuclide and lasted for 4 h. Under direct medical supervision, the radiopharmaceutical was injected by a dedicated radionuclide infusion set over 60 min as slow infusion via the intra-arterial catheter entering the femoral artery access site. The patients were observed clinically, vital parameters (heart rate, blood pressure, temperature, etc.) were monitored and recorded at regular intervals before, during, and after the infusion (Figure 1). The intraarterial catheter was removed by a member of the interventional radiology team according to protocol, and usually about 6 h after insertion. A pressure bandage was placed on the intraarterial insertion site, which was monitored clinically, and removed as per interventional radiology protocol. The administered radioactivity was individually calculated on the basis of the Bad Berka Score; SUV on receptor PET/CT (referrals: OctreoScan K.S.), renal function (GFR and TER / creatinine & BUN), hematological status (blood counts), liver involvement, extrahepatic tumor burden, Ki-67 index/tumor grade, FDG status (glucose hypermetabolism of tumors), tumor dynamics (doubling time, new lesions), Karnofsky performance index, weight loss, time since first diagnosis and functional activity of tumor [27-31]. Patients received IV and IA-PRRT at different stages in their disease; the time interval between cycles did not vary per se with the type of PRRT but was dependent on the Bad Berka score. For IA-PRRT, significant liver involvement, tumor progression, and heavy tumor burden of liver metastases or targeted tumors at other sites, i.e., primary tumors, were the deciding factors.
Toxicity Assessment
All patients were clinically monitored during therapy and for at least 2-4 days thereafter as inpatients for possible side effects. Vital parameters were recorded during therapy and a structured questionnaire documented any delayed complication. Laboratory analyses including hematologic status, renal function, and liver function were performed before and after IA-PRRT, and at each restaging. Details were prospectively documented in a structured database (comprising over 250 items per patient). Treatment-related adverse events were recorded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Additional detailed parameters are shown in the supplementary material.
Response Assessment
The treatment response was evaluated on CT or MR images according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1) [32] and by PET imaging according to the criteria of the European Organization for Research and Treatment of Cancer (EORTC) [33]. Imaging was performed before each IA-PRRT cycle and at restaging. Restaging was performed every 3-4 mo after each cycle of PRRT, and every 6 mo for stable disease or remission after initial follow-up, until disease progression was evident on imaging. The disease control rate was defined as complete remission (CR), partial remission (PR), and stable disease (SD). The best objective response rate was defined as patients achieving CR or PR at follow-up.
Statistical Analysis
Kaplan-Meier survival analysis was performed to determine median progression-free survival (PFS) and median overall survival (OS), defined from the start of PRRT in general to a follow-up time of at least 3 mo after IA-PRRT. The median PFS and OS were compared to the previous cohort with comparable baseline characteristics receiving IV-PRRT at our center (Supplemental Table 1). The log-rank test was used to analyze the survival distribution of subgroups. Continuous variables were denoted as mean ± standard deviation. Differences between paired samples before and after treatment were determined by Student's t-test. For all variables that were proven with the Kolmogorov-Smirnov test to follow the skewed distribution, quantitative data were described in terms of median and range, and nonparametric sign tests were used to determine the significance of differences between parameters before and after treatment. All statistical tests were two-tailed, and a p value of less than 0.05 was considered statistically significant.
Results
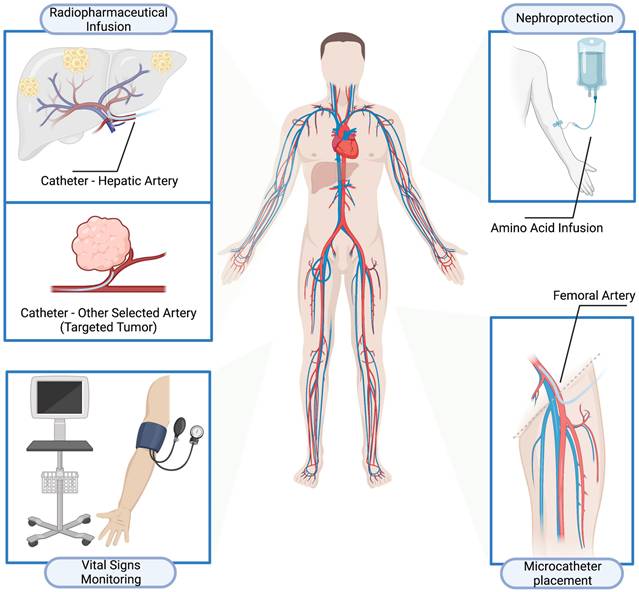
Fifty-two patients (29 men and 23 women; age 19-80 y, mean age 52.4±14.4y) with metastatic SSTR-expressing NENs who received intra-arterial PRRT at Zentralklinik Bad Berka (Germany) from February 1999 to January 2019 were reviewed. The demographics of the patients at baseline are shown in Table 1. Twenty-seven patients (51.9%) were previously treated with systemic 90Y- or 177Lu- IV- PRRT during previous treatment courses. Among them, 13 patients also received IV-PRRT after at least one cycle of IA-PRRT. Five patients (9.6%) received IA-PRRT only, while 20 patients (38.5%) received IA-PRRT followed by IV-PRRT at different stages of their NEN disease (Figure 2). In total, 33 patients (63.5%) patients received IV-PRRT after at least one cycle of IA-PRRT.
The time interval between cycles did not vary per se with the type of PRRT. Treatment cycles and cumulative radioactivity are summarized in Table 2. The median administered activity for 177Lu IA-PRRT per cycle was 6.9±1.1 GBq (range, 5.5-8.5 GBq). The median administered activity for 90Y IA-PRRT per cycle was 4.3±1.1 GBq (range, 1.5-7.3 GBq). The maximum cumulative administered activities were 15.9 GBq and 22.6 GBq for 177Lu IA-PRRT and 90Y IA-PRRT, respectively.
Safety
All patients tolerated the therapy without any serious acute adverse effects. No clinically significant adverse effects were noticed or reported by any patient during hospitalization for therapy or follow-up.
