13.3
Impact Factor
Theranostics 2026; 16(9):4657-4701. doi:10.7150/thno.124716 This issue Cite
Review
Panoramic description of ROS-based nanotechnology for osteomyelitis therapy: Challenges, opportunities, and prospects
1. Department of Spine Surgery, The Affiliated Hospital of Qingdao University, Qingdao 266700, China.
2. Institute of Regenerative Medicine and Laboratory Technology Innovation, Qingdao University, Qingdao 266071, China.
3. Qingdao Key Lab of Common Diseases, University of Health and Rehabilitation Sciences, Qingdao Municipal Hospital, Qingdao 266071, China.
Received 2025-9-5; Accepted 2026-1-13; Published 2026-2-11
Abstract
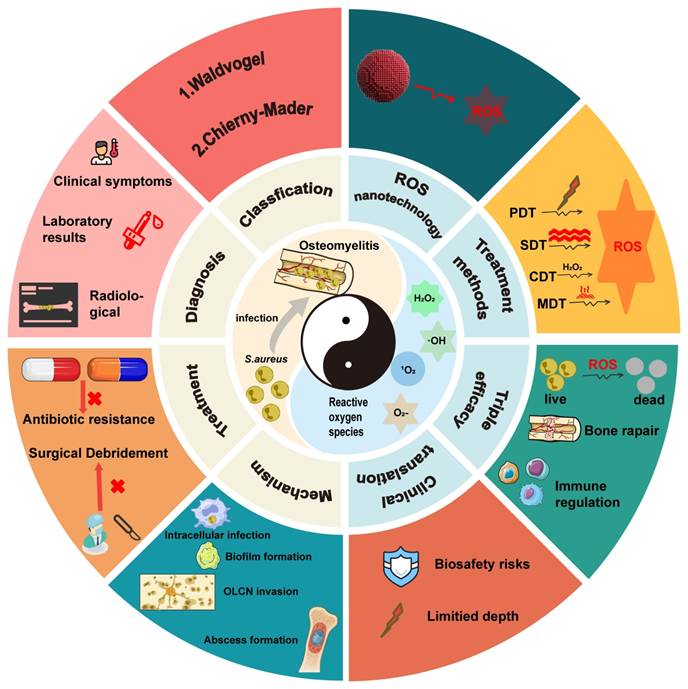
Osteomyelitis, an inflammatory disease of bone and bone marrow caused by infectious microorganisms, has long been a major clinical challenge due to the lack of consistently effective treatment strategies. Conventional therapeutic approaches, such as antibiotic therapy and surgical debridement, are frequently associated with the development of antibiotic resistance and a high risk of disease recurrence, thereby complicating long-term clinical management. In recent years, reactive oxygen species (ROS)-based nanotechnology has emerged as a promising therapeutic modality for osteomyelitis, garnering considerable attention for the potential to overcome antibiotic resistance. This review summarizes the epidemiological characteristics, current treatment approaches, and pathogenic mechanisms of osteomyelitis, and comprehensively examines advances in ROS nanotechnologies for osteomyelitis treatment. In addition, the technical advantages and limitations of major ROS-based strategies, including photodynamic therapy (PDT), sonodynamic therapy (SDT), chemodynamic therapy (CDT), and microwave dynamic therapy (MWDT), are systematically discussed to provide guidance for further optimization of ROS-mediated strategies. Furthermore, the therapeutic potential of these strategies in antimicrobial activity, promotion of tissue repair, and immune regulation is analyzed, offering theoretical support for the integration of ROS-based strategies with existing treatment modalities for improved management of osteomyelitis.
Keywords: osteomyelitis, reactive oxygen species, nanomaterials, nanotechnology
Introduction
Osteomyelitis is widely recognized as an inflammatory disease of bone and bone marrow caused by infectious microorganisms and is closely associated with local bone destruction, necrosis, and impaired new bone formation [1]. The reported annual incidence of osteomyelitis ranges from approximately 4.8 to 21.8 cases per 100,000 population [2]. Considerable variation in incidence exists across different populations and geographic regions, largely influenced by factors such as age distribution, underlying comorbidities, prevalence of diabetes, frequency of post-traumatic and post-operative infections, and regional sanitary conditions [3]. Although the incidence of acute osteomyelitis has declined in many developed countries as a result of improvements in public health measures and nutritional support, higher rates continue to be observed in several developing regions, particularly in parts of Africa, where urbanization and increased motor vehicle use have contributed to a rising number of open injuries [4]. Fracture internal fixation and joint replacement represent common sources of infection leading to osteomyelitis, while elderly individuals with diabetes, neurological disorders, or peripheral vascular disease are at increased risk of developing chronic disease, with approximately 20% of patients with diabetic foot ulcers progressing to osteomyelitis [5]. Despite advances in medical care that have reduced the occurrence of acute infection, the incidence of chronic osteomyelitis continues to increase as a consequence of population aging, a growing number of high-energy traumatic injuries, and the expanding volume of orthopedic surgical procedures [6]. Bone infection following open fractures and periprosthetic joint infection after arthroplasty often progress to chronic osteomyelitis, imposing a substantial financial burden on affected individuals due to prolonged hospitalization and complex treatment requirements [7]. In severe cases, treatment failure may ultimately result in limb amputation or mortality. Consequently, the effective eradication of infection and prevention of disease recurrence have emerged as critical research priorities in the contemporary management of chronic osteomyelitis.
At present, clinically accepted treatment for osteomyelitis remains largely dependent on antibiotic therapy and surgical intervention. Excessive and prolonged use of antibiotics has led to the rapid emergence of antimicrobial resistance and the increasing prevalence of multidrug-resistant bacterial infections. Although surgical debridement enables prompt removal of necrotic and infected tissue, complete eradication of resistant microorganisms remains difficult, thereby increasing the likelihood of disease recurrence [8]. Extensive efforts have therefore been directed towards addressing antibiotic overuse and recurrent infection in osteomyelitis management, including the development of novel antimicrobial agents and improvements in surgical techniques and diagnostic strategies; however, clinical outcomes remain suboptimal [9]. With recent advances in nanomedicine, reactive oxygen species (ROS)-based nanotechnology has emerged as a promising therapeutic approach for chronic osteomyelitis, demonstrating broad-spectrum antimicrobial activity and effective suppression of drug-resistant and multi drug-resistant pathogens [10].
This review provides a comprehensive and systematic discussion of the clinical classification, pathogenesis, diagnostic techniques, and current treatment strategies for osteomyelitis. Particular emphasis is placed on the mechanisms by which Staphylococcus aureus (S. aureus), one of the primary causative agents, facilitates persistent infection and immune evasion at the site of infection, thereby contributing to refractory chronic osteomyelitis. Recent progress in ROS-based nanotechnologies for osteomyelitis treatment is subsequently examined in detail. The technical advantages and inherent limitations of major ROS-mediated strategies, including photodynamic therapy (PDT), sonodynamic therapy (SDT), chemodynamic therapy (CDT), and microwave dynamic therapy (MWDT), are analyzed to provide direction for further optimization. In addition, the therapeutic potential of these approaches in antimicrobial activity, enhancement of tissue repair, and modulation of immune responses is evaluated, offering theoretical support for the integration of ROS-based strategies with existing treatment modalities. Finally, current challenges and future prospects of ROS-based nanotechnology in osteomyelitis therapy are discussed to provide a comprehensive overview of emerging treatment strategies and their potential clinical significance (Figure 1).
Although recent studies have reported the application of ROS-based technologies in the treatment of bone infections, optimization strategies and combination approaches specifically designed for osteomyelitis, together with clearly defined therapeutic mechanisms, remain insufficiently summarized. Furthermore, the development of ROS technologies tailored to the unique pathological microenvironment of osteomyelitis has received limited attention. Within this context, the present review contributes in three major aspects. First, a comprehensive and critical panoramic analysis of ROS nanotechnologies, including PDT, SDT, CDT, and MWDT, is provided, with particular emphasis on multimodal integration strategies to overcome the limitations of single-modality approaches. Second, beyond the direct antimicrobial effects mediated through disruption of microbial cellular structures, the pivotal role of ROS-based approaches in modulating the immunosuppressive bone microenvironment and their synergistic interactions with bone regeneration strategies are systematically elucidated, leading to the proposal of an integrated triple-therapy framework encompassing anti-infection, immune-regulation, and bone-repair as a comprehensive paradigm for osteomyelitis management. Third, critical challenges and future directions for the clinical translation of ROS nanotechnologies are thoughtfully examined, including considerations of material biosafety, targeting efficiency, rational combination therapy design, and translational feasibility. Collectively, this review provides timely and distinctive guidance for researchers and clinicians pursuing innovative therapeutic strategies for osteomyelitis beyond conventional antibacterial approaches.
Osteomyelitis
Pathogenic mechanism of S. aureus in osteomyelitis
Owing to the remarkable capacity for invasion, colonization, and proliferation within bone tissue, S. aureus exhibits distinct pathogenic behavior during skeletal infection. The major mechanisms underlying persistent osteomyelitis include intracellular infection, formation of staphylococcal abscess communities (SACs), invasion of the osteocyte lacuno-canalicular network (OLCN), and biofilm formation [11]. Systematic analysis of these mechanisms not only clarifies the specific pathway by which S. aureus invades and colonizes bone tissue but also provides a conceptual foundation for the development of novel therapeutic strategies for refractory osteomyelitis.
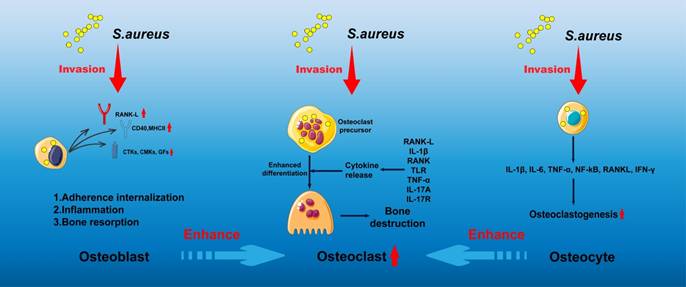
Intracellular infection: Intracellular infection represents a central mechanism contributing to the persistence and recurrence of osteomyelitis, as S. aureus is capable of long-term survival in a dormant state within host cells. Multiple cell types, including macrophages, endothelial cells, keratinocytes, and epithelial cells, have been shown to harbor intracellular S. aureus [12-14]. Infection of macrophages is particularly associated with chronic osteomyelitis. These infected macrophages, often described as "Trojan horse" macrophages, facilitate systemic bacterial dissemination, promote the enrichment of small colony variants (SCV), and attenuate host inflammatory responses [14]. Such phenotypic adaptation enables S. aureus to evade immune surveillance and constitutes a major driver of chronic and recurrent infection. In addition, S. aureus is capable of invading osteoblasts, osteoclasts, and osteocytes (Figure 2), where intracellular persistence eventually leads to host cell apoptosis or sustained intracellular infection, further perpetuating disease progression [15-26].
Schematic illustration of the application of reactive oxygen species-based nanotechnology in the treatment of osteomyelitis. The figure summarizes the principal components addressed in this review, including the pathological microenvironment of osteomyelitis, major ROS-mediated therapeutic strategies (PDT, SDT, CDT, and MWDT), and their core functions in antibacterial activity, immune regulation, and bone regeneration.
Summary diagram illustrating the responses of osteoblasts, osteoclasts, and osteocytes in the presence of S. aureus.
Formation of SACs: S. aureus can achieve effective self-protection within the host microenvironment through the formation of specialized protective barriers, gradually developing into SACs that inhibit immune cell attack and facilitate bacterial survival and dissemination [27]. SACs, representing abscess structures associated with low- virulence bacterial infection, are mainly found within the bone marrow cavity of the long bones in pediatric patients. Structurally, SACs consist of a central bacterial community surrounded a fibrin-rich pseudocapsule and infiltrating immune cells, a configuration that confers high bacterial viability and substantial resistance to both antibiotic treatment and host immune defenses [28]. SAC formation is governed by complex regulatory signaling pathways. Initially, S. aureus activates coagulase (CoA) and von Willebrand factor-binding protein (vWFbp), which bind to and activate prothrombin, thereby promoting the formation of protective fibrin pseudocapsules [29]. Subsequently, clumping factor A (ClfA) and clumping factor B (ClfB) interact with fibrinogen, enabling the pathogen to evade phagocytic recognition and contributing critically to SAC development [30]. In parallel, S. aureus secretes immune evasion proteins, such as Staphylococcal chemoattractant proteins (CHIPS) and Staphylococcal complement inhibitors (SCIN), which suppress acute immune responses [31]. Furthermore, S. aureus induces destruction of neutrophils recruited to the abscess community, generating cellular debris that paradoxically forms an additional physical barrier, thereby preventing subsequent infiltration of host defense cells and further enhancing bacterial persistence [32].
Formation of Biofilm: Biofilms are organized microbial communities that adhere to tissue surfaces or biomaterial interfaces and represent one of the most prevalent complications in orthopedic infections. The surfaces of commonly used orthopedic materials, such as titanium and its alloys, stainless steel, cobalt-chromium alloys, polymeric materials, and polymethyl methacrylate (PMMA) bone cement, provide favorable substrates for biofilm development. Biofilm formation by S. aureus at sites of bone injury significantly restricts the penetration of antibiotics and immune cells, thereby promoting persistent infection [33]. Biofilm development generally proceeds through sequential stages of bacterial attachment, accumulation, and dispersion within host tissues [34-35]. Bacteria released from mature biofilms secrete a broad range of virulence factors, including coagulase, lipase, hyaluronidase, and protein A (SpA), which play important roles in disease progression and osteomyelitis pathogenesis. Among these, toxic shock syndrome toxin-1 (TSST-1), alpha-hemolysin (Hla), and Pantone-Valentine leukocidin (PVL) have been shown to significantly exacerbate osteomyelitis. TSST-1, a potent superantigen that suppresses plasma cell differentiation and antibody responsiveness, markedly inhibits host immune function and induces elevated expression of cytokines associated with toxic shock syndrome, including IFN-γ, TNF, IL-2, and IL-6 [36]. Furthermore, TSST-1 has been demonstrated to enhance osteoclast-mediated bone resorption and accelerate bone tissue destruction [37]. Hla, a secreted cytotoxin synthesized by many S. aureus strains, disrupts early neutrophil recruitment by impairing calcium signaling and calcium-dependent leukotriene B4 (LTB4) production [38]; however, Hla expression is downregulated during persistent bone infection, contributing to bacterial quiescence and the latent nature of recurrent osteomyelitis [39]. PVL, produced by a limited subset of S. aureus strains, lyses leukocytes through membrane disruption and is strongly associated with rapid disease progression and sustained infection [40]. In addition, S. aureus-derived coagulase promotes biofilm stabilization by converting fibrinogen into fibrin, thereby generating a protective barrier that shields bacteria from immune attack. Coagulase further contributes to bone destruction by inhibiting osteoblast proliferation, inducing apoptosis, and reducing mineralization, ultimately aggravating bone loss during infection [41].
OLCN invasion: Invasion of the OLCN by S. aureus represents another critical mechanism underlying the persistence of osteomyelitis, enabling the pathogen to evade host defenses more effectively and substantially increasing the risk of recurrence following treatment of refractory osteomyelitis [11]. Emerging evidence indicates that S. aureus can penetrate cortical bone and persist within the OLCN, a process attributed in part to the remarkable deformability of the bacterial cells [42]. Using transmission electron microscopy (TEM), de Mesy et al. directly visualized the invasion and colonization of cortical bone by S. aureus and demonstrated that the bacteria are capable of deforming to diameters of approximately 0.5 µm to facilitate migration through sub micron bone channels [43]. Consistent with these findings, bacterial deformation and infiltration of the OLCN were subsequently observed in lesion samples from patients with infectious diabetic foot ulcers complicated by chronic S. aureus osteomyelitis [44]. Notably, clinical reports describing reactivation of infection 50 to 75 years after the initial episode further support the capacity of S. aureus for long-term persistence within bone tissue, likely mediated by submicron deformation and slow metabolic activity of S. aureus within the osteocellular canicular system [45-47].
Traditional treatments for osteomyelitis
Effective management of osteomyelitis and prevention of disease recurrence require a comprehensive, interdisciplinary treatment approach encompassing thorough patient evaluation, appropriate antimicrobial therapy, and necessary surgical intervention. Selection of specific treatment modalities is influenced by multiple factors, such as disease pathogenesis, anatomical site, presence of orthopedic implants, and host-pathogen interactions, resulting in significant interindividual variability. Despite these differences, the core objectives of osteomyelitis management remain consistent, namely the eradication of local infection and control of systemic dissemination [48]. This section provides a systematic overview of conventional antibiotic-based and surgical treatment strategies, together with an analysis of their inherent limitations, with the aim of informing optimization of current therapeutic approaches and identifying potential directions for future development.
Antibiotic therapy: Antibiotic administration remains essential in the treatment of osteomyelitis, regardless of whether surgical debridement is performed [49]. Systemic antimicrobial therapy constitutes an important component of disease management, with empirical broad-spectrum antibiotics typically initiated prior to the availability of microbiological culture and sensitivity results. Owing to the high likelihood of rapid resistance development with monotherapy, combination antibiotic regimens are commonly employed when formulating clinical treatment plans [50]. Despite widespread clinical use, systemic antibiotic therapy is associated with substantial limitations. Prolonged high-dose exposure frequently increases the risk of multi-organ toxicity, while long-term treatment contributes to the development of irreversible antimicrobial resistance. The clinical application of local antibiotic delivery for osteomyelitis remains controversial [51-54], and existing localized strategies face additional challenges, including suboptimal drug release kinetics, limited biocompatibility, and the need for secondary surgical procedures.
Surgical Treatment: Surgical management of osteomyelitis is primarily directed toward thorough debridement of infected tissue, reconstruction of bone and soft tissue defects, and prevention of associated complications. In chronic osteomyelitis, debridement typically involves removal of inflammatory tissue, sinus tracts, scar tissue, infected granulation tissue, medullary abscesses, sclerotic bone, and necrotic bone. Clinical outcomes following surgical intervention are strongly influenced by anatomical location, integrity of surrounding soft tissues, presence of orthopedic implants, formation of deep abscesses or biofilms, and host immune status, resulting in considerable interpatient variability [55]. Osteomyelitis associated with diabetic foot infection presents particular therapeutic challenges, often accompanied by severe soft tissue infection, neuroarthritic osteoarthropathy, and compromised vascular supply, which collectively contribute to suboptimal treatment outcomes [56-57]. Consequently, patients with metabolic disorders require highly individualized therapeutic strategies. Moreover, even after technically successful surgical intervention, osteomyelitis remains associated with a substantial risk of recurrence, underscoring the need for adjunctive technologies capable of achieving complete eradication of residual microorganisms while simultaneously promoting restoration of the local microenvironment.
Other Treatments: Hyperbaric oxygen therapy has shown unique therapeutic advantages in the treatment of osteomyelitis. The antibacterial effect of hyperbaric oxygen in osteomyelitis is thought to be closely related to enhanced formation of ROS [58]. In addition, hyperbaric oxygen therapy reduces localized pathogen burden through activation of host immune responses and synergistic interaction with antimicrobial agents, while its anti-inflammatory properties further contribute to the attenuation of bone tissue injury and infection [59]. Beyond oxygen-based approaches, growth factor- and gene-based strategies have also been explored for osteomyelitis treatment. Aliyev et al. demonstrated that vascular endothelial growth factor (VEGF) gene-transfected muscle flaps significantly reduced abscess formation and bone necrosis by inhibiting disease progression and promoting local blood supply, supporting the development of molecular therapies for osteomyelitis [60]. Moreover, a growing number of emerging technologies have reported considerable potential for the treatment of infectious diseases [61-66], among which ROS-based antimicrobial strategies represent particularly promising therapeutic candidates [67].
Application of ROS nanotechnology in osteomyelitis
In recent years, ROS-based antibacterial nanotechnology has attracted increasing attention in the clinical management of osteomyelitis due to its potent antimicrobial efficacy and low propensity for inducing drug resistance. The core mechanism underlying ROS-mediated antibacterial nanotechnology involves exploitation of the physicochemical properties of nanomaterials to generate ROS, thereby disrupting the redox homeostasis of microbial cells and ultimately inducing cell death. This section systematically examines the potential advantages and therapeutic applications of ROS-based antibacterial nanotechnology in osteomyelitis treatment, with the aim of providing valuable insights for the development of next-generation anti-osteomyelitis therapies.
Overview of ROS
ROS are highly reactive and unstable oxygen-derived molecules generated as byproducts of cellular oxygen metabolism, primarily within organelles such as mitochondria, endoplasmic reticulum, and peroxisomes. Major ROS species include free radicals such as superoxide anion (·O2-), hydroxyl radical (·OH), hydroperoxyl radical (·HOO), carbonate radical (·CO3-), and carbon dioxide radical (·CO2-), as well as non-radical oxidants including singlet oxygen (1O2), hydrogen peroxide (H2O2), hypobromous acid (HOBr), and hypochlorous acid (HOCl) [68].
Acting as critical intracellular signaling mediators, ROS regulate multiple canonical signaling pathways involved in inflammation and infection. ROS activate upstream kinases of the mitogen-activated protein kinase (MAPK) cascade, including c-Jun N-terminal kinase (JNK) and extracellular regulatory protein kinase (ERK), thereby promoting activation of the transcription factor AP-1 and inducing expression of genes related to cell proliferation, survival and inflammatory responses [69]. Accumulation of ROS further amplifies phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling, thereby enhancing downstream pathways that support cellular survival and proliferation. Sustained ROS production also maintains continuous activation of the JAK/STAT pathway by stimulating Janus kinases and inhibiting protein phosphatases such as SHP-2, ultimately driving transcription of inflammation-related genes [69]. Within the nuclear factor κB (NF-κB) signaling network, ROS often act as second messengers, oxidizing NF-κB directly or modifying key cysteine residues on IκB, thereby promoting dissociation of the NF-κB-IκB complex and promoting NF-κB nuclear translocation, which culminates in the expression of pro-inflammatory cytokines [70]. In parallel, ROS modulate the antioxidant response through activation of the Nrf2 pathway. Oxidative modification of key cysteine residues on Keap1 leads to Nrf2 release and nuclear translocation, thereby initiating the transcription of antioxidant enzyme, including heme oxygenase-1 (HO-1) and NAD(P)H quinone dehydrogenase 1 (NQO1), to counterbalance oxidative stress. Dysregulation of these interconnected pathways has been observed in chronic inflammatory and infectious conditions, where excessive ROS simultaneously hyperactivate pro-inflammatory cascades such as MAPK, NF-κB, and PI3K/Akt while suppressing Nrf2-mediated antioxidant defenses, thereby driving amplification of tissue-destructive inflammatory responses [69].
Substantial endogenous production of ROS at sites of infection induces oxidative stress and triggers amplification of pro-inflammatory signaling pathways, including MAPK, NF-κB, and NLRP3, thereby reshaping the local microenvironment in osteomyelitis [69,70]. This pathological microenvironment is characterized by persistent inflammation, enhanced cellular apoptosis, and increased bone resorption. The elevated ROS levels within infected bone tissue provide valuable molecular targets for diagnostic applications. For instance, ROS-responsive fluorescent and photoacoustic probes can be selectively "illuminated" within inflamed bone tissues, aiding early lesion localization and dynamic monitoring of disease progression.
Osteomyelitis lesions are further distinguished by persistent microbial colonization and biofilm formation accompanied by immune suppression or dysfunction. ROS exert direct antimicrobial effects by inducing lipid peroxidation and damaging microbial proteins and nucleic acids, thereby serving as a non-antibiotic, broad-spectrum antimicrobial mechanism. Concurrently, physiologically moderate ROS signaling modulates immune responses by activating or reprogramming immune cells. Experimental evidence indicates that ROS promote macrophage polarization toward the M1 phenotype, enhancing bactericidal activity, and enhancing adaptive immune responses through regulation of T-cell activation.
At low to moderate concentrations, ROS promote angiogenesis, osteoblast proliferation and differentiation, and tissue regeneration. In contrast, elevated ROS levels exhibit superior antimicrobial efficacy at infected bone sites but may also induce collateral bone damage. To mitigate ROS-associated osteotoxicity following antibacterial treatment, integrated therapeutic strategies combining ROS-based antimicrobial approaches with osteogenic modalities have been developed, including growth factor delivery, osteoconductive scaffolds, and bioactive ion release systems. These combinatorial platforms enable precise antimicrobial action while simultaneously promoting bone defect repair and reconstruction of the pathological bone microenvironment.