In the short-term following IA-PRRT, grade 3 leukocytopenia was observed in only 1 (1.9%) patient and subsequently improved to CTC grade 2 during follow-up. This patient, with a pancreatic NET, received 3 cycles of 90Y-IA-PRRT (intra-primary tumor, 3.6 GBq, 5 GBq and 2.5 GBq), followed by 1 additional cycle of 177Lu-IV-PRRT (5.6 GBq). Grade 3 leukocytopenia occurred after the first 90Y-IA-PRRT and subsequently improved to CTC grade 2, which was maintained throughout follow-up.
Treatment procedures of intra-arterial peptide receptor radionuclide therapy (IA-PRRT).
Demographic and baseline clinical characteristics of patients with NEN (n = 52)
| Characteristics | Number (n) | Percentage (%) |
|---|---|---|
| Sex - no. (%) | ||
| Male | 29 | 55.8 |
| Female | 23 | 44.2 |
| Age - yr | 52.4±14.4 | |
| Primary tumor site - no. (%) | ||
| CUP | 2 | 3.8 |
| Pancreas | 41 | 78.8 |
| Midgut | 6 | 11.5 |
| Others | 3 | 5.8 |
| Functional vs. Nonfunctional - no. (%) | ||
| Functional NEN | 16 | 30.8 |
| Nonfunctional NEN | 36 | 69.2 |
| Ki-67 index grading | ||
| G1 (Ki-67 <3%) | 10 | 19.2 |
| G2 (Ki-67 =3%-20%) | 26 | 50.0 |
| G3 (Ki-67 >20%) | 2 | 3.8 |
| NA | 14 | 26.9 |
| Primary Tumor Resection | ||
| Yes | 22 | 42.3 |
| No | 30 | 57.7 |
| Tumor metastases | ||
| Liver | 37 | 71.2 |
| Lymph nodes | 22 | 42.3 |
| Lung | 1 | 1.9 |
| Peritoneum | 2 | 3.8 |
| Bone | 8 | 15.4 |
| Others | 7 | 13.5 |
| Microcatheter placement and administration routes for IA-PRRT | ||
| Primary tumors (selected artery) | 15 | 28.8 |
| Pancreas | 13 | 25.0 |
| Ileum | 2 | 3.8 |
| Liver metastases (hepatic artery) | 35 | 67.3 |
| Both primary tumor and liver metastases (hepatic artery and other selected artery) | 2 | 3.8 |
| Previous treatments | ||
| Surgery | 44 | 84.6 |
| Cold somatostatin analogues | 24 | 46.2 |
| Chemotherapy (excl. TACE) | 22 | 42.3 |
| TACE | 8 | 15.4 |
| Immunotherapy | 2 | 3.8 |
| Other radiotherapy (incl. SIRT) | 3 | 5.8 |
| Other (RFA, cryotherapy, other studies) | 8 | 15.4 |
During long-term follow-up with combined IA- and IV-PRRT, 1 patient (1.9%) developed grade 3 anemia. This patient had received 1 cycle of 177Lu-IV-PRRT (4.5 GBq), followed by 1 cycle of 90Y-IA-PRRT (4.5 GBq), and multiple cycle of 177Lu-IV-PRRT (5.4 GBq, 6.8 GBq, 5.6 GBq, 6.6 GBq). Grade 3 anemia occurred after the 6th IV-PRRT cycle, approximately 16 months after the first IV-PRRT. Additionally, 1 patient (1.9%) developed grade 3 thrombocytopenia. This patient underwent 4 cycles of IV-PRRT (177Lu, 9 GBq; 90Y, 4.5 GBq; 90Y, 5 GBq; 177Lu, 5.7 GBq), 1 cycle of 90Y-IA-PRRT (7.3 GBq), and 2 further cycles of IV-PRRT (177Lu, 7.3 GBq; 177Lu, 5 GBq), CTC-3 thrombocytopenia occurred after the 6th of IV-PRRT cycle, approximately 36 months after initiation of IV-PRRT.
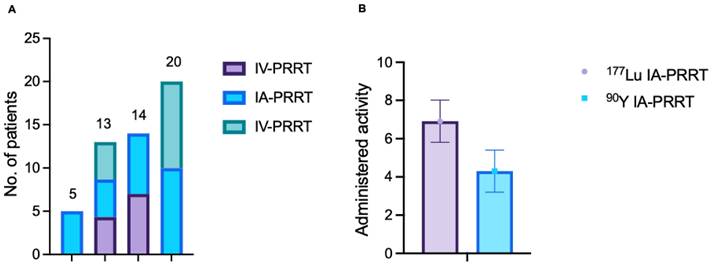
No CTC grade 4 anemia, leukocytopenia or thrombocytopenia was observed. The hematological profile was shown in Table 3. Comparison of hemoglobin, leukocyte count, and platelet count before and after IA-PRRT was shown in Figure 3.
No CTCAE grade 3 or 4 nephrotoxicity was observed in any patient in the short-term following IA-PRRT. Furthermore, among patients presenting with a CTCAE grade 1 or 2 renal dysfunction before IA-PRRT, there was no additional post-therapeutic nephrotoxicity in any patient after IA-PRRT. During long-term follow-up with combined IA- and IV-PRRT, CTC grade 3 nephrotoxicity was documented in 1 of 52 patients (1.9%) with pancreatic NET. This patient had received 1 cycle of 90Y-IV-PRRT (4 GBq), 2 cycles of 90Y-IA-PRRT (intra-primary tumor, 3.5 GBq and 4.6 GBq), 1 additional cycle of90Y-IV-PRRT (5 GBq), and 1 further cycle of 90Y-IA-PRRT (3.5 GBq), resulting in a cumulative administered activity of 20.6 GBq between 2004 and 2005. On restaging follow up, CTC-3 nephrotoxicity was first detected in 2008, 4 years after the initiation of combined IA- and IV- PRRT. No CTC-4 nephrotoxicity was observed (Table 4).