Classification of ROS nanotechnology
Recent advances in nanotechnology, particularly in nanochemistry and nanofabrication, have enabled the development of a diverse range of ROS-generating nanomaterials with microenvironment-responsive and regulatory properties, which are increasingly applied across biomedical fields, including osteomyelitis therapy [71]. These nanomaterials produce distinct ROS upon stimulation by near-infrared (NIR), ultrasound (US), chemical reaction substrate, microwave energy, and related triggers, ultimately inducing irreversible bacterial damage and apoptosis. On the basis of their mechanisms of ROS generation and associated energy sources, ROS-based nanotechnologies are broadly categorized into PDT, SDT, CDT, and MWDT [72] (Table 1).
PDT employs photosensitizers that generate ROS upon irradiation with specific wavelengths of light, thereby directly inactivating pathogenic microorganisms. PDT exhibits broad applicability and a low propensity for inducing drug resistance. Within the hypoxic microenvironment characteristic of osteomyelitis, PDT predominantly generates ·O2- and ·OH through electron transfer-mediated Type I photochemical reactions, enhancing antimicrobial efficacy against drug-resistant bacteria.
Classification of ROS-based nanotechnologies.
| Method | Stimulus | Mechanism | Main ROS types | Advantage | Disadvantage | More suitable type of osteomyelitis | Prospects |
|---|---|---|---|---|---|---|---|
| PDT | Light of specific wavelength | Type II photochemical reaction | ¹O₂ | Non-invasive, highly selective, can be applied locally | Limited tissue penetration depth, may cause phototoxicity, requires external light source | Acute/superficial osteomyelitis | New photosensitizers, wireless micro-LED light sources, and integrated diagnosis and treatment platforms |
| SDT | Ultrasound | US cavitation | OH and ¹O₂ | Deep tissue penetration, strong destructive power on biofilms | May cause tissue damage (such as thermal effects or mechanical damage), sonosensitizer delivery challenges | Chronic/biofilm-associated osteomyelitis | Multifunctional sonosensitizer, ultrasound-multimodal imaging guided therapy, portable device |
| CDT | Microenvironment endogenous substances | Fenton or Fenton-like reaction | ·OH | Does not rely on external energy, self-supplies H₂O₂/regulates the microenvironment | Reaction efficiency depends on the local environment (such as pH, H2O2 concentration) | Acute/chronic osteomyelitis | Intelligent responsive nanocatalytic materials, self-cycling CDT systems, catalytic immunotherapy |
| MWDT | Microwave | Thermal effect and non-thermal effect | ·OH, ¹O₂, or ·O₂⁻ | It has strong penetrating ability, rapid treatment, and is effective for deep and encapsulated biofilms | Thermal damage, complex and expensive equipment, difficult control, and the need for precise temperature monitoring | Chronic/deep/biofilm-associated osteomyelitis | Focus on microwave technology, microwave-responsive smart nanomaterials, and real-time ablation tools as surgical assistants |
SDT utilizes deep-penetrating ultrasound to excite sonosensitizers, leading to ROS production and mechanical effects. SDT demonstrates favorable penetration and targeting properties for deep-seated bone marrow infections while exerting minimal impact on surrounding healthy tissues. Furthermore, cavitation and shear forces generated during SDT disrupt bacterial biofilm architecture, thereby improving antibacterial efficiency. CDT exploits nanozyme-mediated Fenton or Fenton-like reactions within infection tissues to catalyze the conversion of endogenous hydrogen peroxide (H2O2) into highly reactive ·OH, enabling sustained ROS generation for antimicrobial activity without external energy input. In addition, certain catalytic systems incorporating calcium and magnesium release bioactive metal ions during the reaction process, synergistically promoting bone regeneration. MWDT relies on microwave sensitizers that generate both thermal effects and ROS under microwave irradiation, offering advantages of deep tissue penetration and highly efficient sterilization.
PDT for osteomyelitis treatment
Overview of PDT in osteomyelitis treatment
In the early twentieth century, von Tappeiner and Rabb first proposed the potential therapeutic potential of photosensitive synthetic dyes in combination with light and molecular oxygen and introduced the concept of "photodynamic action" [73]. Recently, PDT-based medical devices have been developed and approved for clinical application by the U.S. Food and Drug Administration (FDA), underscoring the growing translational relevance of this modality [74,75].
PDT relies on the synergistic interaction among photosensitizers, tissue oxygen concentration, and an appropriate light source to induce microbial inactivation. Unlike conventional antimicrobial agents, PDT triggers cytotoxic reactions through light-induced excitation of photosensitizers, resulting in the generation of ROS that ultimately disrupt bacterial structures and functions. This mechanism represents a distinct class of anti-infective therapy with demonstrated efficacy against drug-resistant pathogens in both in vivo and in vitro settings. On this basis, photodynamic antimicrobial chemotherapy (PACT) has been developed as a specialized application of PDT. PACT is founded on the selective location of non-toxic photosensitizers within pathogenic microorganisms, thereby minimizing off-target toxicity to surrounding tissues, followed by light activation at specific wavelength to generate highly cytotoxic ROS that inactivate pathogens [76]. PACT is therefore particularly suited for the treatment of microbial infections, including bacterial, fungal, viral, and parasitic diseases. In the context of osteomyelitis therapy, photosensitizers are delivered to the infected bone marrow cavity through systemic administration, such as intraperitoneal or intravenous injection, or by local injection. After a defined dark incubation period, the photosensitizer accumulates preferentially at the site of infection via blood circulation. Subsequent laser irradiation of the affected region induces localized ROS production, leading to efficient bacterial inactivation within the bone marrow cavity and therapeutic resolution of infection. Subsequent studies further confirmed the pronounced antibacterial efficacy of PACT in the bone marrow infections [77]. Notably, the successful application of PACT for the treatment of diabetic foot-associated osteomyelitis reported by Tardivo et al. demonstrated the safety and clinical effectiveness of this approach, offering a promising therapeutic option for refractory osteomyelitis [78].
Development and innovation of PDT for osteomyelitis treatment
Development of photosensitizers for osteomyelitis treatment
An ideal photosensitizer should exhibit high photodynamic efficiency, low intrinsic toxicity, adequate water solubility, and strong targeting capability [75]. Currently, photosensitizers used in PDT for osteomyelitis mainly include 5-aminolevulinic acid (5-ALA) and phenothiazine derivatives. Conventional phenothiazine compounds, such as methylene blue and toluidine blue O (TBO), function as cationic photosensitizers and demonstrate favorable photodynamic antibacterial activity [75]. An in vitro study investigating the inhibitory effects of PDT on mature methicillin-resistant Staphylococcus aureus (MRSA) biofilms in vitro revealed that TBO-mediated PDT induced pronounced morphological alterations, including biofilm shrinkage, fissuring, fragmentation, and thinning, accompanied by significant suppression of bacterial virulence [79].
Despite these advantages, clinical application of non-endogenous photosensitizers in antimicrobial therapy remains constrained by limitations related to incomplete characterization of metabolic pathways and potential cytotoxicity. In contrast, 5-ALA is rapidly metabolized into protoporphyrin IX by ALA dehydratase in vivo, resulting in reduced effective concentrations and heterogenous distribution, which in turn compromise PDT efficiency. Consequently, development of novel photosensitizers specifically tailored for osteomyelitis therapy represents an important task for advancing PDT-based treatment strategies. Yin et al. designed a new cationic photosensitizer, LD4, and evaluated its therapeutic performance in a rabbit tibial acute osteomyelitis model. In vivo results showed that LD4 exhibits good water solubility, low toxicity, targeting, strong targeting capacity, and pronounced antimicrobial activity in the treatment of infectious diseases [80]. Compared with conventional photosensitizers, LD4 exhibited broader photoinactivation efficacy as well as improved biocompatibility and physicochemical stability. Moreover, LD4-mediated PDT showed potential benefits in promoting bone healing and alleviating bone defects. Nevertheless, comprehensive assessment of potential adverse effects and long-term therapeutic outcomes of LD4 requires further validation through extended follow-up and clinical investigation.
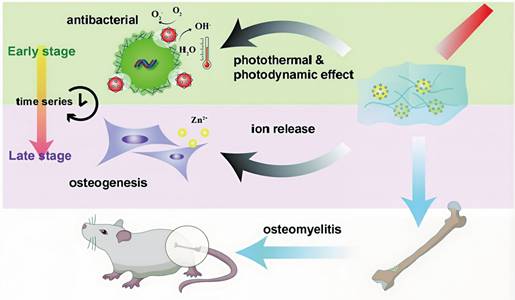
Schematic illustration of ZnO/Ag2S nanoparticles exhibiting combined photothermal and photodynamic effects for osteomyelitis treatment. Adapted with permission from Ref. [83]. Copyright 2023, American Chemical Society.
Development of PDT nanomaterials for osteomyelitis therapy
At present, photosensitizer-mediated PDT, which has been successfully applied in the clinical treatment of tumors and superficial skin infections, has not yet been widely implemented for osteomyelitis management, primarily due to the mismatch between the deep anatomical location of osteomyelitis lesions and the limited issue penetration of conventional light sources. Enhancement of light penetration depth is therefore crucial for extending PDT applicability to osteomyelitis. The wavelength and energy density of the irradiation source exerts decisive influence on PDT efficacy, while tissue density and thickness further modulate penetration depth. NIR light exhibits superior tissue penetration compared with ultraviolet and visible wavelengths. Salehpour et al. reported that whereas the transmittance of a 660 nm laser through the scalp and skull was approximately 5.8%, an 810 nm laser achieved transmittance of (51.41 ± 2.12)% through the skull of male rats [81]. Notzli et al. further demonstrated that the maximum NIR penetration depth in cortical bone, cartilage, and trabecular bone reached 2.9 ± 0.2 mm, 3.5 ± 0.3 mm, and 3.5 ± 0.2 mm, respectively, confirming the feasibility of NIR-mediated PDT for osteomyelitis treatment [82]. Building upon these findings, multiple studies have explored NIR-activated PDT platforms for osteomyelitis therapy. For example, Wu et al. designed a biocompatible core-shell nanomaterial, ZnO/Ag2S nanoparticles, in which incorporation of Ag2S optimized the band-gap structure of ZnO, thereby improving photoelectric efficiency and ROS generation capacity. Density functional theory and ultraviolet photoelectron spectroscopy confirmed the stable photothermal and photodynamic properties of ZnO/Ag2S nanoparticles. These nanomaterials showed potent antibacterial activity upon NIR activation during the acute phase of infection and subsequently released Zn2+ ions during the chronic phase to promote bone regeneration [83] (Figure 3).
Combination of multimodal imaging and PDT in osteomyelitis therapy
In recent years, the combination of PDT with advanced imaging techniques, such as photoacoustic imaging (PAI) and magnetic resonance imaging (MRI), has enabled the development of dual-modal diagnostic and therapeutic platforms for osteomyelitis [84,85]. Compared with conventional optical imaging techniques, PAI offers the advantages of high optical contrast, superior US-based spatial resolution, and increased tissue penetration depth [86]. MRI, in contrast, provides excellent soft-tissue contrast and spatial resolution without the risks associated with ionizing radiation. Notably, PAI compensates for the relatively low spatial resolution of MRI, while MRI offsets the limited penetration depth of PAI. Consequently, the combined application of PAI and MRI during PDT treatment enables accurate delineation of infected regions and real-time guidance of antibacterial therapy, while overcoming the inherent limitations of single-modality imaging. PAI/MRI dual-modal imaging platforms facilitate comprehensive whole-body assessment alongside high-resolution visualization of local tissue architecture, thereby significantly improving early diagnosis and therapeutic precision in osteomyelitis management. For example, in addressing the clinical challenges of early detection and effective intervention, Lu and colleagues developed a multifunctional theranostic agent integrating indomyanine green-mediated PAI with Mn2+-enhanced MRI. This agent selectively accumulated at infection sites and enabled precise lesion identification under both MRI and US imaging, while simultaneously supporting PDT-based therapy. This work represents a successful example of integrated early diagnosis and photodynamic treatment for osteomyelitis [87].
In summary, the spatiotemporal controllability and broad-spectrum antimicrobial activity of PDT render this modality particularly well suited for osteomyelitis treatment. The noninvasive nature of PDT, together with its compatibility with advanced imaging techniques, provides a strong foundation for clinical translation in osteomyelitis treatment.
Challenges and prospects of PDT for osteomyelitis therapy
Despite significant progress, application of PDT in osteomyelitis particularly in deep-seated bone infections, remains constrained by limited light penetration, most notably the inability of conventional light sources to effectively traverse dense cortical bone. Although the introduction of NIR and development of advanced photosensitizers have partially alleviated these technical barriers, such strategies simultaneously raise concerns regarding undefined therapeutic dosages and potential biological safety risks. Furthermore, the oxygen dependence of conventional type II PDT restricts its therapeutic efficacy within the hypoxic microenvironment characteristic of chronic bone infection and biofilm-associated lesions. To address these challenges, several research teams have explored Type I photosensitizers and engineered oxygen-generating or oxygen-carrying nanomaterials; however, these approaches substantially increase the complexity of material design and introduce additional safety considerations. Additionally, comprehensive evaluation of the long-term biosafety, pharmacokinetics, and biodistribution of metal-based nanomaterials and organic photosensitizers remains essential prior to widespread clinical application. Therefore, future breakthroughs in PDT for osteomyelitis treatment hinge on the development of deeply penetrating and/or oxygen-independent photo therapeutic systems, such as NIR-II-responsive platforms and highly efficient self-oxygenating nanostructures, in parallel with the establishment of standardized light delivery protocols optimized for bone tissue and rigorous safety evaluation of next-generation photosensitizers.
In summary, PDT exerts antibacterial effects through light-activated photosensitizers that generate ROS, leading to microbial destruction. Nevertheless, reduced efficacy in deep infections, especially in osteomyelitis, remains a principal limitation. In this context, SDT, which offers superior tissue penetration, is anticipated to complement PDT and compensate for its intrinsic shortcomings, thereby playing an increasingly significant role in the treatment of deep bone infections.
SDT for osteomyelitis therapy
Overview of the application of SDT in osteomyelitis therapy
In 1989, Yumita et al. first reported the responsiveness of hematoporphyrin to US) irradiation and its associated antitumor effects, thereby introducing the therapeutic concept of SDT [88]. As a periodically oscillating mechanical wave, US possesses the capability to penetrate biological tissues to depths of up to approximately 10 cm, supporting the application of SDT in the treatment of deep-seated tumors, deep infections, and other pathological conditions in recent years [89].
The therapeutic mechanisms of SDT involve multiple synergistic processes, including mechanical injury caused by US cavitation, ROS generation mediated by sonosensitizer activation, regulation of intracellular signaling pathways leading to apoptosis, and enhancement of host body immune responses. Among these, US cavitation = and ROS production are considered the principal contributors to the potent antibacterial efficacy of SDT. Cavitation phenomena during SDT are generally classified into non-inertial and inertial cavitation [90]. Under conditions of localized high pressure and temperature, US cavitation within liquid environments produces mechanical forces, such as microstreaming, shock-wave generation, and sonoporation, that induce cellular damage and apoptosis. Sonoporation, in particular, results in the transient formation of reversible micropores on the cell membrane ranging from a few nanometers to several hundred nanometers in diameter, thereby increasing membrane permeability and facilitating improved delivery of therapeutic agents, genes, and bioactive molecules. Two main mechanisms have been proposed to explain ROS generation during SDT. In the first, collapse of cavitation bubbles during inertial cavitation triggers sonoluminescence, which stimulates the sonosensitizer and promotes reactions with molecular oxygen to generate ROS within tissues. In the second, localized high temperatures generated during US irradiation of liquid systems promote dissociation of oxygen-containing molecules, leading to ROS formation [91,92].
Compared with PDT, SDT offers superior tissue penetration and improved biosafety due to the mechanical nature of US energy. A variety of acoustic sensitizers, including precious metal-doped TiO2 (s.g., Pt, Pb, and Au), porphyrin-based single-atom catalysts, metal-organic framework nanoparticles, and piezoelectric nanomaterials such as MoS2 and BaTiO3, have exhibited effective SDT activity and the capacity to induce microbial apoptosis [93]. In recent years, the application of SDT in bacterial infection has expanded substantially, with particular promising outcomes reported in the treatment of osteomyelitis [94,95].
Development and innovation of SDT for osteomyelitis treatment
Development of acoustic sensitizers for osteomyelitis treatment
Over the past few decades, following the discovery of the acoustic activity of hematoporphyrin, an expanding range of acoustic sensitizers has been developed for antitumor and antibacterial applications. Advances in pharmaceutical nanotechnology have not only improved the stability and targeting efficiency of conventional porphyrin-based sensitizers but have also promoted the exploration of sound-responsive characteristics in various nanomaterials, thereby significantly enriching the application potential of sonosensitizing agents. Currently, acoustic sensitizers are mainly classified into organic sensitizers, inorganic sensitizers, and organic-inorganic hybrid sensitizers [96].
Organic acoustic sensitizers, representing the earliest class of agents applied in SDT, exhibit both photosensitive characteristics and sonodynamic activity under US irradiation. This category primarily consists of porphyrins, phthalocyanines, xanthene derivatives, phenothiazine compounds, fluoroquinolones antibiotics, natural products, and other organic small-molecule sonosensitizers [96]. Organic sensitizers are distinguished by high ROS production efficiency, tunable SDT performance, and favorable biodegradability. However, limitations such as poor water solubility, chemical instability, rapid systemic clearance, low bioavailability, and potential phototoxicity have restricted broader clinical application in antimicrobial therapy. In contrast, inorganic sensitizers, represented by TiO2, ZnO2, Bi2MoO6, BaTiO3, and MnWOx, offer superior physicochemical stability and allow flexible regulation of pore structure and surface functionalization, thereby providing notable advantages for in vivo targeted delivery [91]. Nonetheless, the limited biodegradability of inorganic sensitizers may result in cumulative systemic toxicity and long-term biosafety concerns, underscoring the importance and rigorous safety evaluation.
To address the respective limitations of organic and inorganic acoustic sensitizers, organic-inorganic hybrid acoustic sensitizers that integrate the advantages of both classes have been successfully developed and applied in recent years for exploratory clinical use in SDT. Among these, metal-organic framework (MOF)-based acoustic sensitizers have emerged as a major focus of current research [97]. MOF sonosensitizers consist of crystalline structures formed through coordination between metal ions or clusters and organic acoustic-sensitive ligands, featuring well-defined pores and cavities. The controllable cavity dimensions of MOFs enable incorporation of diverse functional components—such as quantum dots, nanoclusters, organic small molecules, or nanoparticles—either during or after synthesis, facilitating construction of multifunctional composite platforms that combine the beneficial properties of organic and inorganic acoustic nanomaterials. In addition, the large specific surface area, adjustable porosity, facile surface modification, and high drug-loading capacity of MOFs provide strong support for their application in antimicrobial SDT. The tunable pore architecture and intrinsic catalytic activity further positioned MOFs as highly promising next-generation sonosensitizers.
SDT nanomaterials for osteomyelitis therapy
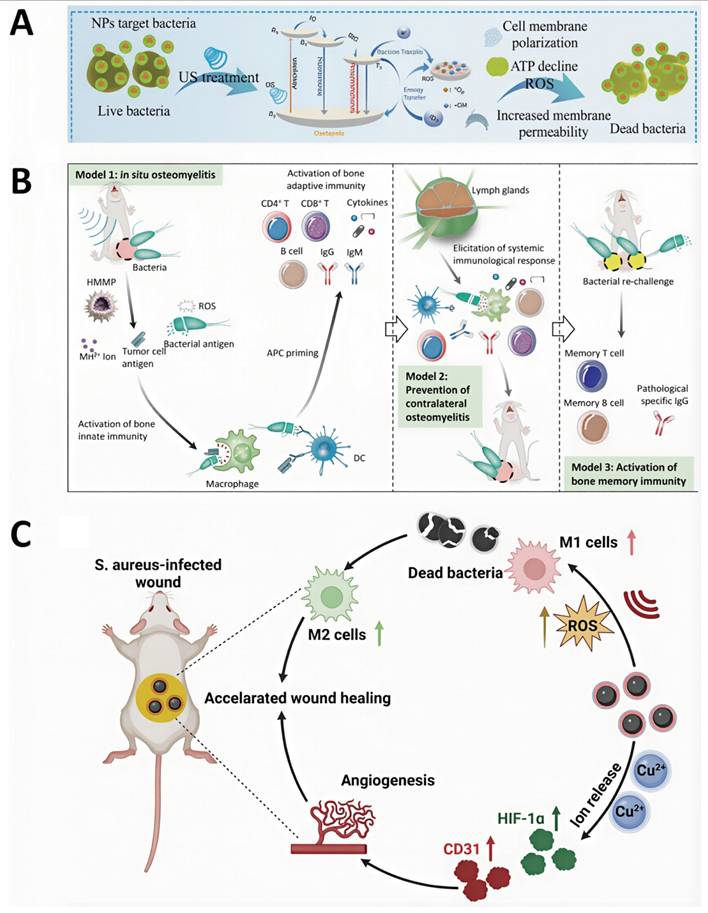
Targeted accumulation of acoustic sensitizers at osteomyelitis lesions is crucial for improving the therapeutic efficacy and bactericidal performance of SDT. For instance, Chen et al. developed cationic starch-modified curcumin nanoparticles (CS@Cur NPs). Owing to their positive surface charge, CS@Cur NPs effectively target bacteria with negatively charged cell membranes, resulting in capture rates of 34.7% for E. coli and 35.8% for S. aureus, respectively. Upon US irradiation, CS@Cur NPs produce substantial levels of ROS, inducing oxidative stress-mediated bacterial death through increased membrane permeability and disruption of ATP synthesis [98] (Figure 4A). In addition, biomimetic nanotechnology has also been used to further improve the targeting efficiency of SDT nanomaterials toward infected bone marrow. Lin et al. designed a biomimetic nanotherapeutic system, HMMP, constructed by combining hybrid membranes derived from macrophages and tumor cells with hollow MnOx nanoparticles encapsulating protoporphyrin IX (PpIX). This platform enabled efficient targeting of osteomyelitis infection sites and controlled ROS release, resulting in highly effective bactericidal activity within infected bone marrow [99] (Figure 4B).
The pathological microenvironment of osteomyelitis is characterized by acidic pH and high GSH levels, and adaptation of nanomaterials to these conditions is critical for improving SDT-mediated therapeutic outcomes. Stability of nanoparticles under acidic conditions represent a key requirement for effective SDT performance. Chen et al. investigated the influence of pH on the antibacterial effect of CS@Cur NPs and showed that these nanoparticles maintained stable and potent antimicrobial activity across a pH range of 3-7 [98] (Figure 4A). To counteract the inhibitory effects of high GSH levels on ROS-mediated antibacterial activity, Cheng et al. designed ultrasmall platinum-copper alloy nanoparticles (PtCu-PEG NPs) with strong GSH-depleting ability. Through enhanced ROS-based bactericidal mechanisms, PTKU-PEG NPs effectively suppressed deep S. aureus-induced osteomyelitis infection [100] (Figure 4C). These findings highlight the importance of microenvironment-responsive acoustic sensitizers in amplifying antibacterial efficacy.
Furthermore, to overcome bacterial antioxidant defenses such as superoxide dismutase (SOD), Yang et al. engineered calcium carbonate-gallium-protoporphyrin IX (CaCO3-Ga-PPIX) nanospheres (CaGaPP NSs) incorporating polyethylene glycol (PEG). the infected microenvironment, CaGaPP NSs selectively released Ga3+ ions that functioned as "Trojan horse" agents to disrupt bacterial metabolism and inhibit SOD activity, thereby further enhancing the antibacterial activity of SDT [101]. This work introduces a promising strategy that combines SDT nanomaterials with targeted suppression of bacterial systems for treatment of osteomyelitis.
In summary, SDT demonstrates significant antibacterial advantages through the combined effects of mechanical disruption mediated by US cavitation and ROS generation. Furthermore, owing to deep tissue penetration of US energy, SDT represents a particularly promising therapeutic strategy for osteomyelitis, especially for infections involving deep bone marrow lesions.
Challenges and prospects of SDT in osteomyelitis therapy
Although SDT strategy has gained increasing recognition for the treatment of osteomyelitis, several critical challenges remain to be addressed. Acoustic sensitizers represent a key determinant of SDT efficacy, yet current limitations related to complex preparation processes and unresolved in vivo biosafety concerns pose substantial barriers to clinical translation. Consequently, the rational design and development of multifunctional acoustic sensitizers remain central for future optimization of SDT-based osteomyelitis therapy. Emerging evidence indicates that MOF-based sonosensitizers integrated with hypoxia-adaptive SDT platforms offer promising solutions for refractory osteomyelitis. In particular, development of oxygen saturation-independent materials that leverage direct energy transfer or ROS catalytic amplification mechanisms has expanded the feasibility of SDT under hypoxic conditions. Future research should therefore prioritize systematic assessment of MOF biosafety and the development of degradable alternatives to support clinical translation. In addition, the existing US delivery systems lack the precision and operational flexibility required for consistent and convenient adjustment of acoustic parameters, limiting their suitability for clinical implementation. Development of next-generation US instruments with standardized and programmable acoustic parameters is thus another important direction for technological advancement. Moreover, the molecular mechanisms underlying SDT-induced apoptosis across different bacterial species remain incompletely understood and warrant further exploration.
In summary, SDT demonstrates considerable therapeutic promise for osteomyelitis owing to its superior tissue penetration ability. However, clinical efficacy is strongly influenced by the performance of US equipment and the stability of acoustic sensitizer delivery. In contrast, CDT, which does not rely on external energy input, enables sustained ROS generation and offers distinct advantages in the management of infections requiring continuous oxidative stress-mediated antimicrobial activity.
CDT for osteomyelitis treatment
Overview of CDT in osteomyelitis treatment
In 2016, Shi et al. formally introduced the concept of CDT [102]. Central to this therapeutic strategy are nanozymes, a class of nanoscale materials possessing intrinsic enzyme-like catalytic activity [103]. Nanozymes integrate the physicochemical properties of nanomaterials with the catalytic functions of natural enzymes, enabling efficient catalysis of biological substrate reactions under mild physiological conditions. Compared with natural enzymes, nanozymes typically exhibit greater thermal and chemical stability, enhanced environmental tolerance, lower production costs, and favorable scalability for mass production [104]. The discovery of nanozymes has challenged the traditional notion of inorganic materials as biologically inert and has revealed the inherent catalytic potential of nanomaterials, thereby expanding their utility across analytical chemistry, biosensing, environmental remediation, and biomedical applications. For example, cerium oxide (CeO2) and iron oxide (Fe3O4) nanoparticles with nanozyme activity have been widely explored for biosensing and therapeutic purposes [71]. Based on material composition, nanoenzymes are broadly classified into metal-based nanoenzymes, carbon-based nanoenzymes, metal-MOF nanoenzymes, and bio-derived nanoenzymes [104]. The core catalytic mechanisms underlying nanoenzyme-mediated CDT involve Fenton and Fenton-like reactions.
(A) Cationic starch-modified curcumin nanoparticles (CS@Cur NPs) for the treatment of MRSA-induced osteomyelitis, exhibiting anti-inflammatory activity and promotion of osteogenic differentiation. Adapted with permission from Ref. [98]. Copyright 2023, Wiley-VCH. (B) Schematic illustration of HMMP construction. Adapted with permission from Ref. [99]. Copyright 2022, American Chemical Society. (C) Scheme representation of PtCu-PEG NP preparation for sonodynamic antibacterial therapy and tissue repair. Adapted with permission from Ref. [100]. Copyright 2023, Wiley-VCH.
During the CDT process, nanocatalytic systems exploit the excess H+ or H2O2 present within pathological lesions to drive Fenton or Fenton-like reactions. In iron-based nanomaterials, Fe2+ ions are released and catalyze the decomposition of endogenous H2O2 into highly reactive ·OH under acidic conditions according to the reaction
Fe2+ + H2O2 → Fe3+ + ·OH + OH-
In addition to iron, other redox-active metal ions, such as Cu+, Mn2+, and Mo6+, can also catalyze H2O2 conversion into ·OH. As a highly cytotoxic Type I ROS, ·OH induces cellular damage through phospholipid peroxidation, mitochondrial dysfunction, and DNA damage, while simultaneously activating caspase-3-dependent apoptotic pathways, ultimately leading to programmed death of both target cells and pathogenic microorganisms. A large number of metal-based nanocatalytic systems has therefore been developed to enhance the efficiency of Fenton and Fenton-like reactions, thereby improving CDT therapeutic efficiency [102,105].
Initially established as an effective strategy for cancer therapy, CDT depends on acidic microenvironments and elevated H₂O₂ concentrations to sustain efficient ROS generation. Owing to the similar pathological features of tumor tissues and infected bone marrow lesions—characterized by mild acidity, hypoxia, and excessive H2O2 —CDT has demonstrated considerable promise for osteomyelitis treatment. Within osteomyelitis lesions, Fenton and Fenton-like catalysts convert endogenous H₂O₂ into ROS, thus exerting potent antibacterial and immunomodulatory effects. Compared with PDT and SDT, CDT-based nanomaterials enable continuous ROS generate without relying on external energy sources, providing a more durable and operationally straightforward therapeutic approach for osteomyelitis management.
Development of CDT nanotechnology for osteomyelitis treatment
CDT nanotechnology for osteomyelitis treatment via H₂O₂ supply
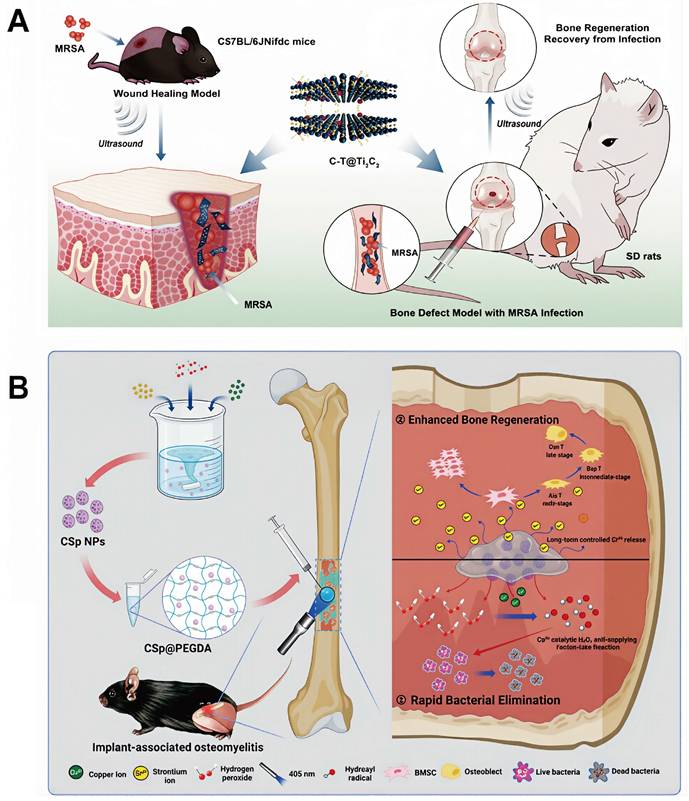
Supply of H₂O₂ at sites of infection improves the therapeutic efficacy of CDT. Numerous studies have therefore leveraged the intrinsic accumulation of H₂O₂ within osteomyelitis lesions to optimize CDT-based treatment strategies. For example, Yu et al. developed two-dimensional TiC nanosheets loaded with CaO2, which showed robust CDT activity through self-supplied H₂O₂ generation, resulting in effective antimicrobial action and substantial therapeutic benefit in osteomyelitis models [106] (Figure 5A).
CDT nanotechnology for osteomyelitis treatment dependent on acidic microenvironment
Efficient Fenton reactions require an acidic environment, typically with pH values ranging from 2 to 4, as elevated pH conditions inhibit nanomaterial decomposition and limit the release of catalytically active metal ions. The acidic microenvironment characteristic of osteomyelitis lesions therefore provides favorable conditions for high-efficiency CDT. For instance, Ge et al. constructed a multifunctional, pH-responsive drug delivery system by integrating zeolitic imidazolate framework-8 (ZIF-8) with celecoxib (CEL@ZIF-8). This platform enabled intelligent release of both metal ions and therapeutic agents in response to the acidic conditions at the infection site, ultimately achieving effective osteomyelitis treatment [107]. Similarly, Guan et al. encapsulated copper-strontium peroxide nanoparticles (CSp) within PEG diacrylate (PEGDA) to generate CSp@PEGDA nanocomposites. These nanoparticles self-supplied H2O2 and released Cu²⁺ ions to initiate Fenton-like reactions, generating a large amount of ·OH for potent antibacterial activity. At the same time, the released Sr2+ ions enhanced osteogenic processes by promoting osteoblast proliferation, increasing alkaline phosphatase activity, facilitating extracellular matrix calcification, and upregulating gene expression related to bone formation [108] (Figure 5B).
Compared with tumor tissues, several intrinsic limitations restrict the application of CDT in osteomyelitis treatment, including relatively low endogenous H2O2 concentrations within bone infections and partial neutralization of the acidic microenvironmental by bone minerals and inflammatory responses. Accordingly, strategies involving autonomous H2O2 supplementation or glutathione depletion have proven effective in enhancing CDT efficiency. Furthermore, potential risks associated with metal accumulation and systemic toxicity arising from extensive use of transition metal catalysts, such as iron, copper, and manganese, warrant sustained investigation. Future development of CDT for osteomyelitis treatment should therefore focus on the design of highly efficient catalysts capable of operating under physiological or near-physiological conditions, the exploration of metal-free CDT nanozymes (e.g., carbon- and nitrogen-based structures), and the construction of intelligent nanoplatforms capable of dynamically sensing and modulating the pathological microenvironment. Integration of CDT strategies with osteogenic elements further represents a promising multifunctional therapeutic direction for osteomyelitis management.
(A) Schematic illustration of the synthesis and therapeutic performance of C-T@Ti3C2 nanosheets, including in vitro and in vivo antibacterial activity and bone defect repair in deep infections. Adapted with permission from Ref. [106]. Copyright 2023, Springer Nature. (B) Schematic illustration of the synthesis, antibacterial activity, and osteogenic performance of the CSp@PEGDA composite. Adapted with permission from Ref. [108]. Copyright 2024, Wiley-VCH.
Challenges and prospects of CDT in osteomyelitis treatment
Development of CDT for osteomyelitis therapy relies heavily on effective exploitation and modulation of the pathological microenvironment characterized by elevated H2O2 levels, increased GSH, and acidic pH. Although CDT has emerged as a promising therapeutic strategy osteomyelitis, several biosafety challenges must be addressed prior to clinical translation. First, the complex and heterogeneous microenvironment of osteomyelitis lesions may introduce unpredictable safety risks during CDT treatment. Second, insufficient targeting accuracy and limited specificity of current CDT nanomaterials increase the potential for off-target effects and damage to normal tissues. Third, extensive use of metal-based components in CDT nanomaterials raised concerns regarding metal accumulation and systemic toxicity in vivo. Consequently, future optimization of CDT for osteomyelitis should prioritize realistic modeling of lesion microenvironments, refinement of nanoscale targeting strategies, and development of non-metallic CDT nanomaterials with improved biosafety profiles.
In summary, the main advantage of CDT lies in its independence from external energy input; however, its antibacterial efficiency remains highly sensitive to local microenvironmental conditions, and reliance on a single therapeutic modality increase the risk of treatment failure. In contrast, MWDT offers the ability to deliver precise microwave-induced hyperthermia while simultaneously generating ROS for effective sterilization. Through the synergistic action of thermal effects and ROS-mediated antibacterial mechanisms, MWDT provides a more comprehensive and efficient therapeutic approach for the management of chronic osteomyelitis, combining deep tissue penetration with rapid therapeutic responses.
MWDT for osteomyelitis treatment
Overview of MWDT in osteomyelitis treatment
Over the past decade, microwave activation has emerged as an increasingly attractive synergistic therapeutic modality due to its ability to induce both thermal effects and ROS, thereby enhancing antitumor and antimicrobial efficacy [109]. In 2017, Fu et al. formally introduced the concept of MWDT, which integrates the hyperthermic advantages of conventional microwave thermal therapy (MTT) with ROS-mediated cytotoxic mechanisms, providing a more comprehensive and effective clinical strategy [110]. ROS generation during MWDT is driven by both thermal and non-thermal effects associated with microwave irradiation. Microwave-induced heating accelerates ion migration and increases interionic collision frequency, leading to localized temperature rise and occurrence of redox reactions. Concurrently, microwave exposure alters cell membrane integrity and intracellular architecture, regulates the intracellular microenvironment, and further promotes ROS generation. Through these combined mechanisms, MWDT enables localized ROS generation at targeted tissue sites by simultaneously promoting redox reactions and interfering with intracellular signaling pathways [111,112].
In recent years, application of MWDT for the treatment of bacterial infections has expanded, with particularly encouraging outcomes reported in the treatment of osteomyelitis [113-119]. Microwave irradiation offers substantial tissue penetration depth and effectively heats tissues with low electrical conductivity, high impedance, and low thermal conductivity, such as bone [120]. Moreover, high microwave-to-thermal conversion efficiency enables rapid achievement of bactericidal temperatures exceeding 100 °C, thereby significantly shortening treatment duration [120]. In parallel, extensive evidence confirms that microwave exposure promotes ROS generation [121]. Collectively, these features establish microwave-responsive nanomaterials with unique physicochemical properties as promising platforms for advanced antimicrobial therapy of osteomyelitis.
Development of MWDT for osteomyelitis treatment
Design of MWDT nanomaterials
Microwave-absorbing materials convert microwave energy into thermal or other forms of energy through absorption and attenuation processes. The main mechanisms underlying microwave absorption include electromagnetic loss, magnetic loss, dielectric loss, and multiple reflection, which may operate individually or synergistically to confer efficient absorption performance [122,123]. At present, microwave-responsive nanomaterials encompass both materials with intrinsic wave-absorbing properties and materials exhibiting microwave dynamic activity [124-126]. Among these, nanomaterials with microwave dynamic properties are most frequently employed in the design and development of MWDT nanomaterials for osteomyelitis treatment. Nanomaterials with microwave dynamic properties usually possess H2O2-like enzymatic behavior or intrinsic microwave catalytic activity. For example, Mn-Zr-doped MOFs have been shown to act as H2O2-mimicking enzymes, catalyzing H2O2 decomposition into ·OH under microwave irradiation [164]. This phenomenon is attributed to microwave-induced acceleration of electron transfer within the material, facilitating excitation of H2O2 molecules from the ground state and ultimately triggering rapid ·OH generation. In contrast, nanomaterials exhibiting microwave catalytic activity, such as Ga/In alloy nanostructures, are capable of directly producing ROS under microwave irradiation. This phenomenon arises from the formation of high-temperature “hot spots” on material surfaces created by localized resonant coupling of microwave energy, where electron transfer from Ga to H2O or O2 is promoted, thereby enhancing production of ·OH and superoxide anion radicals ·O2- [109].
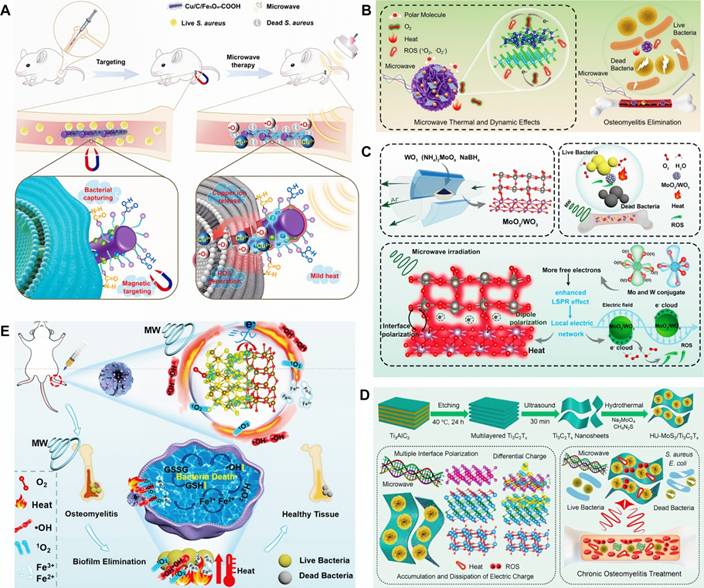
Application of MWDT nanomaterials in osteomyelitis treatment
MWDT nanomaterials used in osteomyelitis therapy are mainly categorized into magnetic loss materials and dielectric loss materials. Magnetic loss materials are typically represented by polymer composites filled with magnetic components such as ferrites or carbonyl iron powder. Notably, Fe3O4 nanoparticles have already received approval from the U.S. FDA for certain medical applications. Ren et al. developed an effective Cu/C/Fe3O4-COOH nanocomposite for osteomyelitis therapy, in which Fe3O4 nanoparticles with strong magnetic loss characteristics improved microwave absorption through optimized impedance matching of the dielectric matrix. Targeting of bacterial cells was achieved via interactions between surface -COOH groups on the nanocomposite and -NH2 groups on the bacterial walls. Under microwave irradiation, Cu/C/Fe3O4-COOH generated both thermal energy and ROS, resulting in disruption of bacterial membrane integrity and permeability. Antibacterial inhibition against S. aureus reached 99.99 ± 0.009% [127] (Figure 6A). Dielectric loss materials depend on tunable dielectric properties of carbonaceous or metallic fillers, which can be regulated through control of material thickness, depth, and filler composition. Jin et al. developed a microwave-responsive nanocomposite composed of MoS2/FeS and emodin (Rhein). Under microwave exposure, MoS2 promoted dipole polarization and ion conduction, resulting in molecular friction and dielectric loss, ultimately generating both thermal energy and ROS. The combined effects of singlet oxygen (1O2), superoxide anion (•O2-) and thermal energy achieved nearly 100% antibacterial efficacy against S. aureus and E. coli [128] (Figure 6B). Furthermore, binary metal oxides exhibit superior electrical conductivity and increased oxygen vacancy density compared with single metal oxides, thereby enhancing electron migration and providing abundant dipolar polarization sites. Based on the principle that interfacial polarization in binary metal oxides improves microwave absorption through enhanced conductivity loss, Zhu et al. designed a microwave-responsive MoO2/WO3 heterojunction for treatment of MRSA-induced osteomyelitis. Owing to tits high electrical conductivity, favorable dielectric loss characteristics, and excellent thermal stability, MoO2/WO3 achieved potent bactericidal activity and effective osteomyelitis treatment through the synergistic action of thermal effects and ROS generation. Inhibition rates against S. aureus and MRSA reached 99.27 ± 0.02% and 99.23 ± 0.43%, respectively [129] (Figure 6C).
In recent years, composite microwave-absorbing materials that integrate both magnetic and dielectric properties have been designed for osteomyelitis treatment to achieve synergistic antibacterial effects. For example, Jin et al. developed a MoS2/Fe3O4 composite to demonstrated effective therapeutic outcomes in osteomyelitis. The synergistic interaction between MoS2 and magnetic Fe3O4 facilitates multiple magnetic loss mechanisms, enhanced dielectric reflection, and interfacial polarization, thus conferring excellent microwave-induced thermal effects and efficient generation of 1O2 and ·O2-, which are essential for successful treatment of S. aureus-infected tibial osteomyelitis [130] (Figure 6D). Similarly, Liao et al. fabricated a Fe2O3/Fe3S4 composite material with strong microwave absorption capacity, enabling effective conversion of electromagnetic energy into heat. Upon microwave irradiation, differential charge accumulation and consumption at the Fe2O3/Fe3S4 interface enhance free electron release and subsequent binding with oxygen adsorbed on the composite surface, ultimately triggering a rapid burst of ROS. The combined magnetic loss and dielectric loss mechanisms promote simultaneous ROS generation and localized hyperthermia, leading to increased bacterial membrane permeability, oxidative stress induction, and eventual bacterial apoptosis. Antibacterial efficiencies of Fe2O3/Fe3S4 reached 99.87% against S. aureus and 99.59% MRSA [131] (Figure 6E).
By leveraging the deep tissue penetration properties of microwave radiation, MWDT enables simultaneous induction of localized hyperthermia and ROS-mediated antibacterial effects, generating a synergistic therapeutic outcome that substantially exceeds the efficacy of either modality alone. The high energy of microwave irradiation overcomes the penetration limitations inherent to phototherapy and demonstrates substantial potential for treating deep-seated and refractory bone infections.
Challenges and prospects of MWDT in osteomyelitis treatment
Several key challenges currently limit the clinical translation of MWDT for osteomyelitis therapy. First, existing MWDT platforms lack precise targeting capability toward infected lesions. Second, currently available microwave sensitizers often exhibit insufficient microwave responsiveness and suboptimal thermal conversion efficiency, thereby limiting therapeutic effectiveness in osteomyelitis management. Third, inadequate control of heat distribution during microwave irradiation introduces the risk of collateral thermal injury to surrounding healthy tissues or vital organs. Consequently, incorporation of advanced imaging probes and targeting moieties is expected to facilitate the development of multifunctional micro-and nanomaterials, enabling more precise, efficient, and safe MWDT techniques for osteomyelitis therapy.
In summary, the four major ROS-based therapeutic strategies discussed above—PDT, SDT, CDT, and MWDT—each exhibit unique advantages while also presenting unavoidable limitations. Future osteomyelitis treatment paradigms are therefore likely to evolve toward multimodal combination therapies to overcome the shortcomings associated with single-modality interventions. Integration of PDT, SDT, CDT, and MWDT provides expanded opportunities for personalized osteomyelitis treatment, with particular promise for addressing chronic, deep-seated, and refractory infections. Continues technological innovation and development of advanced therapeutic modalities are expected to further refine osteomyelitis treatment strategies, ultimately improving clinical outcomes and overall patient quality of life.
Combination of different ROS nanotechnologies for osteomyelitis treatment
Although ROS-based nanotechnologies, including PDT, SDT, CDT, and MWDT, have shown substantial therapeutic potential and promising application prospects in osteomyelitis management, single-modality dynamic treatment strategies remain insufficient to achieve rapid and comprehensive control of complex microbial infections. Consequently, contemporary ROS-based therapeutic approaches for osteomyelitis have gradually shifted from monotherapy toward multimodal combination strategies to enhance infection eradication and promote effective wound healing. Integration of complementary dynamic treatment strategies enables mutual compensation for the inherent limitations of individual approaches and facilitates synergistic interactions among different ROS-generating mechanisms and auxiliary therapeutic components, thereby producing a therapeutic outcome that exceeds the efficacy of any single strategy alone. This section summarizes current combinatorial strategies involving multiple ROS-based nanotechnologies, with the aim of elucidating their synergistic mechanisms and evaluating the potential of integrated therapeutic platforms in addressing the multifaceted challenges posed by osteomyelitis-associated infections.
(A) Schematic diagram of Cu/C/Fe3O4-COOH for treatment of S. aureus-infected osteomyelitis. Adapted with permission from Ref. [127]. Copyright 2024, Wiley-VCH. (B) Microwave-assisted therapeutic strategy for treating S. aureus-infected osteomyelitis using a microwave-responsive MoS2/FeS nanocomposite modified with rhein. Adapted with permission from Ref. [128]. Copyright 2022, Wiley-VCH. (C) Schematic representation of MoO2/WO3 synthesis, microwave-mediated osteomyelitis treatment, and the combined mechanisms of microwave thermal and catalytic effects. Adapted with permission from Ref. [129]. Copyright 2022, American Chemical Society. (D) Microwave-based treatment strategy for COM with S. aureus infection using MoS₂/Ti₃C₂Tₓ heterojunctions generated via hydrothermal treatment, featuring oxygen vacancy-rich TiO2-x interfaces. Adapted with permission from Ref. [130]. Copyright 2022, Wiley-VCH. (E) Schematic illustration of the preparation of Fe2O3/Fe3S4 composite materials. Adapted with permission from Ref. [131]. Copyright 2024, Wiley-VCH.
Combination of PDT and SDT for osteomyelitis treatment
SDT offers superior tissue penetration compared with PDT, whereas PDT benefits from photosensitizers that exhibit hinger intrinsic ROS generation efficiency [132,133]. Consequently, combination of PDT and SDT enables simultaneous optimization of tissue penetration and ROS generation, making this combination particularly suitable for the treatment of osteomyelitis with deep tissue involvement. Ding et al. synthesized barium titanate nanotubes (BNTs) through a combined anodic oxidation and hydrothermal process, achieving efficient ROS generation and potent antibacterial activity under both US and NIR irradiation via synergistic piezoelectric and pyroelectric effects. The coordinated action of PDT and SDT further enhanced charge transfer and electron-hole pair separation, thereby amplifying the coupled pyroelectric-piezoelectric catalytic effect and markedly increasing ROS output. The resulting ROS burst induces extensive structural and functional damage to bacterial DNA and proteins, eventually leading to irreversible bacterial cell death. In vivo experiments showed that the antibacterial efficacy of BNTs reached up to 99% [134].