Treatment cycles and cumulative administered radioactivity for IA-PRRT (n = 52)
| Variables | n | % | Cumulative radioactivity (GBq) | |
|---|---|---|---|---|
| Mean | SD | |||
| Number of IA-PRRT cycles | 52 | 100 | ||
| 1 | 38 | 73.1 | 4.8 | 1.3 |
| 2 | 6 | 11.5 | 9.4 | 4.1 |
| 3 | 5 | 9.6 | 13.6 | 4.1 |
| 4 | 0 | 0 | / | |
| 5 | 1 | 1.9 | 22.6 | / |
| TANDEM (90Y+177Lu) | 2 | 3.8 | 11.8 | / |
| Number of 90Y-IA PRRT cycles | 42 | 80.8 | ||
| 1 | 33 | 63.5 | 4.5 | 1.1 |
| 2 | 4 | 7.7 | 7.4 | 3.2 |
| 3 | 4 | 7.7 | 11.8 | 0.7 |
| 4 | 0 | 0 | / | |
| 5 | 1 | 1.9 | 22.6 | / |
| Number of 177Lu-IA PRRT cycles | 6 | 11.5 | ||
| 1 | 5 | 9.6 | 6.5 | 1.1 |
| 2 | 1 | 1.9 | 14.9 | / |
| Number of 90Y+177Lu-IA PRRT cycles | ||||
| 2 | 1 | 1.9 | 11.8 | / |
| 3 | 1 | 1.9 | 20.9 | / |
| Number of TANDEM cycles | 2 | 3.8 | ||
| 1 cycle of TANDEM + 1 cycle of 90Y | 1 | 1.9 | 15.4 | / |
| 1 cycle of TANDEM | 1 | 1.9 | 8.1 | / |
Hematotoxicity 3-6 months after the last cycle of IA-PRRT§ and long-term follow-up after IA-PRRT and IV-PRRT* according to CTCAE v.5.0 (n = 52)
| Numbers of patients with: | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anemia | Leukocytopenia | Thrombocytopenia | |||||||
| Grade | Before IA-PRRT | After IA PRRT§ | Long-term* | Before IA-PRRT | After IA PRRT§ | Long-term* | Before IA-PRRT | After IA- PRRT§ | Long-term* |
| CTC-1 | 28 | 32 | 31 | 5 | 8 | 6 | 2 | 13 | 11 |
| CTC-2 | 5 | 7 | 10 | 1 | 2 | 3 | 1 | 1 | 0 |
| CTC-3 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| CTC-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CTC-5 | NA | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 |
NA=not applicable before IA-PRRT (grade 5 represents death).
(A) Types of PRRT treatments. Five patients received IA-PRRT only; 14 patients received IV-PRRT followed by IA-PRRT; 13 patients received IV-PRRT, followed by IA-PRRT, and then IV-PRRT again; and 20 patients received IA-PRRT followed by IV-PRRT. (B) Median administered activity per cycle for IA-PRRT was 6.9 ± 1.1 GBq (range, 5.5 - 8.5 GBq) for 177Lu and 4.3 ± 1.1 GBq (range, 1.5 - 7.3 GBq) for 90Y.
Nephrotoxicity 3-6 months after the last cycle of IA-PRRT§ and long-term follow-up after IA-PRRT and IV-PRRT* according to CTCAE v.5.0 (n = 52)
| Numbers of patients with: | |||
|---|---|---|---|
| Nephrotoxicity | |||
| Grade | Before IA-PRRT | After IA PRRT§ | Long-term* |
| CTC-1 | 6 | 5 | 6 |
| CTC-2 | 1 | 1 | 3 |
| CTC-3 | 0 | 0 | 1 |
| CTC-4 | 0 | 0 | 0 |
| CTC-5 | NA | 0 | 0 |
NA=not applicable before IA-PRRT (grade 5 represents death).
There was no evidence of any severe hepatotoxicity during the short-term follow up after IA-PRRT. During long-term follow-up of patients treated with a combination of IA- and IV- PRRT, CTC-3 hepatotoxicity was observed in 2 of 52 patient (3.8%). One patient, who had received 1 cycle of 90Y-IV-PRRT followed by 1 cycle of 90Y-IA-PRRT and 2 cycles of 177Lu-IV-PRRT, developed grade 3 hepatotoxicity 18 months after initiation of combined IA+IV treatment, which subsequently improved to CTC-2. The second patient, who had initially received 1 cycle of 90Y-IA-PRRT without hepatotoxicity, later underwent 8 cycles of 177Lu-IV-PRRT and subsequently developed grade 3 hepatotoxicity 55 months after initiation of combined IA+IV treatment, likely attributable to significant disease progression of liver metastases. Importantly, no cases of CTC grade 4 hepatotoxicity were observed either in the short-term following IA-PRRT or in the long-term follow-up of patients receiving combined IA- and IV-PRRT (Table 5). No evidence of significant synthetic liver dysfunction or any significant enzymatic hepatic dysfunction was observed. There was no significant change in albumin, total protein, Quick test (prothrombin time), AST, ALT, GGT, or bilirubin after IA-PRRT (p > 0.05) (Figure 3).