Combination of SDT and CDT for osteomyelitis treatment
Previous studies have reported that localized turbulence generated by US shock waves can significantly improve the efficiency of Fenton reactions. The observation has provided a strong foundation for the development of synergistic strategies integrating SDT with CDT to achieve antimicrobial outcomes. For example, Cheng et al., conjugated a guanidin-rich polymer (PG) onto gold-doped titanate nanotubes (Au/TNTs) capable of absorbing US, thereby constructing multifunctional nanostructures (Au/TNT@PG) that simultaneously exhibit sonodynamic properties and peroxidase-like catalytic activity. US absorption by Au/TNT@PG markedly enhanced CDT efficiency and ·OH generation. In addition, the biofilm-penetrating characteristics of PG facilitated efficient biofilm disruption and induction of bacterial apoptosis, resulting in superior antibacterial efficacy [135] (Figure 7A).
Combination of MWDT and CDT for osteomyelitis treatment
Microwave-induced hypothermia has been shown to effectively destroy bacterial biofilm structure as well asl cell wall and membrane integrity, thereby improving the antibacterial efficacy of ROS. Building upon this principle, Wei et al. developed a sodium (Na+)-rich Prussian blue (PB) nanomaterial that functions as both a microwave-absorbing agent and an iron ionophore for the treatment of bacterial osteomyelitis. PB nanoparticles absorb microwave energy through dielectric loss and mesoporous structural reflection, generating heat that increases bacterial membrane permeability and accelerates intracellular uptake of iron ions. This process subsequently enhances Fenton reaction efficiency and ROS generation, thus leading to bacterial apoptosis. Antibacterial inhibition rates of PB nanoparticles reached 99.08% against S. aureus and 99.67% against E. coli [136]. In addition, cerium-based compounds, owing to their favorable dielectric polarization, dielectric relaxation, and conductive loss properties, have been identified as promising microwave-responsive antibacterial materials. Sun et al. designed a CuCeOₓ composite oxide for the treatment of S. aureus-induced osteomyelitis. Under microwave irradiation, CuCeOₓ simultaneously produced thermal energy and ROS, thereby increasing Cu2+ thermal sensitivity, enhancing bacterial membrane permeability, and amplifying Fenton-like reaction efficiency, resulting in substantial accumulation of ·OH within bacterial cells. The combined MWDT-CDT treatment achieved an antibacterial efficacy of 99.98% ± 0.02% against S. aureus [137] (Figure 7B).
In summary, investigation of synergistic ROS-based therapeutic strategies, including PDT-SDT, SDT-CDT, and MWDT-CDT combinations, holds significant promise for achieving breakthroughs in the treatment of deep-seated and refractory osteomyelitis.
This review provides a comprehensive analysis examines multimodal ROS-based therapeutic strategies, thereby complementing existing literature on ROS therapies for osteomyelitis, which has largely emphasized single-modality approaches and consequently offered limited insight into enhancing bone infection permeability and microenvironmental modulation. The present work not only elucidates the synergistic mechanisms and representative case studies of diverse ROS-based therapeutic modalities for osteomyelitis management but also critically examines the complementary strengths and translational challenges inherent to each ROS technology. Collectively, these perspectives establish multimodal ROS therapy as a key developmental direction for next-generation osteomyelitis treatment strategies.
(A) Construction and therapeutic mechanism of Au/TNT@PG nanostructures for efficient osteomyelitis treatment. Adapted with permission from Ref. [135]. Copyright 2022, Wiley-VCH. (B) Development of microwave-responsive CuCeOₓ materials for the treatment of S.aureus-infected osteomyelitis. Adapted with permission from Ref. [137]. Copyright 2023, Wiley-VCH.
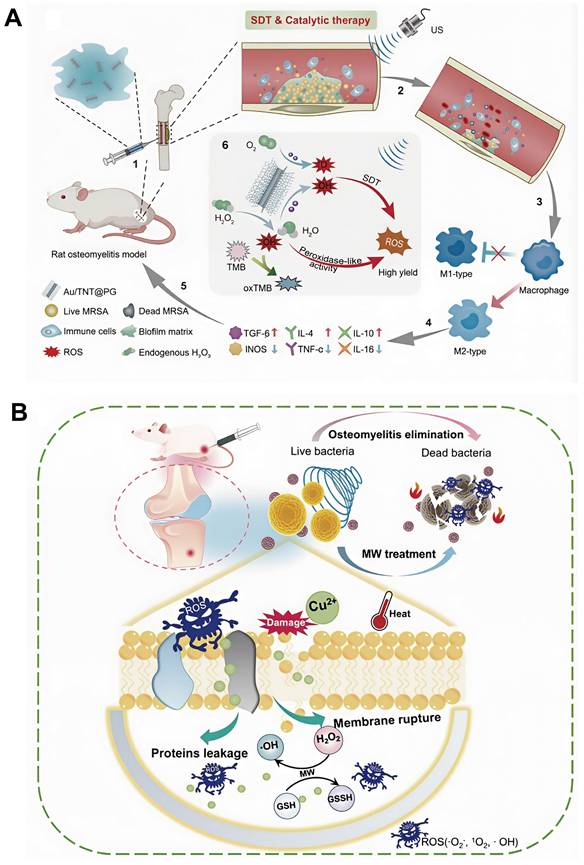
ROS-based diagnostic strategies for osteomyelitis
In recent years, a variety of ROS-responsive diagnostic approaches has been explored, primarily at the preclinical stage, aiming to improve early detection, lesion localization, and therapeutic monitoring of osteomyelitis [138]. Based on detection principles and imaging modalities, current ROS-based diagnostic tools are broadly categorized into ROS-responsive optical imaging probes and ROS-activated MRI contrast agents.
ROS-responsive optical imaging strategies
ROS-responsive optical probes constitute the most extensively studied class of ROS-based diagnostic tools. These probes are typically designed with ROS-sensitive chemical moieties, such as boronate esters, thioketal linkers, and sulfur-containing functional groups, which undergo structural cleavage or oxidation in the presence of elevated ROS levels, thereby generating fluorescence or photoacoustic signals. Although ROS-responsive fluorescent probes have not yet been widely validated in dedicated osteomyelitis models, substantial progress achieved in infection-related and orthopedic infection imaging provides important references and strong translational potential for their future application in osteomyelitis diagnosis and therapeutic monitoring.
Currently, a representative class of ROS-responsive fluorescent probes consists of ROS-activatable NIR fluorescent nanoprobes, which remain optically quenched during systemic circulation but become fluorescent upon oxidative activation at sites of infection. Bouché et al. reported a hybrid nanogel formulation incorporating gold nanoparticles (AuNPs), in which ROS-triggered disintegration or cross-linking of the nanogels enabled quantitative diagnostic detection through corresponding changes in signal intensity [139]. Xu et al. developed fluorescence-quenched TK-CBT nanoparticles containing a thioketal trigger moiety [140]. Elevated ROS levels within S. aureus-infected macrophages induced nanoparticle fragmentation and activated NIR fluorescence, thereby enabling noninvasive in vivo imaging of S. aureus infection. This "ROS-triggered dequenching" paradigm supports the feasibility of exploiting localized oxidative stress as an endogenous activation signal for infection-specific optical diagnosis, which is highly relevant to S. aureus-dominant osteomyelitis. Beyond ROS-activatable probes, studies in orthopedic infection models have also highlighted the utility of NIR fluorescence tracers for precise localization and image-guided surgical debridement, providing valuable reference for osteomyelitis management. Park et al. compared two fluorescent probes targeting staphylococcal biofilm-associated implant infections, including a labeled antibiotic (Vanco-800CW) and an antibody-based tracer (1D9-680) directed against a staphylococcal antigen, and demonstrated their feasibility for fluorescence-guided debridement [141]. Integration of such NIR fluorescence tracers with ROS-responsive probes is anticipated to further enhance the precision of osteomyelitis localization and diagnostic accuracy in future clinical applications.
At present, most ROS-responsive fluorescent probes investigated for osteomyelitis remain at the preclinical stage, primarily constrained by several limitations: (i) limited tissue penetration and optical attenuation in deep bone lesions; (ii) the risks of false-positive signals arising from elevated ROS levels associated with sterile inflammation or post-surgical tissue damage; and (iii) challenges related to batch-to-batch reproducibility and long-term biosafety of nanoprobes. Future developments of ROS-responsive fluorescent probes for osteomyelitis diagnosis are therefore expected to emphasize the use of NIR-I/NIR-II fluorophores, implementation of dual-locked activation designs that combine bacteria targeting with ROS responsiveness, and incorporation of multimodal imaging readouts, such as fluorescence integrated with photoacoustic imaging or MRI, to improve diagnostic robustness under the complex pathological conditions characteristic of osteomyelitis.
ROS-activating MRI contrast agents
Current ROS-activating MRI strategies mainly rely on ROS-triggered release of paramagnetic metal ions and the consequent enhancement of T1-weighted signals. Among reported platforms, MnO2-based nanostructures are considered particularly promising and have been successfully applied in osteomyelitis models. These ROS-activatable MRI contrast agents utilize the elevated H2O2 levels and acidic microenvironment characteristic of infected bone tissue to promote redox-mediated decomposition of MnO2 and generation of paramagnetic Mn2+ ions, thereby significantly enhancing T1-weighted MRI contrast.
Beyond bone-related applications, a broader range of ROS- or enzyme-activatable MRI contrast agents has been developed beyond bone-related models. Mn-based ROS-responsive probes, including Mn-TyEDTA derivatives, showed pronounced increases in longitudinal relaxivity following peroxidase-mediated radical polymerization in the presence of H2O2 [142]. Compared with normal tissues, these probes exhibit selective MRI signal amplification within inflamed regions, supporting the feasibility of synergistic MRI activation through combined enzymatic and ROS-mediated mechanisms.
Accordingly, detection of abnormal ROS levels may serve as a valuable diagnostic indicator for chronic osteomyelitis, enhancing the clinical utility of conventional inflammatory biomarkers. Advances in nanotechnology and development of targeted molecular probes have introduced new possibilities for ROS-driven, disease-specific diagnosis. Nevertheless, challenges related to biosafety, stability, and standardization have confined most ROS-based diagnostic nanoprobes for osteomyelitis to the preclinical stage. Moreover, the nonspecific clinical presentation of atypical osteomyelitis and the limited specificity of ROS-associated signals necessitate confirmatory verification through imaging and histopathological examination during diagnostic decision-making. Integration of ROS-specific molecular imaging approaches, such as responsive nanoprobes, with metabolomic profiling of mitochondrial function and AI-assisted data analysis is expected to substantially enhance the sensitivity of early osteomyelitis detection. Although current ROS-based diagnostic strategies continue to face challenges in specificity and translational feasibility, the central role of ROS in osteomyelitis pathophysiology, combined with emerging technologies and multidimensional data integration, offers strong potential to overcome existing diagnostic bottlenecks and advance the development of personalized treatment strategies.
Combination of ROS nanotechnology with antibiotics for osteomyelitis treatment
As a refractory infection of bone tissue, osteomyelitis presents multiple challenges to the development of effective therapeutic strategies, including limited antibiotic penetration due to bacterial biofilm formation, continuous emergence of drug-resistant strains, and systemic toxicity associated with conventional systemic antibiotic administration. Despite these limitations, antibiotics remain the cornerstone of osteomyelitis management because of their established efficacy and favorable cost profile. However, excessive and prolonged antibiotic use has contributed to the occurrence of chronic osteomyelitis and treatment failure in other infectious diseases, highlighting the urgent need for alternative or adjunctive therapeutic approaches. In this context, ROS-based nanotechnology offers a promising avenue for improving antimicrobial efficacy and overcoming antibiotic resistance. Therefore, increasing attention has been directed toward the development of synergistic treatment strategies that integrate ROS nanotechnology with antibiotic therapy for osteomyelitis. This section systematically discusses the molecular mechanisms underlying microbial antibiotic resistance, recent advances in nanotechnology for reversing antibiotic resistance, and emerging strategies that combine ROS nanotechnology with conventional antibiotics to enhance therapeutic outcomes in osteomyelitis management.
Mechanism of microbial resistance to antibiotics
The emergence and global dissemination of antibiotic-resistant microorganisms represent a critical threat to human health and have evolved into a major public health crisis that requires coordinated international response. Antibiotic resistance compromises the effectiveness of conventional antimicrobial therapies against common pathogens. For instance, infections caused by drug-resistant Mycobacterium tuberculosis necessitate prolonged multidrug treatment regimens, often accompanied by severe side effects [143]. In addition, antibiotic-resistant infections are associated with extended hospitalization, significantly increased healthcare costs, and elevated mortality risks. Notably, MRSA frequently exhibits cross-resistance to multiple antibiotic classes, further complicating treatment and markedly increasing patient mortality [144].
Bacterial antibiotic resistance arises through multiple mechanisms and is generally classified into intrinsic resistance, acquired resistance, and adaptive resistance. Intrinsic resistance refers to the natural insensitivity of certain bacterial species to specific antibiotics, typically due to inherent structural or metabolic characteristics, such as absence of appropriate drug targets. Acquired resistance develops when previously susceptible bacteria undergo genetic mutations or acquire resistance genes through horizontal gene transfer. Adaptive resistance, in contrast, is a transient phenotype induced by environmental stimuli, including antibiotic exposure, growth phase, pH fluctuations, ionic conditions, and nutrient availability. Unlike intrinsic and acquired resistance, adaptive resistance reflects a reversible stress response that diminishes once the inducing environmental pressure is removed. The underlying mechanisms driving microbial antibiotic resistance primarily involve reduction of intracellular antibiotic concentrations, modification of antibiotic targets, enzymatic inactivation or structural modification of antibiotics, and activation of alternative metabolic pathways that bypass antibiotic targets [145-147].
Decrease of intracellular antibiotic concentration
Effective antibacterial activity requires antibiotics to penetrate bacterial cells and reach their intracellular targets. Reduced membrane permeability and enhanced activity of efflux pumps can markedly lower intracellular antibiotic concentrations, thereby promoting the development of bacterial resistance [148,149]. These processes constitute key physicochemical defense strategies that increase bacterial survival by restricting intracellular drug accumulation. Elucidation of these resistance mechanisms provides critical theoretical foundation for the rational design of next-generation antimicrobial agents and strategies aimed at reversing antibiotic resistance, with significant implications for global public health.
Decreased antibiotic permeability: Antibiotic permeability refers to the ability of antimicrobial agents to traverse bacterial cell envelopes and access intracellular targets. Reduced permeability represents an adaptive survival strategy that bacteria developed under antibiotic selective pressure. Decreased permeability of the cell wall or membrane limits antibiotic penetration, thus significantly lowering intracellular drug concentration and diminishing bactericidal efficacy. In Gram-positive bacteria, the cell envelope comprises a thick peptidoglycan layer and a single cytoplasmic membrane. In contrast, Gram-negative bacteria possess a more complex barrier structure consisting of an inner membrane and an additional outer membrane, which confers increased resistance to antimicrobial entry and contributes to the generally lower antibiotic susceptibility observed in Gram-negative bacteria. Porins constitute the primary transport channels that facilitate diffusion of hydrophilic antibiotics, such as β-lactam, fluoroquinolones, tetracyclines, and chloramphenicol, across the outer membrane of Gram-negative bacteria. Consequently, porin loss or decreased porin expression represents a major mechanism of antibiotic resistance. Under physiological conditions, outer membrane proteins OmpF and OmpC in E. coli form nonspecific transmembrane channels that permit entry of antibiotics and other small molecules [150]. However, repeated antibiotic exposure increases the likelihood of mutations in genes encoding OmpF, resulting in reduced channel expression or complete loss of function, thereby conferring resistance to antibiotics such as β-lactams and quinolones [151]. Accordingly, regulation of porin expression represents a key target for the development of future strategies to combat antibiotic resistance.
Promotion of antibiotic efflux: Antibiotic efflux refers to the active expulsion of intracellular antimicrobial agents via efflux pumps. These pumps are transmembrane transport proteins that utilize proton gradient or ATP hydrolysis to export antibiotics either selectively or broadly. Many efflux systems are capable of expelling structurally diverse antibiotics, thereby conferring multidrug resistance. In Gram-negative bacteria, efflux pumps operate in concert with the dual-membrane envelope to produce particularly robust antibiotic resistance phenotypes. Five major transporter families are implicated in antibiotic efflux: resistance-nodulation-division (RND), major facilitator super family (MFS), small multidrug resistance (SMR), multidrug and toxic compound extrusion (MATE), and ATP-binding cassette (ABC) transporters [152]. Alterations in porin expression alone typically induce only low-level resistance; however, reduced antibiotic uptake due to porin loss markedly amplifies the effectiveness of coexisting resistance mechanisms, including efflux pump activity and enzymatic antibiotic degradation, thereby promoting high-level resistance [153]. In addition, efflux pumps exhibit substrate specificity. Consequently, effective reversal of certain antibiotic resistance phenotypes development of therapeutic strategies that specifically target relevant efflux systems, which represent important molecular targets for overcoming antimicrobial resistance [154].
Inactivation or modification of antibiotics
Another common mechanism of bacterial antibiotic resistance involves enzymatic inactivation or structural modification of antimicrobial agents, leading to loss of drug efficacy. β-Lactamases represent a classical example of this mechanism, hydrolyzing the β-lactam ring of β-lactam antibiotics and thereby disrupting the amide bond essential for antimicrobial activity. In addition to β-lactamases, a variety of bacterial enzymes catalyze chemical modifications of antibiotics through group transfer reactions, ultimately conferring resistance. Rifampicin resistance provides a well-characterized illustration of this process. Rifampicin can be rendered inactive through modification by ADP-ribosyltransferases, glycosyltransferase, phosphotransferase, and monooxygenase [155]. In M. abscess, ADP-ribosyltransferase catalyzes ribosylation at the C23 hydroxyl group of rifampicin, obstructing its binding to RNA polymerase, thus promoting rifampicin resistance [156]. Glycosyltransferases can similarly modify the C23 hydroxyl group, producing comparable resistance effects [157]. Furthermore, phosphotransferases convert the C21 position of rifampicin into an inactive form, while monooxygenases diminish antibacterial efficacy by oxygenating the naphthol moiety of rifampicin and preventing its binding to the RpoB subunit of RNA polymerase [158].
Modification of antibiotic targets
High-affinity interaction between antibiotics and their molecular targets is fundamental to antimicrobial efficacy. Genetic mutations or biochemical modifications of antibiotic targets can substantially reduce drug-binding affinity, representing a major mechanism underlying antibiotic resistance. For example, methicillin-resistant S. aureus (MRSA) acquires resistance to β-lactam antibiotics through production of an altered penicillin-binding protein (PBP2a) with markedly reduced affinity for β-lactams [159]. Similarly, ribosomal target modification contributes significantly to antibiotic resistance. In E. coli, ribosomal methyltransferases catalyze mono- or dimethylation of the A2058 residue within 23S rRNA of the large ribosomal subunit, thereby obstructing antibiotic binding and conferring resistance [160]. In addition, increased methylation of 16S rRNA mediated by ribosomal methyltransferases reduces bacterial susceptibility to macrolide antibiotics [161] and is also strongly associated with high-level resistance to aminoglycosides [162].
Evasion of metabolic pathways targeted by antibiotics
To mitigate the inhibitory effects of antibiotics essential metabolic pathways, bacteria employ a series of adaptive strategies, including acquisition of alternative genes that bypass antibiotic targets. Methicillin, for instance, exerts antibacterial activity by binding to penicillin-binding proteins (PBPs) and inhibiting their transeptidase activity, thereby disrupting bacterial cell wall synthesis. However, MRSA overexpresses PBP2a, a homologous variant of PBP that exhibits markedly reduced affinity for methicillin. Binding of methicillin to PBP2a fails to inhibit transpeptidase activity, allowing cell wall synthesis to proceed and conferring high-level resistance.
Nanotechnology for reversing antibiotic resistance
Nanotechnology-based strategies to overcome antibiotic resistance mainly focus on the development of nanoparticles with intrinsic antibacterial ability to either replace conventional antibiotics or potentiate the efficacy of existing antimicrobial agents. In 2011, Huh et al. introduced the concept of “nanoantibiotics”, referring to nanomaterials that exhibit direct antibacterial activity or function as novel antibiotic platforms, showing potent bactericidal effects against drug-resistant pathogens with minimal propensity for resistance development [163]. Nanoantibiotics mitigate antibiotic resistance through multiple mechanisms, including enhancement of intracellular antibiotic uptake, reduction of bacterial efflux, disruption of the biofilm structures, and precise targeting of infection sites [164]. In addition, nanotechnology-based delivery systems reduce the likelihood of resistance development by enabling localized and controlled antibiotic release at infected tissues, while simultaneously improving the stability, solubility, and biocompatibility of pharmacologically challenging antibiotics, thereby enhancing their overall therapeutic efficacy.
Nanotechnology for promotion of antibiotic penetration or reduction of antibiotic efflux
Nanocarriers such as liposomes and dendrimers can simultaneously promote antibiotic penetration across bacterial membranes and suppress antibiotic efflux. Liposomes, which are spherical vesicles composed of one or more lipid bilayers, facilitate antibiotic entry into microbial cells through rapid fusion with bacterial membranes. This mechanism enables accelerated intracellular delivery and achievement of high local antibiotic concentrations, which can saturate and overcome transmembrane efflux pumps responsible for antibiotic extrusion. Consequently, liposomal delivery systems effectively counteract efflux-mediated resistance mechanisms. Dendritic polymers, characterized by highly branched architectures and large specific surface areas, represent another class of efficient antibiotic delivery vehicles. Quaternary ammonium-functionalized dendrimers, bearing abundant surface-positive charges, readily interact with the negatively charged bacterial cell envelope, thereby increasing membrane permeability and promoting intracellular antibiotic accumulation [164].
Nanotechnology for destroying biofilms
Biofilms are highly organized bacterial communities encapsulated within an extracellular polymeric substance (EPS) matrix composed of polysaccharides, proteins, and extracellular DNA. The dense EPS barrier severely restricts antibiotic penetration, allowing conventional therapies to eliminate primary surface-layer bacteria while leaving deeply embedded microbial populations largely unaffected. This limited penetration not only compromises therapeutic efficacy but also accelerates bacterial evolution and emergence of antibiotic resistance. Consequently, effective management of biofilm-associated bacterial infections requires antimicrobial strategies that surpass the capabilities of conventional antibiotics. Recent advances in nanotechnology have demonstrated substantial anti-biofilm potential. Abdelghafar et al. reported that ZnO nanoparticles significantly suppressed biofilm formation by S. aureus, with marked down regulation of key biofilm-related genes, including icaA, sarA, katA, and sigB [165]. Xiu et al. developed an US-responsive catalytic microbubble system composed of a piperacillin-containing shell incorporating Fe3O4 nanoparticles surrounding an air core for the treatment of chronic P. aeruginosa biofilm-associated lung infections. Under US stimulation, these micro bubbles disrupted biofilm architecture and enhanced penetration of both Fe3O4 nanoparticles and piperacillin into deep biofilm layers. Concurrently, Fe3O4 nanoparticles catalyzed H2O2 decomposition to produce free radicals, further degrading the biofilm matrix and synergistically enhancing antibacterial activity [166]. Collectively, these studies highlight the unique advantages of nanomaterials in promoting biofilm penetration, matrix degradation, and regulatory modulation of biofilm-related gene expression. Moreover, they support the development of highly targeted, multimodal antibacterial systems that integrate molecular intervention with physical biofilm disruption, offering promising new strategies for combating biofilm-associated infections.
Nanotechnology targeting the infection site
Infection-targeted nanotechnology enables precise drug delivery to infection sites, thereby enhancing local antimicrobial efficacy while reducing systemic exposure and the likelihood of resistance development. Bacterial targeting strategies commonly exploit physicochemical charge interactions, glycosylated motifs, specific surface antigens, corresponding antibodies, or signature enzymes, and have been successfully evaluated across numerous experimental and clinical investigations [167-170]. For example, Hussain et al. adopted phage display technology to screen out a cyclic nine-amino acid peptide (CARG), with high specificity for S. aureus, facilitating development of targeted antibiotic delivery platforms with enhanced antibacterial effects. CARG-functionalized porous silicon nanoparticles loaded with vancomycin preferentially accumulated in S. aureus-infected lung and skin tissues compared with uninfected tissues or Pseudomonas aeruginosa-infected tissues [171]. This approach significantly reduced required systemic antibiotic doses and associated side effects while improving therapeutic efficacy against multidrug-resistant bacteria, including MRSA. Similarly, Jayawardena et al. enhanced nanoparticle binding and internalization in E. coli by conjugating maltotriose to various nanomaterials, including SiO2 nanoparticles, FeOₓ nanoparticles, and SiO2-coated quantum dots, achieving broad applicability across multiple strains of E. coli [172]. Collectively, infection-targeted nanotechnologies have transformed antimicrobial treatment paradigm through precision delivery strategies that improve eradication of multidrug-resistant pathogens while simultaneously reducing antibiotic selection pressure and resistance evolution, providing an innovative and highly promising solution to the escalating antimicrobial resistance crisis.