Treatment Response
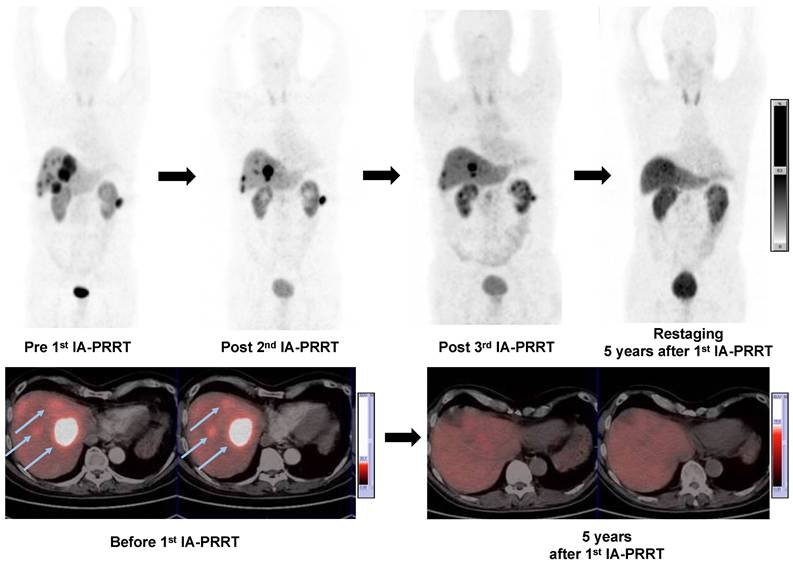
Of the 52 patients, a response evaluation at 3-6 mo after IA-PRRT was possible in 47 patients. According to RECIST 1.1, the disease control rate was 89.4%, including 1 (2.1%) patient with CR, 16 (34.0%) patients with PR and 25 (53.2%) patients with SD, whereas 5 patients (10.6%) had progressive disease (Table 6). The best objective response rate (ORR) was 36.2%. On the basis of the EORTC criteria, 1 (2.1%) patient had CR, 21 patients (44.7%) had PR and 20 patients had SD (42.6%). The disease control rate 3-6 months after IA-PRRT was 89.4%. The best objective ORR was 46.8%. Figure 4 showed a representative example of tumor response after IA-PRRT.
Comparison of hemoglobin, leukocyte count, platelet count, serum creatinine, Quick test (Prothrombin time/PT), albumin, total protein (TP), GOT, GPT, alkaline phosphatase (ALP), gamma-GT, and bilirubin before and after IA-PRRT.
In the long-term follow-up of patients who received both IA- and IV-PRRT, the best ORR and DCR were 48.0% and 90.0%, respectively, according to RECIST 1.1, and 66.0% and 90.0%, respectively, according to EORTC (Table 7). The median time to best observed response was 5.2 months for both EORTC (range, 1.8-86.6 months) and RECIST (range, 1.8-51.5 months). The best observed radiographic response among patients who did not receive IV-PRRT after IA-PRRT was an ORR of 64.7% and a DCR of 82.4% according to EORTC criteria, and an ORR of 41.2% and a DCR of 88.2% according to RECIST 1.1 (Table 7).
Survival
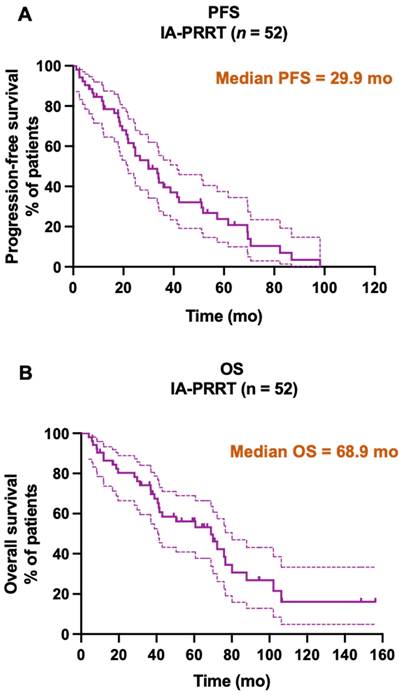
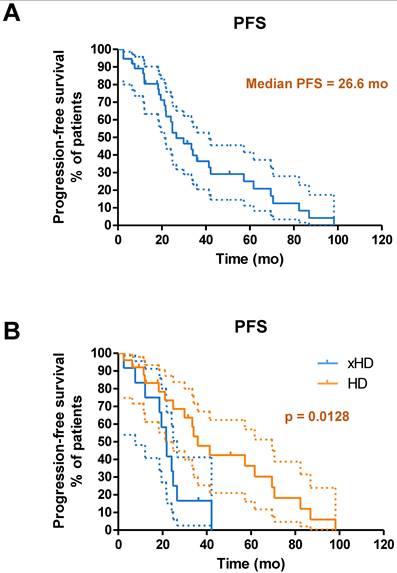
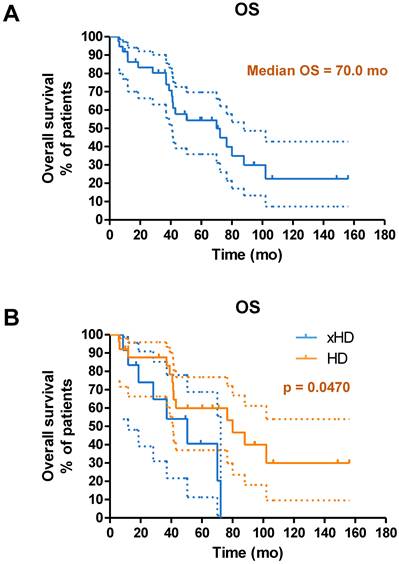
Until the study cutoff date in January 2019, 31 of 52 patients (59.6%) underwent IA-PRRT died. The median follow-up time was 94.4 mo (range, 4.0-156.2 mo). For the entire group of 52 patients receiving IA-PRRT (including those who also underwent additional intravenous PRRT during the extended long-term follow-up period), the median PFS and OS were 29.9 mo and 68.9 mo, calculated from the initiation of the first PRRT (Figure 5). For the 37 patients with neuroendocrine liver metastases (NELM) who underwent IA-PRRT and additional IV-PRRT treatments, the median PFS and OS were 26.6 mo and 70.0 mo, respectively, from the initiation of the first PRRT (Figures 6 and 7).
Discussion
To our knowledge, this is the largest reported cohort of patients with SSTR-expressing NENs treated with IA-PRRT to date. Both short-term safety and responses after IA-PRRT, and long-term safety and survival accounted for by the additional treatments were evaluated. The follow-up (median, 94.4 mo) in this patient cohort is the longest among all published relevant studies. IA-PRRT resulted in excellent tumor response with a disease control rate of 89.4%.