ROS nanotechnology for combined antibiotic treatment of osteomyelitis
Current antibiotic-based treatment strategies for osteomyelitis continue to face substantial clinical limitations. Destruction of cortical bone vasculature often leads to insufficient drug accumulation at infection sites following systemic administration, creating so-called "drug desert” regions. In addition, bacterial biofilm severely restricts antibiotic penetration. Prolonged high-dose antibiotic therapy further contributes to antimicrobial resistance, hepatotoxicity, nephrotoxicity, and other side effects. As discussed earlier, antibiotic resistance may be mitigated through increased intracellular antibiotic uptake, suppression of drug efflux, biofilm disruption, and precise targeting of infection sites. ROS-based nanotechnology offers strong potential to reverse antibiotic resistance due to its capacity for membrane disruption and broad-spectrum antimicrobial action. When combined with targeted ligands or antibiotics, ROS nanoplatforms further demonstrate enhanced therapeutic precision and efficacy. Sustained-release nanomaterials have been widely used in clinical management of chronic osteomyelitis owing to their ability to maintain prolonged minimal inhibitory concentrations at infection sites while minimizing off-target toxicity. For example, Lu et al. synthesized a multifunctional nanoagent composed of bovine serum albumin-manganese dioxide-ubiquicidin29-41-indocyanine green-gentamicin (BMUIG). The antimicrobial peptide ubiquicidin29-41 selectively binds bacterial surfaces, directing the nanocomplex to infected tissues, where abundant ROS generation combined with low-dose gentamicin achieved effective osteomyelitis treatment [87]. Similarly, Jin et al. reported a microwave-responsive magnetic targeting composite system composed of Fe3O4/PB nanoparticles, gentamicin, and biodegradable poly(lactic-co-glycolic acid) (PLGA). Under microwave irradiation, synergistic thermal and ROS effects generated by Fe3O4/PB nanoparticles, together with magnetically guided release of gentamicin, produced potent antibacterial activity against osteomyelitis [173] (Figure 8). Collectively, ROS-responsive drug delivery systems offer dual therapeutic advantages for osteomyelitis management: precise lesion targeting that markedly enhances local drug concentration and sustained antibacterial activity mediated by controlled release, intrinsic bioactivity, and microenvironmental responsiveness. This "precision strike" treatment paradigm represent a fundamental breakthrough beyond conventional systemic antibiotic therapy. Future studies should prioritize elucidation of dynamic nanocarrier release mechanisms within complex infection microenvironments, comprehensive long-term biosafety evaluation, and optimization of clinical translation pathways to enable progression from "passive drug loading" toward truly intelligent diagnostic-therapeutic systems. Moreover, integration of ROS nanotechnology with complementary strategies to further enhance antibiotic uptake and reduce drug efflux warrants continued theoretical development and translational validation.
Schematic diagram of the microwave-responsive, magnetically targeted composite PLGA/Fe3O4/PB/Gent integrating MTT, MDT, and CT for eradication of acute S aureus-infected osteomyelitis. Adapted with permission from Ref. [173]. Copyright 2024, Wiley-VCH.
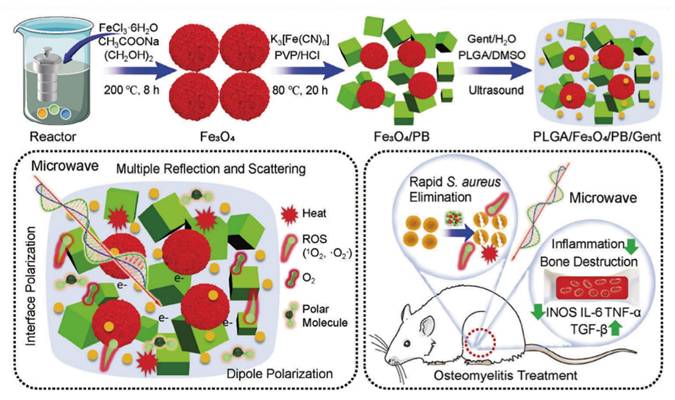
ROS nanotechnology with immune activation for osteomyelitis treatment
The microenvironment of chronic osteomyelitis is often represented by a significant immunosuppressive state, manifested by immune cell dysfunction, imbalance between pro-inflammatory and anti-inflammatory signaling, and impaired immune surveillance [174]. Immune dysregulation within bone marrow lesions not only weakens the host's ability to eradicate pathogens but also leads to therapeutic challenges such as restricted antibiotic penetration and aggravated biofilm formation, thereby representing a central mechanism underlying persistent infection and disease recurrence. As a key mediator of innate immune, ROS serve not only as potent antimicrobial effectors but also as regulators of immune homeostasis within the local microenvironment through regulation of immune cell activation and polarization states. Under conditions of high pathogen load, the dynamic immunomodulatory properties of ROS provide a promising strategy for reversing local immunosuppression and restoring effective host defense mechanisms [175].
Accordingly, application of ROS-based nanotechnology offers and restoring effective host defense mechanisms antibacterial efficacy and modulate the immunosuppressive microenvironment of osteomyelitis. Through effective pathogen eradication, ROS nanoplatforms reduce immune exhaustion, while concurrently promoting conversion of the local microenvironment from a state of immune tolerance to immune activation by activating innate immune responses and restoring immune cell functionality. This dual-mode regulatory mechanism transcends the limitations of conventional antimicrobial therapy and provides both theoretical foundation and technical support for reestablishing dynamic equilibrium between host immune defense and pathogen clearance. The following section first summarizes the immunological characteristics of the osteomyelitis microenvironment, followed by critical analysis of the advantages, limitations, and future development directions of existing ROS nanotechnology-based immune activation strategies, thereby offering theoretical guidance for integrated osteomyelitis treatment paradigms that combine ROS nanotechnology with immunotherapeutic approaches.
Characteristics of the immune microenvironment at the osteomyelitis site
Immune characteristics of acute osteomyelitis
The immune microenvironment of acute osteomyelitis is predominantly characterized by extensive neutrophil infiltration, elevated release of pro-inflammatory mediators, and activation of the complement system. During the early phase of infection, rapid recruitment of neutrophils to the affected site occurs, followed by phagocytic elimination of invading microorganisms. Concurrently, microbial invasion triggers both local and systemic inflammatory responses, resulting in increased production of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). These cytokines enhance vascular permeability and promote further recruitment of immune cells to the infected region. In parallel, activation of the complement cascade facilitates pathogen clearance and amplifies inflammatory signaling, collectively contributing to the acute immune defense response [176,177].
Immune characteristics of chronic osteomyelitis
During the chronic stage of osteomyelitis, the immune microenvironment is primarily characterized by sustained activation of macrophages and T lymphocytes. As infection persists, macrophages progressively become the dominant effector immune cells. Classically activated M1 macrophages play a central role in initiating and maintaining pro-inflammatory responses by secreting large amounts of pro-inflammatory cytokines, thereby facilitating pathogen clearance. Concurrently, M1 macrophages promote differentiation of T helper 1 (Th1) and T helper 17 (Th17) cells, further amplifying immune activation. M1 macrophage polarization is induced by pro-inflammatory stimuli such as interferon-γ (IFN-γ) and lipopolysaccharide (LPS) through the classical activation pathway [178-180]. These signals typically arise from microbial invasion, tissue injury, or activation of immune cells including T lymphocytes and natural killer (NK) cells. Phenotypically, M1 macrophages exhibit elevated surface expression of CD80, CD86 and major histocompatibility complex (MHC) class II molecules, which enhance antigen presentation and reinforce pro-inflammatory immune responses. In addition, M1 macrophages secrete chemokines that recruit other immune cells, such as neutrophils and dendritic cells (DCs), to sites of infection [180,181]. However, while M1-mediated responses contribute to effective pathogen clearance during early infection, prolonged M1 activation promotes persistent inflammation and progressive tissue damage, which is particularly evident in chronic inflammatory and autoimmune conditions.
Alternatively activated M2 macrophages play an important role in inhibiting inflammatory responses, promoting wound healing, and maintaining immune homeostasis [182]. M2 polarization is induced primarily by anti-inflammatory cytokines, such as interleukin-4 (IL-4), interleukin-13 (IL-13), and interleukin-10 (IL-10). During later stages of the immune response, M2 activation contributes to resolution of inflammation and facilitation of tissue repair [182,183]. Phenotypically, M2 macrophages express high levels of CD163 and CD206, markers associated with phagocytic clearance of damaged tissue and promotion of tissue remodeling. In addition, M2 macrophages secrete substantial amounts of anti-inflammatory mediators, such as IL-10 and transforming growth factor-β (TGF-β), which play essential roles in dampening inflammation and accelerating tissue regeneration [182,184].
During the chronic phase of osteomyelitis, activation of T lymphocytes also contributes to host antimicrobial defense. CD4⁺ T helper cells differentiate into multiple subsets, including Th1, Th2, and Th17, based on cytokine secretion profiles, thereby orchestrating distinct immune response pathways. IFN-γ produced by Th1 cells enhances antimicrobial activity, whereas Th17 cells are closely associated with inflammatory and autoimmune responses. Persistent inflammatory stimulation may further promote collagen deposition and fibrotic scar formation, ultimately disrupting normal bone remodeling and metabolic processes [185,186].
ROS nanotechnology for osteomyelitis therapy through immune regulation
Mechanisms of immune regulation
Participation of ROS in shaping the bone marrow immune microenvironment represents a critical determinant of the therapeutic efficacy of ROS-based nanotechnologies for osteomyelitis management. As both endogenous and exogenous signaling mediators, ROS regulate innate and adaptive immune responses by modulating the activity of multiple immune cell populations, including macrophages, DCs, T lymphocytes, and NK cells. These regulatory effects are mediated through activation of key signaling pathways, including TNF-β, mechanistic target of rapamycin (mTOR), ERK, and intracellular calcium signaling cascades. During antibacterial responses, ROS exert dual functions by directly inducing microbial cytotoxicity while simultaneously orchestrating immune cell activation and polarization. Accordingly, systematic examination of ROS-mediated regulatory pathways across different immune cell subsets is essential for elucidating the mechanisms by which ROS nanotechnology modulates the pathological microenvironment of osteomyelitis and enhances therapeutic outcomes.
ROS and macrophages: Polarization of classically activated M1 macrophages is regulated by multiple intracellular signaling pathways, predominantly including the mitogen-activated protein kinase (MAPK)s/NF-κB and Janus kinase-signal transducer and activator of transcription (JAK-STAT) cascades [187]. Members of the nicotinamide adenine dinucleotide phosphate oxidase (NOX) family, especially NOX2 and NOX4, are closely related to ROS generation and M1 macrophage polarization. Activation of NOX2/NOX4 enhances ROS production along the electron transport chain, which subsequently stimulates MAPK and NF-κB signaling, amplifies inflammatory responses, and ultimately drives monocytes differentiation toward M1 phenotype [188,189]. In addition, Cotzomi-Ortega et al. found that ROS-dependent macrophage migration inhibitor (MIF) induces M1 polarization via paracrine signaling mechanisms [190]. Zhou et al. further demonstrated that iron overload-induced ROS upregulates p53 acetylation, thereby facilitating M1 macrophage polarization [191].
ROS and T cells: Accumulating evidence demonstrates that ROS critically regulate T-cell proliferation, differentiation, apoptosis, and effector function. Low physiological levels of ROS are essential for effective T-cell activation. Endogenous ROS generation in T lymphocytes arises primarily from mitochondrial metabolism and NOX [192,193]. Mitochondria-derived ROS (mROS), produced by respiratory chain complexes I, II, and III, are indispensable for T-cell activation and promote signaling through nuclear factor of activated T cells (NFAT), NF-κB, and AP-1, thereby influencing secretion of IL-2 and IL-4, and regulating subsequent T-cell proliferation and differentiation [194]. The contribution of NOX-derived ROS to T-cell activation remains an area of ongoing investigation. Exogenous ROS enhance T-cell activation by stimulating T-cell receptor (TCR) signaling and reinforcing transcriptional programs mediated by NFAT, AP-1, and NF-κB [192,195]. TCR engagement induces rapid production of H2O2 and •O2-, which subsequently modulate ERK signaling and the human apoptosis-related factor ligand (Fas ligand, FasL/CD95L) apoptotic pathway, respectively [193].
T cell differentiation involves antigen-driven activation of CD4⁺ T cells or CD8⁺ T cells into helper T cells (Th) and cytotoxic T lymphocyte (CTLS) subsets. ROS have been shown to promote differentiation of Th1, Th2, and Th17 cells [193-195]. NOX activation increases intracellular ROS levels and promotes polarization toward Th1 and Th17 phenotypes [199]. Furthermore, ROS-mediated activation of ERK1/2 signaling enhances IL-4 production, thereby promoting Th2 differentiation [200]. Abimannan et al. demonstrated that oxidative stress induced by pro-oxidants such as plumbagin and H2O2 upregulates Th1 and Th17 differentiation through enhanced ERK1/2-dependent oxidative phosphorylation [201]. Kim et al further identified a pivotal role for ROS in regulating inflammatory functions of natural killer T (NKT) cells, a unique lymphocyte subset sharing features of both T cells and NK cells. ROS signaling promoted differentiation toward NKT1 and NKT17 subtypes while inhibiting NKT2 differentiation [202].
In addition to regulating activation and differentiation, ROS also contribute critically to T cell activation-induced cell death (AICD), a specialized apoptotic process that maintains immune homeostasis following T-cell activation. ROS-mediated AICD is primarily regulated through the Fas ligand (FasL) pathway and the ERK signaling pathway [203,204]. TUpon TCR engagement, H2O2 production is initially catalyzed by dual oxidase-1 (Duox-1), which amplifies early TCR signaling. Sustained TCR activation subsequently promotes mitochondrial ·O2- generation, leading to increased FasL expression on T cells and triggering Fas-dependent apoptotic signaling. Concurrently, TCR stimulation elevates intracellular H2O2 levels and activates ERK 1/2 phosphorylation, thus promoting apoptotic cascades and execution of AICD [205].
ROS and neutrophils: ROS play a central role in regulating neutrophil antimicrobial activity and programmed cell death [203,204]. Upon pathogen invasion, neutrophils are rapidly recruited to infected tissues under the guidance of chemokines and eliminate pathogens primarily through phagocytosis. Internalization of microorganisms triggers a robust "respiratory burst”, characterized by a sharp increase in O2 consumption and massive ROS production [208]. This process is driven by the high expression and activation of NADPH oxidase 2 (NOX2) in neutrophils, which constitutes one of the most powerful antimicrobial mechanisms of innate immunity [209]. Within phagosomes, myeloperoxidase (MPO) and other antibacterial enzymes utilize H2O2 and halide or pseudo-halide ions to generate highly cytotoxic oxidants, including HOCl, HOBr, and hypothiocyanous acid (HOSCN). These oxidants induce extensive lipid peroxidation and oxidative DNA damage in engulfed pathogens, ensuring efficient microbial killing [210].
Apoptosis represents the predominant non-lytic death pathway of neutrophils and is tightly regulated by NOX-derived ROS [211]. Scheel-Toellner et al. proved that NOX-generated ROS activate acidic sphingomyelase, promoting clustering of death receptors such as CD95 (Fas) and subsequent caspase-8 activation, ultimately driving neutrophil apoptosis [212]. Conus et al. further confirmed that ROS facilitate the release of cathepsin D from lysosomes, which then activates caspase-8 and reinforces apoptotic signaling [213]. Beyond apoptosis, NOX-regulated ROS production also critically influences neutrophil pyroptosis and necroptosis. Ryu et al. found that NOX2 deficiency reduces ROS levels, leading to aberrant activation of the P2X7 receptor-dependent noncanonical inflammasome pathway, culminating in enhanced neutrophil pyroptosis [214]. Collectively, these findings establish ROS as pivotal regulators of both neutrophil antimicrobial function and fate determination within infected tissues.
ROS and B cells: ROS play a critical regulatory role in B-cell maturation, activation, differentiation, and programmed cell death. B cells originate from pluripotent hematopoietic stem cells in the bone marrow and undergo a tightly regulated developmental program comprising pre-B cells, immature B cells, mature B cells, activated B cells, and plasma cells [215, 216]. The early differentiation of pre-B and immature B cells is antigen-independent and occurs primarily within the bone marrow microenvironment [217]. Accumulating evidence indicates that ROS are integral to B-cell receptor (BCR) signaling and B-cell activation. ROS modulate the phosphorylation status of BCR-associated kinases, thereby influencing downstream signaling cascades essential for B-cell activation. Upon antigen engagement, mature B cells undergo profound metabolic reprogramming characterized by increased mitochondrial biogenesis and PI3K-dependent glucose uptake, which in turn fuels the pentose phosphate pathway, providing reducing equivalents for oxidative stress control and substrates for nucleotide synthesis required during clonal expansion. Vené et al reported that ROS depletion significantly impairs BCR-mediated B-cell activation and proliferation, highlighting the indispensable role of ROS in sustaining B-cell immune responses [218]. Similarly, Yang et al. demonstrated that sustained NOX-dependent ROS production following BCR stimulation enhances the activation of the NF-κB and AKT signaling pathways, thereby promoting B-cell proliferation and survival [219].
B cells can be further classified into B1 and B2 subpopulations, which originate from distinct hematopoietic precursors: B1 cells primarily derive from fetal liver-derived hematopoietic stem cells (HSCs), whereas B2 cells originate from bone marrow-derived HSCs [220]. During B-cell differentiation, endoplasmic reticulum-derived ROS are greatly amplified, a process that supports efficient immunoglobulin (Ig) synthesis and secretion. Moreover, ROS generated through mitochondrial depolarization play a crucial role in promoting the differentiation of activated B cells into long-lived memory B cells, thereby sustaining humoral immune memory [221].
Apoptosis, the predominant mode of B-cell death, is tightly regulated by intracellular ROS levels. Physiological (low) ROS concentrations are indispensable for maintaining B cell activation and function, whereas excessive ROS accumulation triggers oxidative stress, leading to cellular injury and programmed cell death. Mechanistically, elevated ROS levels activate caspase-9, leading to upregulation of XAF1, which subsequently induces apoptosis in EBV-transformed B cells. In parallel, ROS activate the JNK/p38-MAPK signaling pathway, promoting mitochondrial translocation of Bax, disruption of mitochondrial membrane potential, and subsequent activation of caspase-9 and caspase-3 [222]. In addition to apoptosis, ROS also contribute to pyroptotic B-cell death. ROS-mediated activation of inflammasomes, including NLRP3, NLRP6, NLRC4, and AIM2, enhances caspase-1-dependent pyroptosis, further influencing B-cell survival and immune homeostasis [223].
ROS and DCs: As specialized antigen-presenting cells, DCs play a central role in initiating immune responses, regulating immune homeostasis, and maintaining immune tolerance. Immature DCs undergo activation and maturation to acquire potent antigen-processing and T-cell-priming capacity. Consequently, precise regulation of DCs immune function is crucial for the treatment of immune-related disorders, including autoimmune diseases, infections, and cancer. Accumulating evidence indicates that ROS significantly affects the differentiation, activation, and functional programming of DC subsets, including monocyte-derived DCs (MoDCs) and plasmacytoid DCs (pDCs) [224]. For MoDCs, maintenance of physiological ROS levels is essential for their activation, maturation, migration, cytokine secretion, and T-cell stimulatory capacity. Peng et al. revealed that ROS depletion inhibits the double-stranded RNA-dependent protein kinase (PKR), protein kinase C (PKC), and p38/MAPK signaling pathways, resulting in downregulation of NF-κB, co-stimulatory molecules (CD40, CD80, CD86), and pro-inflammatory cytokines ( IL-1β, TNF-α and IL-12) [225]. Complementary studies have shown that ROS elevation—induced by the xanthine-xanthine oxidase system or cationic liposomes—promotes MoDC activation and maturation. Cheong et al. further found that increased ROS enhances MoDC maturation via phosphorylation of p38, JNK, and ERK, accompanied by nuclear translocation of NF-κB, thereby augmenting the capacity of MoDCs to activate allogeneic CD8+ T cells and CD4+ Th1 cells, while concurrently inhibiting Th2 differentiation [226]. For pDCs, ROS homeostasis is likewise crucial for their activation and immune function. Oberkampf et al. reported that ROS downregulation significantly inhibits the expression of CD69, CD40, CD80, and CD86, and the secretion of IFN-α, underscoring the necessity of ROS for normal pDC physiology [227]. Moreover, while elevated ROS does not markedly alter Th1 and Th17 differentiation induced by resting pDCs, it significantly enhances Th2 polarization, indicating that ROS exerts context-dependent regulatory effects on T-cell differentiation mediated by pDCs [228].
ROS and NK cells: NK cells are cytotoxic lymphocytes lymphoid progenitors and constitute a critical component of the innate immune system, providing rapid first-line defense against invading pathogens and malignant cells. Increasing evidence indicates that ROS levels exert a tightly regulated, bidirectional influence on NK cell survival and effector function. While physiological, low-level ROS are necessary to sustain NK cell viability and functional competence, excessive oxidative stress severely compromises NK cell activity. Harmon et al. verified that extracellular acidification induces mitochondrial dysfunction in NK cells, resulting in elevated intracellular ROS accumulation, which in turn triggers NK cell apoptosis and impairs antitumor immune responses. Conversely, activated NK cells actively suppress intracellular ROS to preserve their cytotoxic function. For example, IL-15-induced NK cells up regulate the thioredoxin antioxidant system, effectively reducing intracellular ROS levels and protecting NK cells from oxidative damage. These findings highlight a dynamic, bidirectional regulatory relationship between ROS and NK cells, in which balanced redox homeostasis is essential for maintaining NK cell-mediated immune surveillance and host defense [229].
Application of ROS nanotechnology for immune regulation
ROS play an important role in both the physiological function and immune regulation of bone marrow cells, making ROS nanotechnology a powerful tool for reshaping the immune microenvironment in osteomyelitis therapy. First, ROS directly exert potent antimicrobial effects through oxidative damage to microbial membranes, proteins, and nucleic acids. Simultaneously, ROS participate in the dynamic regulation of inflammatory responses by modulating macrophage polarization. By activating signaling pathways such as AMPK, ROS can promote the transition of monocytes toward the pro-inflammatory M1 phenotype during early infection and subsequently facilitate the shift from M1 to M2 macrophages during later stages of tissue repair, thereby orchestrating both pathogen clearance and wound resolution. Second, ROS act as key signaling mediators that bridge innate and adaptive immunity. Elevated ROS levels at infection site induce Immunogenic cell death (ICD) of pathogens and damaged host cells, generating abundant antigenic signals that stimulate immune activation. During ICD, multiple danger-associated molecular patterns and cytokines are released, including membrane-associated signaling molecules and pro-inflammatory mediators, which collectively amplify immune surveillance. Moreover, ROS directly promote the activation and maturation of DCs by triggering intracellular signaling pathways, including TNF-β, mTOR, ERK, and calcium signaling cascades. Importantly, ROS facilitate efficient antigen processing and presentation by enhancing lysosomal escape of internalized antigens and protecting them from degradation, thereby improving cytoplasmic antigen delivery. This process significantly strengthens antigen cross-presentation and induces robust CD8⁺ T-cell responses, further reinforcing antimicrobial immunity. Through the coordinated regulation of antimicrobial activity, immune cell activation, inflammatory resolution, and tissue regeneration, ROS-based therapeutic strategies generate profound immune feedback that critically determines overall outcome of osteomyelitis treatment.
In view of the pivotal roles of M1 and M2 macrophages in antimicrobial defense and wound healing, respectively, macrophage-targeted ROS nanomaterials have been actively developed for osteomyelitis therapy. Fu et al. constructed a microwave-responsive engineered pseudo-macrophage-coated Fe3O4/Au nanoparticle (M-Fe3O4/Au), which weakened inflammatory responses and induced M2 macrophage polarization within osteomyelitis lesions by neutralizing pro-inflammatory cytokines and reducing intracellular ROS production [230] (Figure 9A).
Similarly, Cai et al. developed a novel US-responsive heterojunction array p-ZnO/TiO2-x, of which nanorod morphology activated the Fn-Integrin-α5β1-PI3K-AKT1 signaling pathway in macrophages, thereby inducing M1-to-M2 polarization and alleviating local inflammation, as evidenced by increased IL-10 levels and decreased IL-1β expression in surrounding tissues [231]. In parallel, ROS nanomaterials capable of promoting M1 macrophage polarization have also been engineered to enhance antibacterial efficacy. Zhang et al. integrated a PEG-based photosensitive nitric oxide donor (PEG-b-pCouNO) with an antimicrobial peptide (Nisin) onto Cs3Cu2I5 nanoscintillators, forming Cs3Cu2I5@(Nisin+PEG-b-pCouNO) nanoparticles (SNP NPs). Upon activation, PEG-b-pCouNO released NO and depleted intracellular GSH, thereby relieving tissue hypoxia and enhancing ROS production. Concurrently, NO signaling drove the polarization of immunosuppressive M0 macrophages toward the pro-inflammatory M1 phenotype. This synergistic strategy of combining immunomodulation with chemo-radiotherapeutic augmentation, showed highly effective therapeutic outcomes in MRSA-infected osteomyelitis [232] (Figure 9B).
In addition, macrophage polarization, the exploration of other immune activation pathways provides important support for the development of next-generation immunotherapeutic strategies for osteomyelitis. For example, Lin et al. developed a biomimetic nanotherapeutic platform termed HMMP, in which PpIX was encapsulated within hollow MnOx nanoparticles and subsequently cloaked with a hybrid membrane derived from macrophages and tumor cell lines. This multifunctional system not only activated innate immunity responses to enhance direct pathogenic bacterial clearance, but also stimulated adaptive immunity through antigen burst release and subsequent APC activation. Importantly, the resulting activation of immune memory effectively prevented infection recurrence, demonstrating durable therapeutic protection in osteomyelitis models [99] (Figure 9C).
Therefore, the optimization of chronic osteomyelitis treatment fundamentally depends on overcoming the dual barriers of pathogen persistence and local immunosuppression. Conventional antimicrobial strategies predominantly focus on microbial eradication while largely neglecting immune microenvironment remodeling. In contrast, ROS-responsive nanotechnology enables the spatiotemporally controlled integration of antibacterial effect and immune activation, thereby establishing a synergistic therapeutic paradigm for osteomyelitis management. This approach not only introduces an innovative "antibacterial and immunomodulatory" strategy for refractory bone infections, but also underscores the importance of restoring host-pathogen homeostasis as a central objective in future anti-infective therapies. By integrating materials engineering with immune regulation, these intelligent delivery systems define a new paradigm for targeted immunometabolic intervention, with broad translational potential for the treatment of chronic infections and immune dysregulation-associated diseases.
ROS nanotechnology with bone repair for osteomyelitis treatment
Chronic osteomyelitis is usually accompanied by varying degrees of bone defects. During the self-repair process, adequate new bone formation is necessary for the reconstruction of skeletal structure and function. The difficulty of bone defect repair is closely related to defect size. When the defect area exceeds the intrinsic osteogenic healing capacity, rapidly migrating fibrous connective tissue occupies the defect site before osteoblast infiltration, while persistent bacterial infection further compromises bone regenerative potential, ultimately leading to adverse clinical sequelae [233]. Currently, conventional management of extensive osteomyelitis-associated defects relies on infection control using systemic or local antibiotics combined with defect reconstruction via autografts or allografts. However, such strategies are often limited by prolonged treatment duration, incomplete eradication of infection, and suboptimal regenerative outcomes. In response to these limitations, numerous novel nanobiomaterials possessing both ROS-mediated antibacterial activity and osteoinductive capacity have recently been developed for osteomyelitis treatment. The following section systematically delineates the molecular mechanisms underlying bone metabolic imbalance during osteomyelitis, examines latest advances in ROS-based nanotechnologies for reconstruction of the bone regeneration microenvironment, and critically discusses the advantages, limitations, and future directions of the emerging “antibacterial-anti-inflammatory-osteogenic" trinity therapeutic paradigm, thereby providing theoretical guidance for integrated osteomyelitis treatment strategies combining ROS nanotechnology with bone repair approaches.
Mechanism of bone loss in osteomyelitis progression
When bone tissue is invaded by bacteria, the host immune system is rapidly activated and releases a broad spectrum of inflammatory mediators and cytokines to eliminate invading pathogens. However, these inflammatory factors exert dual pathological effects on bone metabolism. On the one hand, they significantly enhance osteoclast differentiation and activity, thereby accelerating bone resorption; on the other hand, they simultaneously suppress osteoblast function, impairing new bone formation and regeneration [174] (Figure 10). The combined imbalance between excessive bone resorption and insufficient bone formation ultimately leads to progressive bone loss and structural destruction at osteomyelitis lesions. In this section, the specific molecular and cellular mechanisms through which increased osteoclast activity and decreased osteoblast function contribute to bone loss during osteomyelitis progression are discussed in detail, providing a theoretical basis for the development of targeted therapeutic strategies.
(A) Schematic illustration of the fabrication process of engineered pseudo-macrophage nanoparticles and the biological property and characterization of the microwave-responsive nanoplatform. Adapted with permission from Ref. [230]. Copyright 2021, Wiley-VCH. (B) Schematic design of the SNP nanoradiosensitizer and its anti-biofilm and immunomodulatory functions for osteomyelitis therapy. Adapted with permission from Ref. [232]. Copyright 2024, Wiley-VCH. (C) Schematic representation of HMMP-mediated in situ nanovaccination for immune activation in osteomyelitis treatment. Adapted with permission from Ref. [99]. Copyright 2022, American Chemical Society.
Schematic illustration of inflammation-induced imbalance in the bone immune microenvironment during osteomyelitis.
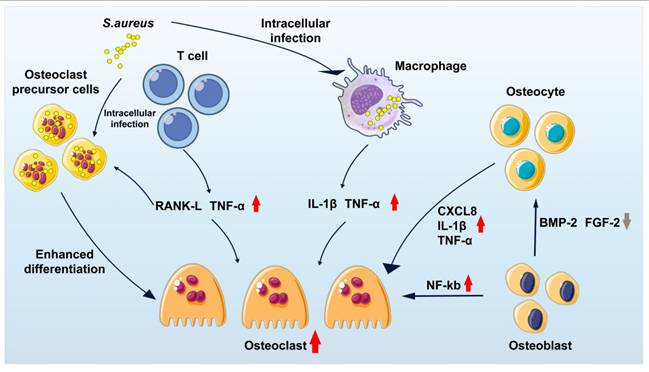
Enhanced osteoclast activity
Osteoclasts are multinucleated cells derived from the monocyte-macrophage lineage that mediate bone resorption through the secretion of proteolytic enzymes and H+ ions. Osteoclasts activity is tightly regulated by immune cells, including macrophages, neutrophils, and T lymphocytes.
Macrophage and osteoclast activity: Upon pathogenic stimulation, macrophages markedly up regulate pro-inflammatory factors to fight infection and initiate immune responses. In particular, macrophage-derived TNF-α and IL-1β aggravate osteocyte apoptosis. Apoptotic osteocytes subsequently release receptor activator of nuclear factor-κB ligand (RANKL), a key signaling molecule that drives osteoclast proliferation and differentiation [234]. This cascade promotes excessive osteoclast accumulation at the site of infection and accelerates pathological bone destruction. Furthermore, during S. aureus invasion of bone marrow, macrophages activate he NOD-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome, leading to caspase-1 activation and enhanced IL-1β maturation, which further stimulates osteoclastogenesis via the c-Jun JNK and NF-κB signaling pathways [235].
Neutrophil and osteoclast activity: Neutrophils are highly motile phagocytes that constitute the earliest cellular responder during microbial invasion. Following bacterial infiltration of bone marrow, chemokines produced by host cells and pathogens rapidly recruit neutrophils from the bloodstream, facilitating transendothelial migration toward the infected site. Experimental studies have demonstrated that, upon S. aureus infection of bone tissue, infiltrating neutrophils secrete a large number of inflammatory cytokines, such as IL-6, which activate the NF-κB signaling pathway and promote differentiation of osteoclast precursors into mature bone-resorbing osteoclasts [26]. Moreover, sustained neutrophil accumulation within inflamed bone tissues produces excessive levels of ROS, nitric oxide synthase products, and neutrophil extracellular traps (NETs). This oxidative overload disrupts redox homeostasis, trigger aberrant inflammatory responses, and ultimately induces cytotoxicity, tissue injury, and progressive bone loss [195].
T cell and osteoclast activity: Within infected bone marrow, activated macrophages exhibiting elevated expression of IFN-α and IL-12 activate NK cells and induce the differentiation of CD4⁺ T cells toward the Th1 lineage. Although Th1 cells contribute to pathogen clearance through secretion of IFN-γ and TNF-α, the concomitant upregulation of RANKL and TNF-α constitutes a major stimulus for osteoclast differentiation. In parallel, IL-6 and transforming growth factor-β (TGF-β) promote maturation of Th17 cells and upregulate IL-17 production, which further increases RANKL expression in both osteoclast precursors and osteoblasts, thereby accelerating osteoclastogenesis and bone resorption [174].
Other elements and osteoclast activity: Beyond immune cell regulation, multiple additional factors participate in controlling osteoclast activity during osteomyelitis. Toxic shock syndrome toxin-1 (TSST-1), a superantigen produced by S. aureus to suppress host immunity, has been shown to directly enhance the bone-resorptive capacity of osteoclasts [37]. Trouillet-Assant et al. confirmed that, compared with osteoclast precursors, mature osteoclasts show a significantly greater capacity to internalize S. aureus, which in turn promotes osteoclast fusion and further augments bone resorption [236]. Moreover, Somayaji et al. found that osteoblasts infected with S. chrysosa significantly upregulate the prostaglandin E2 (PGE2) production, a potent activator of osteoclastic bone resorption mediated via the RANKL-dependent signaling pathway [237].
Collectively, osteoclast activity is governed by a complex network of immune mediators, microbial virulence factors, and bone-derived signals. Within the inflammatory microenvironment of osteomyelitis, this regulatory balance becomes severely disrupted, resulting in excessive osteoclast activation and accelerated bone loss. Targeting key regulatory pathways involved in osteoclast differentiation and function therefore represents a promising therapeutic strategy to mitigate bone loss and preserve skeletal integrity during osteomyelitis progression.
Decreased osteoblast activity
S. aureus infection of bone tissue leads to robust production of chemokines, such as CXCL8, CXCL9, and CXCL10. Among these mediators, CXCL8 significantly upregulates the release of IL-1β and TNF-α. Elevated TNF-α subsequently activates t NF-κB signaling in osteoblasts while simultaneously inhibiting the expression of key osteogenic regulators, such as BMP-2 and fibroblast growth factor 2 (FGF-2). In addition, CXCL8 promotes neutrophil chemotaxis and upregulates the expression of matrix metalloproteinases (MMP), which actively participate in extracellular matrix degradation and bone resorption [235]. Consequently, osteoblast dysfunction constitutes a critical contributor to impaired bone regeneration and progressive skeletal destruction during osteomyelitis. Preservation or restoration of osteoblast activity therefore represents an essential therapeutic objective for effective bone repair and structural reconstruction. Targeted regulation of inflammatory signaling and osteogenic factor expression offers a promising intervention for preventing pathological bone loss and improving long-term skeletal outcomes in osteomyelitis.
ROS nanotechnology for osteomyelitis therapy by promoting bone repair
ROS contribute to bone regeneration through multiple interconnected mechanisms, including modulation of the immune microenvironment, stimulation of angiogenesis, initiation of cellular proliferation and differentiation, and regulation of intracellular signaling pathways. First, controlled levels of ROS provide essential exogenous stimuli that enhance macrophage activity within the hyperglycemic and immunosuppressive microenvironment characteristic of chronic osteomyelitis. ROS promote macrophage metabolic reprogramming by activating glycolytic and glucose metabolism pathways, thereby strengthening the reparative function of M2 macrophages during bone healing [238]. Activated M2 macrophages subsequently establish a pro-regenerative milieu by secreting anti-inflammatory cytokines and angiogenic factors that support tissue reconstruction [239]. Second, ROS regulate vascular endothelial cell function at bone defect sites by activating the PI3K/Akt signaling pathway, thereby stimulating neovascularization, which is indispensable for nutrient delivery and metabolic waste clearance during bone regeneration [240]. Moreover, ROS influence local oxidative phosphorylation and Wnt signaling pathways, indirectly contributing to vascular remodeling and tissue integration [241]. Additionally, transient elevation of ROS during the early phase of tissue damage activates apoptotic signaling and initiates osteoprogenitor regeneration via compensatory proliferation mechanisms [242]. Physiological concentrations of ROS further participate in directing osteogenic differentiation of mesenchymal stem cells and directly promote extracellular matrix deposition and bone formation [243]. Finally, as key second messengers, ROS orchestrate redox-sensitive signaling networks that govern bone development and remodeling by coordinating the functional activities of mesenchymal cells, osteoblasts, osteoclasts, and endothelial cells, thereby maintaining dynamic skeletal homeostasis [244].
These findings collectively highlight the significant therapeutic potential of ROS in bone repair. Future development of ROS-based nanoplatforms is expected to enable precise loading and controlled release of osteogenic agents, thereby improving local drug bioavailability and therapeutic efficacy. Such nanoplatforms not only stimulate the secretion of osteogenic factors but also accelerate bone formation by activating key osteogenic signaling pathways. Moreover, ROS nanotechnology can suppress osteoclast activity and effectively attenuate excessive bone resorption, contributing to the preservation of bone mass and structural stability. Consequently, ROS-based nanotherapeutic systems establish a synergistic “antibacterial-bone regenerative" treatment paradigm that simultaneously eradicates infection while promoting functional bone regeneration and tissue.
ROS nanotechnology for osteomyelitis therapy by loading osteogenic drugs
Integration of ROS nanotechnology with osteogenic drug delivery enables simultaneous antimicrobial therapy and bone regeneration at osteomyelitis lesions. Dexamethasone (Dex), a well-established osteogenic inducer capable of promoting osteogenic differentiation and bone formation in mesenchymal stem cells, has been widely explored in this context. Wu et al. developed a multifunctional nano system by co-loading antibacterial silver (Ag) nanoparticles and Dex into polydopamine-functionalized mesoporous silica nanoparticles (MSNs), which were subsequently incorporated into a poly-L-lactic acid (PLLA) scaffold for osteomyelitis treatment. The resulting Ag-pMSNs@Dex/PLLA nanosystem exhibited efficient ROS generation and significantly enhanced activation of bone marrow mesenchymal stem cells (BMSCs), thereby promoting robust bone regeneration while maintaining strong antibacterial efficacy [245].
ROS nanotechnology for osteomyelitis therapy by inducing osteogenic factors
ROS-based nanotechnology can further enhance bone regeneration by upregulating osteogenic factor expression and activating osteogenic signaling pathways. Ma et al. designed a Nb2C nanosheet modified porphyrin metal-organic frame hollow nanotubes (HNTM/Nb2C), which not only stimulated BMSCs through controlled ROS production and Nb2+ ion release but also significantly increased the expression of osteogenic genes, such as BMP2 and RUNX2, thereby promoting osteogenic differentiation and bone formation [246] (Figure 11A).
ROS nanotechnology for osteomyelitis therapy by inhibiting osteoclast activity
Bone tissue is mainly composed of nano-hydroxyapatite and organic matrix, and the structural characteristics of hydroxyapatite critically influence the osteogenic microenvironment and subsequent bone regeneration. Alendronate sodium (ALN), a clinically approved bisphosphonate, selectively binds bone mineral hydroxyapatite and inhibits osteoclast-mediated bone repair. Leveraging this mechanism, Ma et al. constructed a defect-engineered porphyrin-based MOF acoustic sensitizer (HN25) capable of simultaneously delivering ALN. The HN25 system regulated the bone marrow microenvironment via acoustodynamically mediated ROS generation and promoted bone repair by increasing chromatin accessibility of osteogenesis-related genes and forkhead box protein O1 (FOXO1_ [247] (Figure 11B).
Beyond direct antibacterial activity, the combined effects of reversing immunosuppression during remodeling of the bone marrow abscess microenvironment and promoting bone repair constitute a major therapeutic advantage of ROS-based technologies for osteomyelitis treatment. Recent comprehensive reviews have extensively addressed novel delivery systems for antibiotics or osteogenic factors in bone defects, as well as the general immune responses elicited by infection. However, systematic investigations focusing on the ROS-mediated reconstruction of the specific immune landscape in osteomyelitis—such as macrophage polarization, DC activation, and T-cell regulation within infected bone marrow—and its synergistic integration with targeted bone regeneration strategies remain limited.
ROS strategies in different types of osteomyelitis
Osteomyelitis represents a heterogeneous group of disorders of which clinical manifestations and therapeutic responses vary substantially according to underlying systemic conditions, including diabetes mellitus, peripheral vascular disease, and immunocompromised states. Among these, diabetic foot osteomyelitis (DFO) constitutes one of the most refractory forms, in which conventional treatment strategies frequently fail because of compromised blood perfusion, peripheral neuropathy, and impaired immune function. These pathological features not only accelerate disease progression but also severely limit drug delivery and host-mediated pathogen clearance, thereby necessitating disease-specific and mechanism-guided therapeutic approaches.
ROS strategies in diabetic foot osteomyelitis
ROS-based therapeutic strategies, including PDT, SDT, CDT, and MWDT, exhibit promising potential in overcoming the treatment challenges of DFO, particularly those associated with persistent infection and impaired tissue regeneration. PDT has demonstrated efficacy in diabetic foot infections by selectively targeting bacterial cells and biofilms within infected tissues. In addition, SDT has emerged as a potential modality for the management of biofilm-associated infections, which represent a hallmark of chronic DFO. US-activated nanomaterials generate ROS upon acoustic stimulation, leading to the disruption of antibiotic-resistant biofilms and enhancing bacterial eradication. Furthermore, the superior tissue penetration capacity of US compared with light enables SDT to effectively reach deep-seated osseous lesions, rendering this approach particularly suitable for the treatment of advanced DFO involving deeper bone structures.
(A) Proposed mechanism underlying sonodynamic antibacterial treatment and ROS-mediated bone regeneration. Adapted with permission from Ref. [246]. Copyright 2023, Springer Nature. (B) Schematic illustration of ALN-mediated defect-engineered MOF for targeted repair of infected bone tissue through sono-epigenetic modulation of chromatin accessibility. Adapted with permission from Ref. [247]. Copyright 2024, Wiley-VCH.
ROS strategies in other types of osteomyelitis
The application of ROS-based therapeutic modalities extends beyond DFO to encompass osteomyelitis resulting from trauma, open fractures, and post-surgical infections, conditions that are often characterized by complex polymicrobial colonization and dense biofilm formation. In such refractory cases, CDT offers distinct advantages by generating highly cytotoxic ·OH that directly disrupt bacterial cell walls and biofilms matrices. Moreover, the oxygen-independent nature of CDT renders it particularly well suited for osteomyelitis lesions, which commonly exhibit hypoxic environment. MWDT provides additional advantages in anatomically complex regions, where the synergistic combination of localized hyperthermia and ROS-mediated antimicrobial activity is especially beneficial for the eradication of infections. The application of microwave-responsive nanomaterials enables deep tissue penetration and precise thermal control, facilitating effective treatment of chronic infections, including those associated with orthopedic implants and prosthetic devices.
Challenges and prospects
The rapid development and expanding application of ROS-based nanomedicine in the biomedical field have created significant opportunities to improve therapeutic outcomes in osteomyelitis treatment. Nevertheless, translation of these promising technologies into routine clinical practice remains constrained by multiple scientific, technical, and regulatory challenges. The major limitations and future directions for ROS-based nanomedicine in osteomyelitis therapy are summarized as follows.
Biosafety risks
The clinical translation of ROS-based nanotechnology remains limited by significant biosafety concerns. Many platforms employed in PDT, SDT, CDT, and MWDT incorporated metal components, raising the risk of long-term metal retention and accumulation within the body. Moreover, although ROS exhibit potent, non-drug-resistant antimicrobial activity, excessive ROS generation may induce oxidative stress and collateral damage in healthy bone tissue. Consequently, rigorous evaluation of the biosafety profile and metabolic behavior of ROS nanomaterials is particularly important for their clinical application in osteomyelitis treatment. While short-term toxicity and biodistribution of various ROS nanomaterials have been studied, long-term biological effects and latent safety risks remain insufficiently characterized. Future development should prioritize nanomaterials with highly biodegradability or efficient renal clearance to meet clinical safety requirements. Several key parameters require careful optimization. Particle size is widely recognized as a critical determinant of nonmaterial toxicity [248]; therefore, precise control of nanoparticle size and morphology is necessary to minimize systemic toxicity, undesired organ accumulation, and excessive cellular uptake. In addition, positively charged ROS nanoparticles typically exhibit higher cytotoxicity than neutral counterparts due to enhanced membrane disruption and mitochondrial stress induction [249], suggesting that the modulation of surface charge represents an effective approach for reducing biological toxicity. Furthermore, rational surface modification and functionalization can substantially improve biocompatibility. For instance, Yu et al. reported that GSH encapsulation greatly enhanced renal clearance efficiency of fluorescent gold nanoparticles, thereby reducing systemic retention and toxicity [249].
Elucidation of the specific mechanism underlying ROS therapy
Current studies of ROS-based therapies for osteomyelitis have predominantly emphasized therapeutic efficiency, whereas the underlying molecular mechanisms governing ROS-mediated antimicrobial and regenerative effects remain incompletely understood. Integration of computational chemistry with materials science offers a powerful strategy for elucidating these mechanisms at the molecular level. Emerging reports have demonstrated the potential of such interdisciplinary approaches to predict ROS-related reaction pathways, thereby guiding rational design and synthesis of novel redox-active nanomaterials while providing a theoretical framework for experimental verification [250]. With continued advances in cheminformatics and molecular modeling, computational methodologies are expected to delivery increasingly detailed insights into ROS-driven therapeutic processes, facilitating mechanistic understanding the accelerating translation of ROS-based technologies into clinically effective osteomyelitis treatments.
Design and synthesis of new ROS nanomaterials
Stability of nanomaterials
The global healthcare sector is rapidly advancing toward an era of precision medicine, imposing increasingly stringent requirements on the rational design and synthesis of ROS-based nanomaterials. Future research and development of ROS nanotechnology for osteomyelitis therapy should focus on the optimization of nanomaterials with well-defined structures, compositions, and surface properties. Such platforms must accommodate individualized therapeutic demands while exhibiting excellent biocompatibility, high targeting efficiency, and clearly characterized in vivo metabolic behavior. The therapeutic efficacy of ROS-based treatment relies heavily on precise targeting and efficient delivery of nanomaterials to infected bone tissues. However, in vivo transport of ROS nanoparticles remains constrained by multiple physiological barriers, including systemic circulation dynamics and immune-mediated clearance, which collectively limit delivery efficiency and targeting precision [251]. Moreover, uncertainties surrounding in vivo biodistribution, mechanisms of action, and long-term biological effects necessitate continued investigation. Equally critical is the physicochemical stability of ROS nanomaterials throughout synthesis, storage, and clinical application. Development of effective stabilizing strategies is therefore indispensable for preserving nanoparticle integrity. A variety of stabilizers, including surfactants, silica coatings, biomolecules, polymers, and metal shells, have been employed to maintain structural stability and prevent aggregation or premature dissolution of nanoparticles, thereby enchanting their functional reliability in biomedical applications [252,253].
Design of intelligent nanocarriers
Future development of ROS-based nanotechnology should incorporate the concept of "intelligent nanocarriers" stop enable more precise and personalized therapeutic interventions. Integration of artificial intelligence (AI) and machine learning algorithms into nanomaterial design and delivery planning offers a powerful framework for optimizing therapeutic performance. Specifically, AI-driven modeling can rapidly identify optimal nanoparticle parameters—including particle size, surface functionalization, and delivery strategies—thereby simultaneously enhancing ROS production efficiency and minimizing safety risks. Moreover, AI-assisted analysis of patient-specific clinical data, such as infecting microbial strains, genetic profiles, and local blood perfusion characteristics, can inform individualized carrier design and delivery protocols, enabling truly personalized osteomyelitis treatment. When combined with high-throughput experimental screening and computational simulation, these approaches allow prediction of nanomedicine biodistribution and therapeutic efficacy across diverse patient populations, thereby accelerating translational development and improving clinical outcomes [254].