A 57-y-old man with well-differentiated, nonfunctioning metastatic pancreatic NEN. Maximum-intensity-projection (MIP) (top-left, Pre 1st IA-PRRT) images from 68Ga-SSTR PET/CT showed SSTR expression in liver metastases (arrows) and lymph nodes metastases with SUVmax of 41.4. Patient was treated with 3 cycles of 90Y-DOTATATE IA-PRRT with cumulative administered radioactivity of 12.7 GBq. After the 2nd IA-PRRT and 3rd IA-PRRT, 68Ga-SSTR PET/CT showed significant regression of the liver and paracolic lymph node metastases (PR). MIP images from restaging 68Ga-SSTR PET/CT 5 years after 1st IA-PRRT showed complete remission of the lesions. No adverse effects were observed during and after the treatment with long-term follow-up.
Hepatotoxicity 3-6 months after the last cycle of IA-PRRT§ and long-term follow-up after IA-PRRT and IV-PRRT* according to CTCAE v.5.0 (n = 52)
| Numbers of patients according to: | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Albumin | Quick | AST | ALT | ALP | GGT | Bilirubin | |||||||||
| Grade | Before IA-PRRT | After IA PRRT§ | Long-term* | After IA PRRT§ | Long-term* | After IA PRRT§ | Long-term* | After IA PRRT§ | Long-term* | After IA PRRT§ | Long-term* | After IA PRRT§ | Long-term* | After IA PRRT§ | Long-term* |
| CTC-1 | 4 | 4 | 6 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| CTC-2 | 1 | 3 | 3 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 5 | 1 | 3 | 0 | 2 |
| CTC-3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 |
| CTC-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CTC-5 | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
NA=not applicable before IA-PRRT (grade 5 represents death).
Treatment response at 3-6 months after IA-PRRT (n = 47)
| Response after IA-PRRT (n=47) | Total | including TANDEM* | 90Y-IA PRRT | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| EORTC - SSTR imaging response - no. (%) | n=47 | n=9 | n=38 | |||
| Complete response | 1 | 2.1 | 1 | 11.1 | 0 | 0 |
| Partial response | 21 | 44.7 | 6 | 66.7 | 15 | 39.5 |
| Stable disease | 20 | 42.6 | 2 | 22.2 | 18 | 47.4 |
| Progressive disease | 5 | 10.6 | 0 | 0 | 5 | 13.2 |
| ORR | 22 | 46.8 | 7 | 77.8 | 15 | 39.5 |
| DCR | 42 | 89.4 | 9 | 100 | 33 | 86.8 |
| RECIST - CT and/or MRI response - no. (%) | n=47 | n=9 | n=38 | |||
| Complete response | 1 | 2.1 | 1 | 11.1 | 0 | 0 |
| Partial response | 16 | 34.0 | 3 | 33.3 | 13 | 34.2 |
| Stable disease | 25 | 53.2 | 5 | 55.6 | 20 | 52.6 |
| Progressive disease | 5 | 10.6 | 0 | 0 | 5 | 13.2 |
| ORR | 17 | 36.2 | 4 | 44.4 | 13 | 34.2 |
| DCR | 42 | 89.4 | 9 | 100 | 33 | 86.8 |
* Numbers of patients underwent 177Lu-IA PRRT, 90Y+177Lu-IA PRRT or TANDEM PRRT
Kaplan-Meier curves for PFS (A, median PFS = 29.9 mo) and OS (B, median OS = 68.9 mo) from start of PRRT in general for all patients receiving IA-PRRT (not excluding those who also underwent additional IV-PRRT during the long-term follow-up period) in the present study (n = 52).
(A) Kaplan-Meier curves for PFS from start of PRRT in general for patients with neuroendocrine liver metastases receiving IA-PRRT (not excluding those who also underwent additional IV-PRRT during the long-term follow-up period) (n = 37). (B) Kaplan-Meier curves for PFS for patients with hepatic disease only (with or without lymph nodes metastases, HD, n = 25) and patients with extrahepatic tumor metastases (other organ involvement except lymph nodes, xHD, n = 12) (PFS, 35.9 mo vs. 21.6 mo, p = 0.0128).
(A) Kaplan-Meier curves for OS from start of PRRT in general for patients with neuroendocrine liver metastases receiving IA-PRRT (not excluding those who also underwent additional IV-PRRT during the long-term follow-up period) (n = 37). (B) Kaplan-Meier curves for OS for patients with hepatic disease only (with or without lymph nodes metastases, HD, n = 25) and patients with extrahepatic tumor metastases (other organ involvement except lymph nodes, xHD, n = 12) (OS, 80.1 mo vs. 50.5 mo, p = 0.0470).
The excellent treatment response to IA-PRRT was likely attributable to the combination of direct therapeutic effect of the radiopharmaceutical to the dominant tumors and systemic therapeutic effect to all the SSTR-expressing tumors. Kratochwil et al. reported a head-to-head intraindividual comparison of selective arterial versus venous 68Ga-DOTATOC PET/CT in 15 patients with GEP-NETs. They demonstrated several-fold higher uptake in the primary tumor as well as 3.75-fold higher uptake in liver metastases of NENs after selective IA administration in comparison with IV injection. Pool et al. also demonstrated increased radionuclide uptake of SSTR analog 111In-DTPAOC by using IA administration via the hepatic artery as compared to IV in NET liver metastases [34]. Dosimetry studies further demonstrated high intratumoral concentration and prolonged absorbed tumor doses with IA administration [23, 35].
Best tumor response after IA-PRRT and IV-PRRT in all patients (n = 50) and in patients who did not receive IV-PRRT after IA-PRRT (n = 17)
| Response after IA-PRRT and IV-PRRT | Total | |
|---|---|---|
| n | % | |
| EORTC - SSTR imaging response - no. (%) | n=50 | |
| Complete response | 2 | 4.0 |
| Partial response | 31 | 62.0 |
| Stable disease | 12 | 24.0 |
| Progressive disease | 5 | 10.0 |
| ORR | 33 | 66.0 |
| DCR | 45 | 90.0 |
| RECIST - CT and/or MRI response - no. (%) | n=50 | |
| Complete response | 2 | 4.0 |
| Partial response | 22 | 44.0 |
| Stable disease | 21 | 42.0 |
| Progressive disease | 5 | 10.0 |
| ORR | 24 | 48.0 |
| DCR | 45 | 90.0 |
| Response among patients who did not receive IV-PRRT after IA-PRRT | Total | |
| n | % | |
| EORTC - SSTR imaging response - no. (%) | n=17 | |
| Complete response | 1 | 5.9 |
| Partial response | 10 | 58.8 |
| Stable disease | 3 | 17.6 |
| Progressive disease | 3 | 17.6 |
| ORR | 11 | 64.7 |
| DCR | 14 | 82.4 |
| RECIST - CT and/or MRI response - no. (%) | n=17 | |
| Complete response | 1 | 5.9 |
| Partial response | 6 | 35.3 |
| Stable disease | 8 | 47.1 |
| Progressive disease | 2 | 11.8 |
| ORR | 7 | 41.2 |
| DCR | 15 | 88.2 |
A more recent study by Lawhn-Heath et al. evaluated a single treatment using 90Y-DOTATOC and the comparison between IA and IV 68Ga-DOTATOC infusion in 5 patients, showing that IA 68Ga-DOTATOC failed to demonstrate increased uptake by hepatic metastases compared to IV [22]. However, the time points differed between IA and IV infusion (63±7 min after IV injection vs. 90±20 min from the midpoint of the IA infusion), the result of which might not be suitable for a direct comparison; in addition, the IA 68Ga-DOTATOC could be influenced as it was administered concurrently with the therapeutic 90Y-DOTATOC. Lawhn-Heath et al. also reported in 10 patients that the single treatment using hepatic intraarterial administration of 90Y-DOTATOC did not induce tumor shrinkage, indicating that more treatment cycles might be required [22]. In contrast, McStay et al. [20] and Kolasińska-Ćwikła et al. [21] reported hepatic intraarterial PRRT of 90Y-DOTA-lanreotide and 90Y-DOTATATE, being safe and effective for patients with progressive SSTR-positive liver metastases from NETs [20]. Thakral et al. demonstrated that IA administration of 177Lu-DOTATATE resulted in higher concentration and absorbed dose in hepatic metastases of GEPNETs as compared to a single dose of PRRT administered through standard IV route. In a cohort with 12 patients, Limouris et al. reported that repeated, trans-hepatic high doses of 177Lu-DOTA-TATE resulted in a high tumor response with a PR in 75% of the treated patients in unresectable metastatic SSTR-positive liver lesions [24]. Kratochwil et al. also reported the high rate of objective radiologic response in NET patients treated with arterial infusion of 90Y-/177Lu-DOTATOC compared favorably with systemic chemotherapy and intravenous radiopeptide therapy in a cohort of 15 patients with liver metastases arising from GEP-NETs [25]. More recently, a multicenter, randomized controlled trial in 26 NET patients is being conducted to investigate whether IA-PRRT with 177Lu-DOTATATE results in a higher activity concentration in liver metastases compared to IV administration [36]. Our data are in accordance with the reported results, we observed a remarkably high response rate after IA-PRRT, likely due to the previously reported high first-pass effect, by delivering more concentrated doses of the agent to the dominant tumors, followed by a systemic therapeutic effect to other tumor locations as radioactivity is further distributed in the systemic circulation [18].
In the present study, both 90Y and 177Lu were used for IA-PRRT, with the concept of individualized precision oncology. Typically, we utilized 90Y for bulkier tumors due to the higher energy and 177Lu for smaller tumors. We also took into account the influence of the longer range of 90Y to normal organs such as bone marrow reserve, to maximize the patient benefits given the different physical properties of radioisotopes [12, 37]. The administered radioactivity was individually calculated on the basis of the Bad Berka Score [12, 27, 28, 38], and the timing of IA-PRRT was related to disease spread as seen on PET/CT, especially regarding liver metastases. Although IA PRRT was not by itself an indication to give PRRT at a higher frequency, is not impossible that patients with more aggressive liver disease progression received an IA cycle earlier than they would have received an IV cycle had they had less aggressive disease.
The majority of patients in this cohort received IA-PRRT via hepatic artery. Accordingly, potential liver adverse effects could be expected. However, in this study, no severe (grade 3 or 4) hepatotoxicity was observed after 1-5 cycles of IA-PRRT. During long-term follow-up of patients treated with a combination of IA- and IV- PRRT, grade hepatotoxicity was observed in 2 of 52 patient. These events were transient, improving on subsequent follow-up, or were attributable to disease progression from liver metastases following IV-PRRT. Notably, no cases of grade 4 hepatotoxicity were observed either in the short-term after IA-PRRT or during long-term follow-up after combined IA- and IV-PRRT, underscoring the favorable safety profile of IA-PRRT.
The long-term outcomes of patients in the present cohort were very encouraging. For comparison, our previously reported cohort of 1048 patients who exclusively received intravenous PRRT at the same medical center, utilizing the identical EORTC criteria for PFS evaluation, had a median PFS and OS of 19 mo and 51 mo, respectively (Supplemental Table 2) [12]. In contrast, the median OS and PFS in the present IA-PRRT cohort appeared to be improved compared with the prior standard IV-PRRT cohort. However, this study is limited by its nature as a single-center retrospective study, and the cohorts have not been case-control matched. Prospective studies are naturally needed in the future for further verification. In addition, we observed that as compared to patients with neuroendocrine liver metastases plus extrahepatic disease, patients with neuroendocrine liver metastases who underwent IA-PRRT and additional IV-PRRT treatments had a better outcome. These results might be partly attributable to the high first-pass effect of IA-PRRT treatment, by delivering more concentrated doses of the agent to the dominant tumors followed by the redistribution of radioactivity after the first pass effect in the systemic circulations as discussed above [18].
This study has a few limitations. One limitation of the present study is that it is a retrospective analysis (however, with prospective data sampling using a structured database). No strict pretest criteria for the selection of patients were applied, and the patient group was heterogeneous. Another limitation is the lack of availability of the exact Ki-67 index in 14 patients; these patients were referred from other centers and were characterized by histopathologically confirmed NENs and with tumor uptake on 68Ga-SSTR PET imaging. After IA-PRRT, per long-term follow-up, patients also received additional IV-PRRT given the remission of the dominant tumors after IA-PRRT or other consideration, and those patients were considered suitable for IV-PRRT at the given time. Therefore, the long-term outcome, in term of overall survival, was actually from the combination of IA-PRRT and IV-PRRT. There were variations in radioisotopes and SSTR affinities because different radiopharmaceuticals were used. Further prospective and controlled studies are certainly warranted.
Conclusion
This study confirms that intra-arterial PRRT is well-tolerated, safe, and effective in patients with SSTR-expressing NENs. The median OS and PFS appear promising, particularly in patients with hepatic tumor burden. No additional severe hematotoxicity, nephrotoxicity, or hepatotoxicity as expected was observed after IA-PRRT and during long-term follow-up. Prospective studies are warranted to verify these results.
Abbreviations
NET: neuroendocrine tumor; NEN: Neuroendocrine neoplasm; NEC: neuroendocrine carcinoma; SSTR: somatostatin receptor; IA-PRRT: Intra-arterial peptide receptor radionuclide therapy; IV: intravenous; PET/CT: positron emission tomography combined with computed tomography; GEP-NETs: gastroenteropancreatic neuroendocrine tumors; NELM: neuroendocrine liver metastases; CUP: cancer of unknown primary; DOTA: 1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid; LM4: p-Cl-Phe-cyclo[DCys-Pal-Daph(Cbm)-Lys-Thr-Cys]DTyr-NH2; SUV: standardized uptake value; CTCAE: Common Terminology Criteria for Adverse Events; RECIST: Response Evaluation Criteria in Solid Tumors; EORTC: European Organization for Research and Treatment of Cancer; CR: complete remission; PR: partial remission; SD: stable disease; PFS: progression-free survival; OS: overall survival; ORR: objective response rate.
Supplementary Material
Supplementary materials and methods, tables.
Acknowledgements
This study was supported by the National University of Singapore Start-up Grant (NUHSRO/2021/097/Startup/13, NUHSRO/2023/008/NUSMed/TCE/LOA, NUHSRO/2021/034/TRP/09/Nanomedicine), National Medical Research Council (MOH-001483-00, MOH-001334-00, MOH-001254-01), Singapore Ministry of Education (FY2022) Tier 1 Grant (NUHSRO/2022/093/T1/Seed-Sep/06) and National Research Foundation (NRF-000352-00), the International Centers for Precision Oncology (ICPO) Foundation (NUS-P21), and 2024 Tsinghua-NUS Joint Research Initiative Fund.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV. et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72
2. Huguet I, Grossman AB, O'Toole D. Changes in the Epidemiology of Neuroendocrine Tumours. Neuroendocrinology. 2017;104:105-11
3. Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P. et al. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J Clin Oncol. 2016;34:588-96
4. Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K. et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J Nucl Med. 2018;59:66-74
5. Lee ST, Kulkarni HR, Singh A, Baum RP. Theranostics of Neuroendocrine Tumors. Visc Med. 2017;33:358-66
6. Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP. et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124-30
7. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B. et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-35
8. Zhang J, Singh A, Kulkarni HR, Schuchardt C, Muller D, Wester HJ. et al. From Bench to Bedside-The Bad Berka Experience With First-in-Human Studies. Semin Nucl Med. 2019;49:422-37
9. Ballal S, Yadav MP, Bal C, Sahoo RK, Tripathi M. Broadening horizons with (225)Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to (177)Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging. 2020;47:934-46
10. Panda A, Garg I, Johnson GB, Truty MJ, Halfdanarson TR, Goenka AH. Molecular radionuclide imaging of pancreatic neoplasms. Lancet Gastroenterol Hepatol. 2019;4:559-70
11. Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Horsch D, O'Dorisio MS. et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800-16
12. Baum RP, Kulkarni HR, Singh A, Kaemmerer D, Mueller D, Prasad V. et al. Results and adverse events of personalized peptide receptor radionuclide therapy with (90)Yttrium and (177)Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget. 2018;9:16932-50
13. Hope TA, Bodei L, Chan JA, El-Haddad G, Fidelman N, Kunz PL. et al. NANETS/SNMMI Consensus Statement on Patient Selection and Appropriate Use of (177)Lu-DOTATATE Peptide Receptor Radionuclide Therapy. J Nucl Med. 2020;61:222-7
14. Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M. et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With (177)Lu-Dotatate in the Phase III NETTER-1 Trial. J Clin Oncol. 2018;36:2578-84
15. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-59
16. Frilling A, Sotiropoulos GC, Li J, Kornasiewicz O, Plockinger U. Multimodal management of neuroendocrine liver metastases. HPB (Oxford). 2010;12:361-79
17. Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753-81
18. Kratochwil C, Giesel FL, Lopez-Benitez R, Schimpfky N, Kunze K, Eisenhut M. et al. Intraindividual comparison of selective arterial versus venous 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors. Clin Cancer Res. 2010;16:2899-905
19. Buscombe JR. Interventional nuclear medicine in hepatocellular carcinoma and other tumours. Nucl Med Commun. 2002;23:837-41
20. McStay MK, Maudgil D, Williams M, Tibballs JM, Watkinson AF, Caplin ME. et al. Large-volume liver metastases from neuroendocrine tumors: hepatic intraarterial 90Y-DOTA-lanreotide as effective palliative therapy. Radiology. 2005;237:718-26
21. Kolasinska-Cwikla A, Nowicki ML, Sankowski AJ, Palucki JM, Buscombe JR, Glinka L. et al. Radiological and Clinical Efficacy of Intra-Arterial (90)Y-DOTATATE in Patients with Unresectable, Progressive, Liver Dominant Neuroendocrine Neoplasms. J Clin Med. 2021 10
22. Lawhn-Heath C, Fidelman N, Chee B, Jivan S, Armstrong E, Zhang L. et al. Intraarterial Peptide Receptor Radionuclide Therapy Using (90)Y-DOTATOC for Hepatic Metastases of Neuroendocrine Tumors. J Nucl Med. 2021;62:221-7
23. Thakral P, Sen I, Das SS, Manda D, Cb V, Malik D. Dosimetric analyses of intra-arterial versus standard intravenous administration of 177Lu-DOTATATE in patients of well differentiated neuroendocrine tumor with liver-dominant metastatic disease. Br J Radiol. 2021;94:20210403
24. Limouris GS, Karfis I, Chatzioannou A, Paphiti MI, Lyra M, Gennatas K. et al. Super-selective hepatic arterial infusions as established technique ('ARETAIEION' Protocol) of [177Lu]DOTA-TATE in inoperable neuroendocrine liver metastases of gastro-entero-pancreatic (GEP) tumors. Q J Nucl Med Mol Imaging. 2012;56:551-8
25. Kratochwil C, Lopez-Benitez R, Mier W, Haufe S, Isermann B, Kauczor HU. et al. Hepatic arterial infusion enhances DOTATOC radiopeptide therapy in patients with neuroendocrine liver metastases. Endocr Relat Cancer. 2011;18:595-602
26. Wehrmann C, Senftleben S, Zachert C, Muller D, Baum RP. Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm. 2007;22:406-16
27. Baum RP, Kulkarni HR, Carreras C. Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin Nucl Med. 2012;42:190-207
28. Zhang J, Kulkarni HR, Singh A, Niepsch K, Muller D, Baum RP. Peptide Receptor Radionuclide Therapy in Grade 3 Neuroendocrine Neoplasms: Safety and Survival Analysis in 69 Patients. J Nucl Med. 2019;60:377-85
29. Baum RP, Kulkarni HR. THERANOSTICS: From Molecular Imaging Using Ga-68 Labeled Tracers and PET/CT to Personalized Radionuclide Therapy - The Bad Berka Experience. Theranostics. 2012;2:437-47
30. Zhang J, Kulkarni HR, Singh A, Baum RP. Successful Intra-arterial Peptide Receptor Radionuclide Therapy of DOTATOC-Negative High-Grade Liver Metastases of a Pancreatic Neuroendocrine Neoplasm Using 177Lu-DOTA-LM3: A Somatostatin Receptor Antagonist. Clin Nucl Med. 2020;45:e165-e8
31. Baum RP, Zhang J, Schuchardt C, Muller D, Macke H. First-in-Humans Study of the SSTR Antagonist (177)Lu-DOTA-LM3 for Peptide Receptor Radionuclide Therapy in Patients with Metastatic Neuroendocrine Neoplasms: Dosimetry, Safety, and Efficacy. J Nucl Med. 2021;62:1571-81
32. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-47
33. Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA. et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773-82
34. Pool SE, Kam BL, Koning GA, Konijnenberg M, Ten Hagen TL, Breeman WA. et al. [(111)In-DTPA]octreotide tumor uptake in GEPNET liver metastases after intra-arterial administration: an overview of preclinical and clinical observations and implications for tumor radiation dose after peptide radionuclide therapy. Cancer Biother Radiopharm. 2014;29:179-87
35. Puranik AD, Rangarajan V, Shetty NS, Gala K, Kulkarni S, Mohite A. et al. Intra-arterial PRRT with Lu-177 DOTATATE in Liver-dominant Metastatic Neuroendocrine Tumors: Early Assessment of Efficacy and Toxicity. Indian J Nucl Med. 2024;39:71-6
36. Ebbers SC, Braat A, Moelker A, Stokkel MPM, Lam M, Barentsz MW. Intra-arterial versus standard intravenous administration of lutetium-177-DOTA-octreotate in patients with NET liver metastases: study protocol for a multicenter, randomized controlled trial (LUTIA trial). Trials. 2020;21:141
37. Gabriel M, Andergassen U, Putzer D, Kroiss A, Waitz D, Von Guggenberg E. et al. Individualized peptide-related-radionuclide-therapy concept using different radiolabelled somatostatin analogs in advanced cancer patients. Q J Nucl Med Mol Imaging. 2010;54:92-9
38. Schuchardt C, Kulkarni HR, Prasad V, Zachert C, Muller D, Baum RP. The Bad Berka dose protocol: comparative results of dosimetry in peptide receptor radionuclide therapy using (177)Lu-DOTATATE, (177)Lu-DOTANOC, and (177)Lu-DOTATOC. Recent Results Cancer Res. 2013;194:519-36
Author contact
![]() Corresponding authors: Jingjing Zhang, MD, PhD. Department of Diagnostic Radiology, National University of Singapore. National University Hospital, Main Building, Lobby F, #04-398, 5 Lower Kent Ridge Road, Singapore 119074, Singapore. Phone: +65 84353534. E-mail: j.zhangedu.sg; Richard P. Baum, MD, PhD. Curanosticum Wiesbaden-Frankfurt, Center for Advanced Radiomolecular Precision Oncology, DKD HELIOS Klinik Wiesbaden Aukammallee 33, 65191 Wiesbaden, Germany. Email: baumrpcom.
Corresponding authors: Jingjing Zhang, MD, PhD. Department of Diagnostic Radiology, National University of Singapore. National University Hospital, Main Building, Lobby F, #04-398, 5 Lower Kent Ridge Road, Singapore 119074, Singapore. Phone: +65 84353534. E-mail: j.zhangedu.sg; Richard P. Baum, MD, PhD. Curanosticum Wiesbaden-Frankfurt, Center for Advanced Radiomolecular Precision Oncology, DKD HELIOS Klinik Wiesbaden Aukammallee 33, 65191 Wiesbaden, Germany. Email: baumrpcom.
 Global reach, higher impact
Global reach, higher impact