Design of multimodal treatment strategies
Strategic integration of disease-stage-specific requirements with spatiotemporally controlled multimodal ROS nanotechnology represents a promising therapeutic paradigm for osteomyelitis. Multimodal treatment strategies are anticipated to become a central direction in the future development of ROS-based therapies. In particular, combining ROS nanotechnology with complementary modalities such as immune modulation and genome-editing approaches, offers substantial potential for improving therapeutic outcomes in complex and refractory osteomyelitis.
ROS-based nano platforms have demonstrated the capacity to significantly enhance the efficacy of immunotherapy, thereby reinforcing antibacterial responses. This confirms the feasibility of dual-modality treatment strategies that combine ROS nanotechnology with immunotherapeutic interventions, especially in the treatment of chronic and drug-resistant osteomyelitis. However, the therapeutic synergy between ROS nanotechnology and immunotherapy remains constrained by local immunosuppression, immune tolerance, and bacterial immune evasion within the infection microenvironment. Accordingly, continued optimization of ROS -immunotherapy combination strategies represents a critical frontier in osteomyelitis treatment development. Emerging approaches, such as co-administration of ROS nanotechnology with immune checkpoint inhibitors, may further strengthen host immune recognition and clearance of pathogenic bacteria while mitigating immune escape mechanisms. Moreover, future integration of ROS-based systems with advanced immunotherapeutic technologies—including chimeric antigen receptor (CAR) T-cell therapy, gene-editing platforms, or cytokine-based therapies—may provide transformative solutions for refractory osteomyelitis management [255].
Gene-editing technologies, particularly CRISPR-Cas9, have become powerful tools for correcting gene mutations, regulating gene expression, and intervening in disease-related molecular pathways [256]. Integration of ROS-based nanotechnology with gene-editing approaches is expected to enhance localized ROS generation and amplify immune activation within infected tissues. Genetically engineered immune cells exhibit increased sensitivity to pathogenic microorganisms, thereby facilitating bacterial clearance and inhibiting immune evasion. However, current applications of gene-editing therapies remain constrained by limited delivery efficiency, potential off-target effects, and suboptimal cellular stability. Next-generation CRISPR systems with improved precision and specificity, such as CRISPR-Cas12 and CRISPR-Cas13 platforms, are expected to provide more clinically viable alternatives for therapeutic gene modulation in the future [257].
Clinical translation challenges and potential of ROS technology
Although ROS-based nanotechnology has shown remarkable efficacy in immune activation and eradication of drug-resistant bacteria, its clinical translation remains constrained by potential toxicity to host tissues. Excessive ROS production may induce oxidative stress, tissue injury, and undesirable inflammatory responses. Therefore, precise spatiotemporal control over generation, both in damage and duration, in essential for ensuring therapeutic safety and efficacy. Equally important is the targeted delivery of ROS nanomaterials to infected lesions, which strongly depends on the physicochemical stability, biological half-life, and tissue-specific targeting capabilities of the nanoplatforms. Moreover, the long-term toxicological risks of ROS nanotechnology, including potential carcinogenicity and teratogenicity, require extensive clinical validation before widespread application. To address these challenges, priority should be given to the development of biodegradable nanomaterials and renally clearable nanoparticles to minimize systemic accumulation and long-term toxicity. Concurrently, image-guided monitoring systems and active-targeting strategies, such as biomimetic membrane coatings or pathogen-specific ligands, provide crucial support for improving delivery prevision and safety during clinical translation of ROS technologies in osteomyelitis treatment [257]. Future optimization of ROS-based osteomyelitis therapies should focus on the construction of "triple-function" nanoplatforms that integrate antibacterial ROS generation, immunomodulation, and osteogenic stimulation. In parallel, intelligent closed-loop systems capable of dynamically adjusting ROS production in response to infection biomarkers hold promise for achieving personalized and adaptive therapy. Finally, standardized preclinical models and unified evaluation criteria for antibacterial efficacy will be essential to accelerate regulatory approval and clinical adoption.
The clinical translation of ROS-based technologies for osteomyelitis therapy requires systematic validation across multiple experimental models. Although in vitro studies provide critical insights into antibacterial mechanisms, they are insufficient to predict actual in vivo antimicrobial efficacy. Small animal models enable preliminary evaluation of immunomodulatory potential; however, their thin cortical bone structure and distinct bone remodeling dynamics do not adequately recapitulate the complex pathophysiology of human osteomyelitis. Consequently, large animal and preclinical studies are indispensable for accelerating the transition of ROS nanotechnology from laboratory research to clinical application. While large animal models offer anatomical and biomechanical relevance, their high cost and limited scalability impede high-throughput evaluation. To overcome these limitations, human-scale bone infection models should be promptly established, incorporating clinically relevant heterogeneity factors like diabetes, vascular insufficiency, and immune dysfunction into experimental design [258]. Furthermore, priority should be given to ROS-based technologies that exhibit dual antibacterial and osteogenic effects, thereby maximizing translational efficiency. Emerging new approach methodologies (NAMs) that complement or replace traditional animal testing, including organ-on-chip systems, computational modeling, and advanced imaging techniques, are increasingly applied in osteomyelitis research. Organ-on-chip models can reconstruct interactions between human bone, immune cells, and pathogens, providing high-fidelity evaluation of therapeutic responses at cellular and tissue levels. When combined with human-derived cell-based 3D-printed bone constructs, these systems enable precise testing of local drug delivery strategies and biomaterial performance. Meanwhile, computational models allow simulation of osteomyelitis progression and prediction of treatment effects on infection dynamics, immune responses, and bone regeneration. The integration of NAMs with conventional animal models is expected to substantially enhance experimental reliability, translational relevance, and ethical sustainability, thereby establishing a comprehensive and efficient research framework for the development of ROS-based therapies and next-generation osteomyelitis treatments [259-261].
The clinical application of ROS nanotechnology in osteomyelitis treatment continues to face significant regulatory and ethical challenges. Owing to the unique physicochemical properties and potential biological risks associated with nanomaterials, the establishment of a dedicated regulatory framework is necessary to ensure biosafety, quality control, and standardized manufacturing. Future clinical translation of ROS nanotechnology will therefore require strict standardization of production processes, comprehensive toxicological evaluation, and robust quality assurance systems. Furthermore, the efficacy, safety, and manufacturing consistency of ROS nanomaterials must be systematically benchmarked against existing clinical therapeutics within the same application domains to demonstrate non-inferiority or superiority. Only through rigorous comparative assessment and compliance with regulatory standards can ROS-based therapies achieve reliable, reproducible, and clinically acceptable outcomes, thereby supporting their eventual integration into mainstream osteomyelitis treatment strategies.
Conclusion
This review comprehensively summarizes the epidemiology, current diagnosis and therapeutic approaches, and pathogenic mechanisms of osteomyelitis, with a particular focus on the evolving role of ROS-based nanotechnologies in its treatment. This review systematically analyzed the development strategies, technical advantages, and inherent limitations of existing ROS-based treatment modalities, including PDT, SDT, CDT, and MWDT, thereby providing an integrative framework to guide the optimization of ROS-driven interventions for osteomyelitis. Furthermore, this review evaluated ROS nanotechnology from multiple functional perspectives—antibacterial activity, immune modulation, and tissue regeneration—and highlighted how the integration of ROS-based strategies with complementary therapeutic modalities can address the multifactorial pathophysiology of osteomyelitis. Such multimodal treatment paradigms offer substantial promise for overcoming the limitations of single-mode therapies and advancing personalized therapeutic solutions. Finally, we critically discussed the remaining challenges of ROS-based technologies, including biosafety concerns, targeting efficiency, mechanistic uncertainties, and translational barriers, while outlining future directions for the development of customized ROS-based therapies tailored to diverse osteomyelitis subtypes and clinical scenarios. Overall, this review provides a panoramic and mechanistically grounded overview of ROS-based osteomyelitis treatment over the past two decades, integrating technological evolution with biological insight. It offers important theoretical practical guidance for advancing our understanding of osteomyelitis pathogenesis and accelerating the development and clinical translation of innovative, next-generation treatment strategies.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2023YFC2812004), the Qingdao Natural Science Foundation (Grant No. 24-4-4-zrjj-154-jch), the Technological Innovation Capability Improvement Project for Small and Medium-Sized Technology-Based Enterprises in Shandong Province (Grant No. 2023TSGC0511), and the Qingdao City Healthcare Key Discipline Construction Project. No AI tools were used in the preparation of this manuscript.
Author contributions
Conceptualization: XK, JS, TW; Methodology: XK, JS, TW; Data curation: WW; Validation: WW, XK, JS, TW; Supervision: TW; Writing—original draft: WW; Writing—review & editing: WW, XK, JS, TW. All authors read and approved the final manuscript.
Competing interests
The authors have declared that no competing interest exists.
References
1. Camp L, Weber M-A. [osteomyelitis in adults]. Radiol Heidelb Ger. 2025;65:656-65
2. Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ, Huddleston PM. Trends in the epidemiology of osteomyelitis: A population-based study, 1969 to 2009. J Bone Jt Surg. 2015;97:837-45
3. Carroll R. Vertebral osteomyelitis and epidural abscess. Med Clin North Am. 2025;109:601-14
4. Yang Z, Lin B, Ren H, Liu Y, Huang K, Guo Q. et al. Risk factors for osteomyelitis: a systematic review and meta-analysis. Int J Surg Lond Engl. 2025;111:5606-22
5. Ghieh F, Bizri AR, Beaineh P, Chalhoub R, Abu Sittah G. Systematic review of the microbiology of osteomyelitis associated with war injuries in the middle east and north africa. Med Confl Surviv. 2023;39:150-61
6. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984-91
7. Scolari IR, Granero GE. Narrative review of the opportunities for bone tissue regeneration and osteomyelitis treatment: Transition metal complexes with antibiotics as proof-of-concept. Regen Med Rep.: 10.4103/REGENMED.REGENMED.
8. Wang X, Zhang M, Zhu T, Wei Q, Liu G, Ding J. Flourishing antibacterial strategies for osteomyelitis therapy. Adv Sci. 2023;10:2206154
9. Gornitzky AL, Kim AE, O'Donnell JM, Swarup I. Diagnosis and management of osteomyelitis in children: A critical analysis review. Jbjs Rev. 2020;8:e19.202-e19.202
10. Huang Y, Guo X, Wu Y, Chen X, Feng L, Xie N. et al. Nanotechnology's frontier in combatting infectious and inflammatory diseases: Prevention and treatment. Signal Transduct Target Ther. 2024;9:34
11. Masters EA, Ricciardi BF, Bentley KLDM, Moriarty TF, Schwarz EM, Muthukrishnan G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat Rev Microbiol. 2022;20:385-400
12. Dziewanowska K, Patti JM, Deobald CF, Bayles KW, Trumble WR, Bohach GA. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of staphylococcus aureus by epithelial cells. Fischetti VA, Ed. Infect Immun. 1999;67:4673-8
13. Kintarak S, Whawell SA, Speight PM, Packer S, Nair SP. Internalization of staphylococcus aureus by human keratinocytes. Infect Immun. 2004;72:5668-75
14. Garzoni C, Kelley WL. Return of the trojan horse: Intracellular phenotype switching and immune evasion by staphylococcus aureus. EMBO Mol Med. 2011;3:115-7
15. Ellington JK, Harris M, Webb L, Smith B, Smith T, Tan K. et al. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J Bone Joint Surg Br. 2003 85-B: 918-21
16. Sendi P, Proctor RA. Staphylococcus aureus as an intracellular pathogen: The role of small colony variants. Trends Microbiol. 2009;17:54-8
17. Lee J, Mashayamombe M, Walsh TP, Kuang BKP, Pena GN, Vreugde S. et al. The bacteriology of diabetic foot ulcers and infections and incidence of staphylococcus aureus small colony variants. J Med Microbiol. 2023 72
18. Josse J, Velard F, Gangloff SC. Staphylococcus aureus vs. Osteoblast: Relationship and consequences in osteomyelitis. Front Cell Infect Microbiol. 2015;5:85
19. Jauregui CE, Mansell JP, Jepson MA, Jenkinson HF. Differential interactions of Streptococcus gordonii and Staphylococcus aureus with cultured osteoblasts. Mol Oral Microbiol. 2013;28:250-66
20. Leibbrandt A, Penninger JM. Novel functions of RANK(L) signaling in the immune system. Adv Exp Med Biol. 2010;658:77-94
21. Roper PM, Shao C, Veis DJ. Multitasking by the OC lineage during bone infection: Bone resorption, immune modulation, and microbial niche. Cells. 2020;9:2157
22. Gunn NJ, Kidd SP, Solomon LB, Yang D, Roscioli E, Atkins GJ. Staphylococcus aureus persistence in osteocytes: Weathering the storm of antibiotics and autophagy/xenophagy. Front Cell Infect Microbiol. 2024;14:1403289
23. Yang D, Wijenayaka AR, Solomon LB, Pederson SM, Findlay DM, Kidd SP. et al. Novel insights into staphylococcus aureus deep bone infections: The involvement of osteocytes. Smeltzer MS, Novick RP, Eds. mBio. 2018;9:e00415-18
24. Zhang Z, Song Y, Wang SI, Ha SH, Jang KY, Park BH. et al. Osteoblasts/osteocytes sirtuin6 is vital to preventing ischemic osteonecrosis through targeting VDR-RANKL signaling. J Bone Miner Res. 2020;36:579-90
25. Hardy E, Fernandez-Patron C. Destroy to rebuild: The connection between bone tissue remodeling and matrix metalloproteinases. Front Physiol. 2020;11:47
26. Wang T, He C. TNF-α and IL-6: The link between immune and bone system. Curr Drug Targets. 2020;21:213-27
27. Brodie BC. Pathological researches respecting the diseases of joints. Medico-Chir Trans. 1813;4:210-80
28. Hofstee MI, Riool M, Terjajevs I, Thompson K, Stoddart MJ, Richards RG. et al. Three-dimensional in vitro staphylococcus aureus abscess communities display antibiotic tolerance and protection from neutrophil clearance. Freitag NE, Ed. Infect Immun. 2020;88:e00293-20
29. Jiang Y, Hou J, Liu C, Zhao C, Xu Y, Song W. et al. Inhibitory effect of salicin on staphylococcus aureus coagulase. ChemMedChem. 2023;18:e202300302
30. Foster TJ. The MSCRAMM family of cell-wall-anchored surface proteins of gram-positive cocci. Trends Microbiol. 2019;27:927-41
31. Binsker U, Palankar R, Wesche J, Kohler TP, Prucha J, Burchhardt G. et al. Secreted immunomodulatory proteins of staphylococcus aureus activate platelets and induce platelet aggregation. Thromb Haemost. 2018;118:745-57
32. Kobayashi SD, Malachowa N, DeLeo FR. Pathogenesis of staphylococcus aureus abscesses. Am J Pathol. 2015;185:1518-27
33. Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol Ijmm. 2002;292:107-13
34. Ricciardi BF, Muthukrishnan G, Masters E, Ninomiya M, Lee CC, Schwarz EM. Staphylococcus aureus evasion of host immunity in the setting of prosthetic joint infection: Biofilm and beyond. Curr Rev Musculoskelet Med. 2018;11:389-400
35. Donlan RM. Biofilm formation: A clinically relevant microbiological process. Clin Infect Dis Off Publ Infect Dis Soc Am. 2001;33:1387-92
36. Cunningham R, Cockayne A, Humphreys H. Clinical and molecular aspects of the pathogenesis of staphylococcus aureus bone and joint infections. J Med Microbiol. 1996;44:157-64
37. Flammier S, Rasigade J-P, Badiou C, Henry T, Vandenesch F, Laurent F. et al. Human monocyte-derived osteoclasts are targeted by staphylococcal pore-forming toxins and superantigens. PLOS One. 2016;11:e0150693
38. Jahn K, Handtke S, Palankar R, Kohler TP, Wesche J, Wolff M. et al. α-hemolysin of staphylococcus aureus impairs thrombus formation. J Thromb Haemost Jth. 2022;20:1464-75
39. Silva MP, Rodrigues CG, Machado DC, Nogueira RA. Long-term memory in staphylococcus aureus α-hemolysin ion channel kinetics. Eur Biophys J EBJ. 2023;52:661-71
40. Badiou C, Dumitrescu O, George N, Forbes AR, Drougka E, Chan KS. et al. Rapid detection of staphylococcus aureus panton-valentine leukocidin in clinical specimens by enzyme-linked immunosorbent assay and immunochromatographic tests. J Clin Microbiol. 2010;48:1384-90
41. Jin T, Zhu YL, Li J, Shi J, He XQ, Ding J. et al. Staphylococcal protein a, panton-valentine leukocidin and coagulase aggravate the bone loss and bone destruction in osteomyelitis. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2013;32:322-33
42. Ondusko DS, Nolt D. Staphylococcus aureus. Pediatr Rev. 2018;39:287-98
43. de Mesy Bentley KL, Trombetta R, Nishitani K, Bello-Irizarry SN, Ninomiya M, Zhang L. et al. Evidence of staphylococcus aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2017;32:985-90
44. de Mesy Bentley KL, MacDonald A, Schwarz EM, Oh I. Chronic osteomyelitis with staphylococcus aureus deformation in submicron canaliculi of osteocytes: A case report. JBJS Case Connect. 2018;8:e8
45. Al-Maiyah M, Hemmady MV, Shoaib A, Morgan-Jones RL. Recurrence of chronic osteomyelitis in a regenerated fibula after 65 years. Orthopedics. 2007;30:403-4
46. Kitamura H, Fukui T, Oe K, Matsushita T, Kuroda R, Niikura T. Osteomyelitis of the pubis treated by masquelet technique in a football player: A case report. J Orthop Case Rep. 2022;12:39-42
47. Masters EA, de Mesy Bentley KL, Gill AL, Hao SP, Galloway CA, Salminen AT. et al. Identification of penicillin binding protein 4 (PBP4) as a critical factor for staphylococcus aureus bone invasion during osteomyelitis in mice. PLOS Pathog. 2020;16:e1008988
48. Bury DC, Rogers TS, Dickman MM. Osteomyelitis: Diagnosis and treatment. Am Fam Physician. 2021;104:395-402
49. Han D, Li Z, Li R, Tan P, Zhang R, Li J. mNGS in clinical microbiology laboratories: On the road to maturity. Crit Rev Microbiol. 2019;45:668-85
50. Zimmerli W, Sendi P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob Agents Chemother. 2019;63:e01746-18
51. Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop. 2004:86-93
52. Soldevila-Boixader L, Fernández AP, Laguna JM, Uçkay I. Local antibiotics in the treatment of diabetic foot infections: A narrative review. Antibiot Basel Switz. 2023;12:124
53. Wassif RK, Elkayal M, Shamma RN, Elkheshen SA. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021;28:2392-414
54. Riesgo AM, Park BK, Herrero CP, Yu S, Schwarzkopf R, Iorio R. Vancomycin povidone-iodine protocol improves survivorship of periprosthetic joint infection treated with irrigation and debridement. J Arthroplasty. 2018;33:847-50
55. Simpson AH, Deakin M, Latham JM. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br. 2001;83:403-7
56. Expert Panel on Musculoskeletal Imaging, Walker EA, Beaman FD. et al. ACR appropriateness criteria® suspected osteomyelitis of the foot in patients with diabetes mellitus. J Am Coll Radiol JACR. 2019;16:S440-50
57. Arias M, Hassan-Reshat S, Newsholme W. Retrospective analysis of diabetic foot osteomyelitis management and outcome at a tertiary care hospital in the UK. PloS One. 2019;14:e0216701
58. Memar MY, Yekani M, Alizadeh N, Baghi HB. Hyperbaric oxygen therapy: Antimicrobial mechanisms and clinical application for infections. Biomed Pharmacother Biomedecine Pharmacother. 2019;109:440-7
59. Hart BB. Hyperbaric oxygen for refractory osteomyelitis. Undersea Hyperb Med J Undersea Hyperb Med Soc Inc. 2021;48:297-321
60. Aliyev M, Aykan A, Eski M, Arslan N, Kurt B, Şengezer M. Effects of transpositional muscle flaps transfected with vascular endothelial growth factor gene in the treatment of experimental osteomyelitis. Ulus Travma Ve Acil Cerrahi Derg Turk J Trauma Emerg Surg Tjtes. 2016;22:205-14
61. Franzini M, Valdenassi L, Tirelli U, Ricevuti G, Pandolfi S, Vaiano F. et al. Post-surgical wounds treated with ozone: A preliminary case series. Med Gas Res. 2024;14:225-7
62. Li X-R, Cui J-J, Ge W-P, Wang Z-W, Chu Y-C, Zheng G-R. Ozonated autohemotherapy combined with pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia in older adults: A retrospective study. Med Gas Res. 2024;14:12-8
63. Li H, Yang S, Wang J, You W, Xu J, Chen G. The potential role of nitrogen dioxide inhalation in Parkinson's disease. Med Gas Res. 2024;14:153-5
64. Wang Z, Chu Y, Du J, Hu Y, Wang H, Liu H. et al. Accelerating repair of infected bone defects through post-reinforced injectable hydrogel mediated antibacterial/immunoregulatory microenvironment at bone-hydrogel interface. Carbohydr Polym. 2025;351:123082
65. Du J, Chu Y, Hu Y, Liu J, Liu H, Wang H. et al. A multifunctional self-reinforced injectable hydrogel for enhancing repair of infected bone defects by simultaneously targeting macrophages, bacteria, and bone marrow stromal cells. Acta Biomater. 2024;189:232-53
66. Liu Q, Yu Y, Liu C, Liu Y, Yuan L, Wang Z. et al. Effect of La3+ and Mg2+ combined system on bioactivity and osteogenesis of bioinspired la-doped magnesium phosphate composites prepared utilizing the precursor method. J Mater Res Technol. 2023;24:9523-36
67. Schmidmaier G, Kerstan M, Schwabe P, Südkamp N, Raschke M. Clinical experiences in the use of a gentamicin-coated titanium nail in tibia fractures. Injury. 2017;48:2235-41
68. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909-50
69. Manful CF, Fordjour E, Ikumoinein E, Abbey L, Thomas R. Therapeutic Strategies Targeting Oxidative Stress and Inflammation: A Narrative Review. BioChem. 2025;5(4):35
70. Krylatov AV, Maslov LN, Voronkov NS, Boshchenko AA, Popov SV, Gomez L. et al. Reactive oxygen species as intracellular signaling molecules in the cardiovascular system. Curr Cardiol Rev. 2018;14:290-300
71. Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev. 2019;119:4881-985
72. Chen Z, Xing F, Yu P, Zhou Y, Luo R, Liu M. et al. Metal-organic framework-based advanced therapeutic tools for antimicrobial applications. Acta Biomater. 2024;175:27-54
73. Robinson JR. Photodynamic insecticides: a review of studies on photosensitizing dyes as insect control agents, their practical application, hazards, and residues. Residue Rev. 1983;88:69-100
74. Lee HP, Gaharwar AK. Light-responsive inorganic biomaterials for biomedical applications. Adv Sci Weinh Baden-Wurtt Ger. 2020;7:2000863
75. Correia JH, Rodrigues JA, Pimenta S, Dong T, Yang Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13:1332
76. Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother. 1998;42:13-28
77. Dos Reis JA, Dos Santos JN, Barreto BS, de Assis PN, Almeida PF, Pinheiro ALB. Photodynamic antimicrobial chemotherapy (PACT) in osteomyelitis induced by staphylococcus aureus: microbiological and histological study. J Photochem Photobiol B. 2015;149:235-42
78. Tardivo JP, Baptista MS. Treatment of osteomyelitis in the feet of diabetic patients by photodynamic antimicrobial chemotherapy. Photomed Laser Surg. 2009;27:145-50
79. Park D, Choi EJ, Weon K-Y, Lee W, Lee SH, Choi JS. et al. Non-invasive photodynamic therapy against -periodontitis-causing bacteria. Sci Rep. 2019;9:8248
80. Yin X, Fang Z, Fang Y, Zhu L, Pang J, Liu T. et al. Antimicrobial photodynamic therapy involving a novel photosensitizer combined with an antibiotic in the treatment of rabbit tibial osteomyelitis caused by drug-resistant bacteria. Front Microbiol. 2022;13:876166
81. Salehpour F, Cassano P, Rouhi N, Hamblin MR, De Taboada L, Farajdokht F. et al. Penetration profiles of visible and near-infrared lasers and light-emitting diode light through the head tissues in animal and human species: A review of literature. Photobiomodulation Photomed Laser Surg. 2019;37:581-95
82. Nötzli HP, Swiontkowski MF, Thaxter ST, Carpenter GK, Wyatt R. Laser doppler flowmetry for bone blood flow measurements: Helium-neon laser light attenuation and depth of perfusion assessment. J Orthop Res Off Publ Orthop Res Soc. 1989;7:413-24
83. Wu J, Wei H, Wei Y, Deng T, Wang Y, Qiu Y. et al. Spatiotemporal synergism in osteomyelitis treatment with photoactivated core-shell zinc oxide/silver sulfide heterogeneous nanoparticles. ACS Appl Mater Interfaces. 2024;16:11194-205
84. Qu M, Mallidi S, Mehrmohammadi M. et al. Magneto-photo-acoustic imaging. Biomed Opt Express. 2011;2:385-96
85. Jin Y, Jia C, Huang S-W, O'Donnell M, Gao X. Multifunctional nanoparticles as coupled contrast agents. Nat Commun. 2010;1:41
86. Gonzalez EA, Bell MAL. Photoacoustic imaging and characterization of bone in medicine: Overview, applications, and outlook. Annu Rev Biomed Eng. 2023;25:207-32
87. Lu X, Chen R, Lv J, Xu W, Chen H, Ma Z. et al. High-resolution bimodal imaging and potent antibiotic/photodynamic synergistic therapy for osteomyelitis with a bacterial inflammation-specific versatile agent. Acta Biomater. 2019;99:363-72
88. Yumita N, Nishigaki R, Umemura K, Umemura S. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn J Cancer Res Gann. 1989;80:219-22
89. Rengeng L, Qianyu Z, Yuehong L, Zhongzhong P, Libo L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn Ther. 2017;19:159-66
90. McHale AP, Callan JF, Nomikou N, Fowley C, Callan B. Sonodynamic therapy: Concept, mechanism and application to cancer treatment. Adv Exp Med Biol. 2016;880:429-50
91. He Z, Du J, Miao Y, Li Y. Recent developments of inorganic nanosensitizers for sonodynamic therapy. Adv Healthc Mater. 2023;12:e2300234
92. Guo J, Pan X, Wang C, Liu H. Molecular imaging-guided sonodynamic therapy. Bioconjug Chem. 2022;33:993-1010
93. Pan X, Wang H, Wang S, Sun X, Wang L, Wang W. et al. Sonodynamic therapy (SDT): A novel strategy for cancer nanotheranostics. Sci China Life Sci. 2018;61:415-26
94. Guo Y, Mao C, Wu S, Wang C, Zheng Y, Liu X. Ultrasound-triggered piezoelectric catalysis of zinc oxide@glucose derived carbon spheres for the treatment of MRSA infected osteomyelitis. Small Weinh Bergstr Ger. 2024;20:e2400732
95. Li J, Liu X, Zheng Y, Cui Z, Jiang H, Li Z. et al. Achieving Fast Charge Separation by Ferroelectric Ultrasonic Interfacial Engineering for Rapid Sonotherapy of Bacteria-Infected Osteomyelitis. Adv Mater. 2023;35:2210296
96. Li D, Yang Y, Li D, Pan J, Chu C, Liu G. Organic sonosensitizers for sonodynamic therapy: From small molecules and nanoparticles toward clinical development. Small Weinh Bergstr Ger. 2021;17:e2101976
97. Liu W, Ni C, Gao M, Zhao X, Zhang W, Li R. et al. Metal-organic-framework-based nanoarrays for oxygen evolution electrocatalysis. ACS Nano. 2023;17:24564-92
98. Chen C, Yang Z, Mao C, Jin L, Wu S, Zheng Y. et al. A smart nanovector of cationic starch modified curcumin with excellent targeting and sonodynamic properties for effective therapy of MRSA-induced osteomyelitis. Adv Funct Mater. 2023;33:2308437
99. Lin H, Yang C, Luo Y, Ge M, Shen H, Zhang X. et al. Biomimetic nanomedicine-triggered in situ vaccination for innate and adaptive immunity activations for bacterial osteomyelitis treatment. ACS Nano. 2022;16:5943-60
100. Cheng S, Chen L, Gong F, Yang X, Han Z, Wang Y. et al. PtCu nanosonosensitizers with inflammatory microenvironment regulation for enhanced sonodynamic bacterial elimination and tissue repair. Adv Funct Mater. 2023;33:2212489
101. Yang K, Shi C, Yin Z, Xiu W, Yuwen L, Pan S. et al. Iron metabolism interference-enhanced sonodynamic therapy of methicillin-resistant staphylococcus aureus-induced osteomyelitis by CaCO3-ga-PPIX@PEG nanospheres. Nano Today. 2024;56:102299
102. Zhang C, Bu W, Ni D, Zhang S, Li Q, Yao Z. et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew Chem Int Ed Engl. 2016;55:2101-6
103. Rais Sadati SMM, Zamanian J, Moshiri M, Ghayour Mobarhan M, Abnous K, Taghdisi SM. et al. Advancing cancer treatment with nanozyme frameworks: Integrating photothermal, photodynamic, sonodynamic, and chemodynamic therapies. Iran J Basic Med Sci. 2025;28:533-52
104. Feng Z, Guo Y, Zhang Y, Zhang A, Jia M, Yin J. et al. Nanozymes: a bibliometrics review. J Nanobiotechnology. 2024;22:704
105. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1-13
106. Yu Y, Sun H, Lu Q, Sun J, Zhang P, Zeng L. et al. Spontaneous formation of MXene-oxidized sono/chemo-dynamic sonosensitizer/nanocatalyst for antibacteria and bone-tissue regeneration. J Nanobiotechnology. 2023;21:193
107. Ge Y, Wang K, Liu J, Tian Y, Li H, Wang H. et al. A ZIF-8-based multifunctional intelligent drug release system for chronic osteomyelitis. Colloids Surf B Biointerfaces. 2022;212:112354
108. Guan X, Wu S, Ouyang S, Ren S, Cui N, Wu X. et al. Remodeling microenvironment for implant-associated osteomyelitis by dual metal peroxide. Adv Healthc Mater. 2024;13:e2303529
109. Wu Q, Xia N, Long D, Tan L, Rao W, Yu J. et al. Dual-functional supernanoparticles with microwave dynamic therapy and microwave thermal therapy. Nano Lett. 2019;19:5277-86
110. Fu C, Zhou H, Tan L, Huang Z, Wu Q, Ren X. et al. Microwave-activated mn-doped zirconium metal-organic framework nanocubes for highly effective combination of microwave dynamic and thermal therapies against cancer. ACS Nano. 2018;12:2201-10
111. Chen Y, Cai F, Liu Y, Fan W, Wang J, Yin G. et al. Construction of BaTiO3-TiO2 hollow sphere heterojunctions for enhanced microwave dynamic therapy in cancer treatment. Phys Chem Chem Phys Pccp. 2024;26:14131-9
112. Jin L, Liu X, Zheng Y, Zhang Y, Li Z, Zhu S. et al. Interfacial and defect polarization enhanced microwave noninvasive therapy for staphylococcus aureus-infected chronic osteomyelitis. ACS Nano. 2023;17:18200-16
113. Zhang W-J, Jin L-G, Wu S-L, Wang C-F, Zheng Y-F, Li Z-Y. et al. Microwave excited hyperthermy and catalysis of heterostructured au/cu-BTA for effective bacteria killing by accelerating charge separation. Rare Met. 2024;43:5186-201
114. Wang Y, Jin L, Wang C, Liu H, Mao C, Liu X. et al. Microwave-excited thermoelectric catalysis of organic-inorganic nanohybrid for highly effective treatment of staphylococcus aureus-infected osteomyelitis. Small. 2025;21:e2500915
115. Shi M, Jin L, Wang C, Mao C, Liu X, Li Z. et al. Diverse loss mechanism enhanced microwave thermal effects of capacitor-like structure Ti3C2Tx MXene/CNT for rapid treatment of osteomyelitis. Chem Eng J. 2025;507:160540
116. Jin L, Liu H, Mao C, Wang C, Wu S, Lai K. et al. Multivariant interfacial/ferroelectric/dipole polarization strengthened microwave-catalysis eradicates deep bacteria-infected osteomyelitis. J Mater Sci Technol. 2025;232:313-24
117. Jin L, Liu H, Wang C, Liu X, Mao C, Zhang Y. et al. A bacterial capturing, neural network-like carbon nanotubes/prussian blue/puerarin nanocomposite for microwave treatment of staphylococcus aureus-infected osteomyelitis. Small. 2024;20:e2407113
118. Wang C, Wang C, Wu S, Cui Z, Zheng Y, Li Z. et al. Microwave catalytic and thermal effects of Ti3C2Tx/ZnO-PPy enhanced by interfacial polarization for rapid treatment of MRSA-induced osteomyelitis. Nano Today. 2024;58:102439
119. Jin L, Wu S, Mao C, Wang C, Zhu S, Zheng Y. et al. Rapid and effective treatment of chronic osteomyelitis by conductive network-like MoS2/CNTs through multiple reflection and scattering enhanced synergistic therapy. Bioact Mater. 2024;31:284-97
120. Chen X, Tan L, Liu T, Meng X. Micro-nanomaterials for tumor microwave hyperthermia: Design, preparation, and application. Curr Drug Deliv. 2017;14:307-22
121. Manthe RL, Foy SP, Krishnamurthy N, Sharma B, Labhasetwar V. Tumor ablation and nanotechnology. Mol Pharm. 2010;7:1880-98
122. Xing H, Liu Z, Lin L, Wang L, Tan D, Guan Y. et al. Excellent microwave absorption properties of fe ion-doped SnO2/multi-walled carbon nanotube composites. RSC Adv. 2016;6:41656-64
123. Wang W, Cao M. Ni3sn2 alloy nanocrystals encapsulated within electrospun carbon nanofibers for enhanced microwave absorption performance. Mater Chem Phys. 2016;177:198-205
124. Xu J, Cheng X, Tan L, Fu C, Ahmed M, Tian J. et al. Microwave responsive nanoplatform via P-selectin mediated drug delivery for treatment of hepatocellular carcinoma with distant metastasis. Nano Lett. 2019;19:2914-27
125. Fatima SF, Sabouni R, Husseini G, Paul V, Gomaa H, Radha R. Microwave-responsive metal-organic frameworks (MOFs) for enhanced in vitro controlled release of doxorubicin. Nanomater Basel Switz. 2024;14:1081
126. Cui H, Zhao Y-Y, Wu Q, You Y, Lan Z, Zou KL. et al. Microwave-responsive gadolinium metal-organic frameworks nanosystem for MRI-guided cancer thermotherapy and synergistic immunotherapy. Bioact Mater. 2024;33:532-44
127. Ren J, Qiao Y, Jin L, Mao C, Wang C, Wu S. et al. A smart bacteria-capture-killing vector for effectively treating osteomyelitis through synergy under microwave therapy. Small. 2024;20:e2307406
128. Jin L, Liu X, Zheng Y, Li Z, Zhang Y, Zhu S. et al. Interface polarization strengthened microwave catalysis of MoS2/FeS/rhein for the therapy of bacteria-infected osteomyelitis. Adv Funct Mater. 2022;32:2204437
129. Zhu H, Li B, Liu X, Qiao Y, Lv Y, Zheng Y. et al. Interfacial mo, W-conjugated polarization, and oxygen vacancies of MoO2/WO3 in enhanced microwave therapy for MRSA-induced osteomyelitis. ACS Nano. 2022;16:21098-110
130. Jin L, Zheng Y, Liu X, Zhang Y, Li Z, Liang Y. et al. Magnetic composite rapidly treats staphylococcus aureus -infected osteomyelitis through microwave strengthened thermal effects and reactive oxygen species. Small. 2022;18:2204028
131. Liao S, Wu S, Mao C, Wang C, Cui Z, Zheng Y. et al. Electron aggregation and oxygen fixation reinforced microwave dynamic and thermal therapy for effective treatment of MRSA-induced osteomyelitis. Small Weinh Bergstr Ger. 2024;20:e2312280
132. McEwan C, Nesbitt H, Nicholas D, Kavanagh ON, McKenna K, Loan P. et al. Comparing the efficacy of photodynamic and sonodynamic therapy in non-melanoma and melanoma skin cancer. Bioorg Med Chem. 2016;24:3023-8
133. Liu S, Wen M, Huang M, Wang H, Chen Z, Yu N. Nanoscale hematoporphrin-based frameworks for photo-sono synergistic cancer therapy via utilizing al (III) as metal nodes rather than heavy metals. J Colloid Interface Sci. 2022;616:23-33
134. Ding T, Liu F, Xin H, Chen Y, Kong L, Han J. et al. Pyro-piezoelectric effect of BaTiO3 bio-nanocarrier for osteomyelitis therapy. Nano Today. 2024;54:102069
135. Cheng Y, Zhang Y, Zhao Z, Li G, Li J, Li A. et al. Guanidinium-decorated nanostructure for precision sonodynamic-catalytic therapy of MRSA-infected osteomyelitis. Adv Mater Deerfield Beach Fla. 2022;34:e2206646
136. Wei S, Qiao Y, Wu Z, Liu X, Li Y, Cui Z. et al. Na+ inserted metal-organic framework for rapid therapy of bacteria-infected osteomyelitis through microwave strengthened fenton reaction and thermal effects. Nano Today. 2021;37:101090
137. Sun W, Wang C, Wan D, Zheng Y, Wu S, Shen J. et al. CuCeO bimetallic oxide rapidly treats staphylococcus aureus-infected osteomyelitis through microwave strengthened microwave catalysis and fenton-therapy. Small Methods. 2023;7:e2300203
138. Li J, Zhang L, Peng J, Zhao C, Li W, Yu Y. et al. Mitochondrial metabolic regulation of macrophage polarization in osteomyelitis and other orthopedic disorders: Mechanisms and therapeutic opportunities. Front Cell Dev Biol. 2025;13:1604320
139. Bouché M, Pühringer M, Iturmendi A, Amirshaghaghi A, Tsourkas A, Teasdale I. et al. Activatable hybrid polyphosphazene-AuNP nanoprobe for ROS detection by bimodal PA/CT imaging. ACS Appl Mater Interfaces. 2019;11:28648-56
140. Xu L, Zhan W, Deng Y, Liu X, Gao G, Sun X. et al. ROS Turn Nanoparticle Fluorescence on for Imaging Staphylococcus aureus Infection In vivo. Adv Healthc Mater. 2022;11:e2200453
141. Park HY, Zoller SD, Hegde V, Sheppard W, Burke Z, Blumstein G. et al. Comparison of two fluorescent probes in preclinical non-invasive imaging and image-guided debridement surgery of Staphylococcal biofilm implant infections. Sci Rep. 2021;11:1622
142. Li Y, Xia Q, Zhu C, Cao W, Xia Z, Liu X. et al. An activatable Mn (II) MRI probe for detecting peroxidase activity in vitro and in vivo. J Inorg Biochem. 2022;236:111979
143. Opperman CJ, Wojno J, Goosen W, Warren R. Phages for the treatment of mycobacterium species. Prog Mol Biol Transl Sci. 2023;201:41-92
144. Giacomini E, Perrone V, Alessandrini D, Paoli D, Nappi C, Degli Esposti L. Evidence of antibiotic resistance from population-based studies: A narrative review. Infect Drug Resist. 2021;14:849-58
145. McManus MC. Mechanisms of bacterial resistance to antimicrobial agents. Am J Health-Syst Pharm AJHP Off J Am Soc Health-Syst Pharm. 1997;54:1420-33 quiz 1444-6
146. Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322-32
147. Gauba A, Rahman KM. Evaluation of antibiotic resistance mechanisms in gram-negative bacteria. Antibiot Basel Switz. 2023;12:1590
148. da Silva Dantas A. Antimicrobial resistance. Mol Microbiol. 2022;117:959-60
149. Poole K. Efflux-mediated antimicrobial resistance. J Antimicrob Chemother. 2005;56:20-51
150. Davin-Regli A, Pagès J-M, Vergalli J. The contribution of porins to enterobacterial drug resistance. J Antimicrob Chemother. 2024;79:2460-70
151. Pratt LA, Hsing W, Gibson KE, Silhavy TJ. From acids to osmZ: Multiple factors influence synthesis of the OmpF and OmpC porins in escherichia coli. Mol Microbiol. 1996;20:911-7
152. Du D, Wang-Kan X, Neuberger A, van Veen HW, Pos KM, Piddock LJV. et al. Multidrug efflux pumps: Structure, function and regulation. Nat Rev Microbiol. 2018;16:523-39
153. Fernández L, Hancock REW. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin Microbiol Rev. 2012;25:661-81
154. Rahman T, Yarnall B, Doyle DA. Efflux drug transporters at the forefront of antimicrobial resistance. Eur Biophys J. 2017;46:647-53
155. Rominski A, Roditscheff A, Selchow P, Böttger EC, Sander P. Intrinsic rifamycin resistance of mycobacterium abscessus is mediated by ADP-ribosyltransferase MAB_0591. J Antimicrob Chemother. 2017;72:376-84
156. Spanogiannopoulos P, Thaker M, Koteva K, Waglechner N, Wright GD. Characterization of a rifampin-inactivating glycosyltransferase from a screen of environmental actinomycetes. Antimicrob Agents Chemother. 2012;56:5061-9
157. Spanogiannopoulos P, Waglechner N, Koteva K, Wright GD. A rifamycin inactivating phosphotransferase family shared by environmental and pathogenic bacteria. Proc Natl Acad Sci. 2014;111:7102-7
158. Koteva K, Cox G, Kelso JK, Surette MD, Zubyk HL, Ejim L. et al. Rox, a rifamycin resistance enzyme with an unprecedented mechanism of action. Cell Chem Biol. 2018;25:403-412.e5
159. Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1549-55
160. Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016 4
161. Bhujbalrao R, Anand R. Deciphering determinants in ribosomal methyltransferases that confer antimicrobial resistance. J Am Chem Soc. 2019;141:1425-9
162. Doi Y, Wachino J-I, Arakawa Y. Aminoglycoside resistance: The emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin North Am. 2016;30:523-37
163. Huh AJ, Kwon YJ. 'nanoantibiotics': A new paradigm for treating infectious diseases using nanomaterials in the antibiotic's resistant era. J Control Release Off J Control Release Soc. 2011;156:128-45
164. Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65:1803-15
165. Abdelghafar A, Yousef N, Askoura M. Zinc oxide nanoparticles reduce biofilm formation, synergize antibiotics action and attenuate staphylococcus aureus virulence in host; an important message to clinicians. BMC Microbiol. 2022;22:244
166. Xiu W, Ren L, Xiao H, Zhang Y, Wang D, Yang K. et al. Ultrasound-responsive catalytic microbubbles enhance biofilm elimination and immune activation to treat chronic lung infections. Sci Adv. 2023;9:eade5446
167. van Oosten M, Schäfer T, Gazendam JAC, Ohlsen K, Tsompanidou E, de Goffau MC. et al. Real-time in vivo imaging of invasive- and biomaterial-associated bacterial infections using fluorescently labelled vancomycin. Nat Commun. 2013;4:2584
168. Tang EN, Nair A, Baker DW, Hu W, Zhou J. In vivo imaging of infection using a bacteria-targeting optical nanoprobe. J Biomed Nanotechnol. 2014;10:856-63
169. Romero Pastrana F, Thompson JM, Heuker M, Hoekstra H, Dillen CA, Ortines RV. et al. Noninvasive optical and nuclear imaging of staphylococcus-specific infection with a human monoclonal antibody-based probe. Virulence. 2018;9:262-72
170. Kwon H-Y, Liu X, Choi EG, Lee JY, Choi SY, Kim JY. et al. Development of a universal fluorescent probe for gram-positive bacteria. Angew Chem Int Ed Engl. 2019;58:8426-31
171. Hussain S, Joo J, Kang J, Kim B, Braun GB, She ZG. et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat Biomed Eng. 2018;2:95-103
172. Jayawardena HSN, Jayawardana KW, Chen X, Yan M. Maltoheptaose promotes nanoparticle internalization by escherichia coli. Chem Commun Camb Engl. 2013;49:3034-6
173. Jin L, Liu H, Wang C, Mao C, Wu S, Zhang Y. et al. Interface/dipole polarized antibiotics-loaded Fe3O4/PB nanoparticles for non-invasive therapy of osteomyelitis under medical microwave irradiation. Adv Mater. 2024;36:2410917
174. Qin L, Yang S, Zhao C, Yang J, Li F, Xu Z. et al. Prospects and challenges for the application of tissue engineering technologies in the treatment of bone infections. Bone Res. 2024;12:28
175. Kuo C-L, Ponneri Babuharisankar A, Lin Y-C, Lien HW, Lo YK, Chou HY. et al. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J Biomed Sci. 2022;29:74
176. Funk SS, Copley LAB. Acute hematogenous osteomyelitis in children. Orthop Clin North Am. 2017;48:199-208
177. Lew DP, Waldvogel FA. Osteomyelitis. Lancet Lond Engl. 2004;364:369-79
178. Urish KL, Cassat JE. Staphylococcus aureus osteomyelitis: Bone, bugs, and surgery. Ottemann KM, Ed. Infect Immun. 2020;88:e00932-19
179. Beck-Broichsitter BE, Smeets R, Heiland M. Current concepts in pathogenesis of acute and chronic osteomyelitis. Curr Opin Infect Dis. 2015;28:240-5
180. Rafferty BA, Thakrar P. Chronic recurrent multifocal osteomyelitis. Med Clin North Am. 2024;108:227-39
181. Hofmann SR, Kapplusch F, Girschick HJ, Morbach H, Pablik J, Ferguson PJ. et al. Chronic recurrent multifocal osteomyelitis (CRMO): Presentation, pathogenesis, and treatment. Curr Osteoporos Rep. 2017;15:542-54
182. Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090
183. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450-62
184. Chen T, Cao Q, Wang Y, Harris DCH. M2 macrophages in kidney disease: Biology, therapies, and perspectives. Kidney Int. 2019;95:760-73
185. Wang Y, Wang J, Meng J, Jiang H, Zhao J, Qian H. et al. Epigenetic modification mediates the increase of LAG-3+ T cells in chronic osteomyelitis. Inflammation. 2017;40:414-21
186. Wu Y, Tang Y, Liang X, Lin Y, Yang W, Ma Y. et al. The role of increased frequency of treg cells in patients with chronic osteomyelitis. Orthopedics. 2011;34:98
187. Zhang Q, Deng Y, Lai W, Guan X, Sun X, Han Q. et al. Maternal inflammation activated ROS-p38 MAPK predisposes offspring to heart damages caused by isoproterenol via augmenting ROS generation. Sci Rep. 2016;6:30146
188. Yang Y-F, Zhou Y-D, Hu J-C, Luo FL, Xie Y, Shen YY. et al. Ficolin-a/2, acting as a new regulator of macrophage polarization, mediates the inflammatory response in experimental mouse colitis. Immunology. 2017;151:433-50
189. Lee H-W, Lee CG, Rhee D-K, Um SH, Pyo S. Sinigrin inhibits production of inflammatory mediators by suppressing NF-κB/MAPK pathways or NLRP3 inflammasome activation in macrophages. Int Immunopharmacol. 2017;45:163-73
190. Cotzomi-Ortega I, Nieto-Yañez O, Juárez-Avelar I, Rojas-Sanchez G, Montes-Alvarado JB, Reyes-Leyva J. et al. Autophagy inhibition in breast cancer cells induces ROS-mediated MIF expression and M1 macrophage polarization. Cell Signal. 2021;86:110075
191. Zhou Y, Que K-T, Zhang Z, Yi ZJ, Zhao PX, You Y. et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7:4012-22
192. Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015;22:85
193. Weyand CM, Shen Y, Goronzy JJ. Redox-sensitive signaling in inflammatory T cells and in autoimmune disease. Free Radic Biol Med. 2018;125:36-43
194. Padgett LE, Anderson B, Liu C, Ganini D, Mason RP, Piganelli JD. et al. Loss of NOX-derived superoxide exacerbates diabetogenic CD4 T-cell effector responses in type 1 diabetes. Diabetes. 2015;64:4171-83
195. Chávez MD, Tse HM. Targeting mitochondrial-derived reactive oxygen species in T cell-mediated autoimmune diseases. Front Immunol. 2021;12:703972
196. Zhu J. T helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol. 2018;10:a030338
197. Henning AN, Roychoudhuri R, Restifo NP. Epigenetic control of CD8+ T cell differentiation. Nat Rev Immunol. 2018;18:340-56
198. Yang W, Chen X, Hu H. CD4+ T-cell differentiation in vitro. Methods Mol Biol Clifton Nj. 2020;2111:91-9
199. Shatynski KE, Chen H, Kwon J, Williams MS. Decreased STAT5 phosphorylation and GATA-3 expression in NOX2-deficient T cells: Role in T helper development. Eur J Immunol. 2012;42:3202-11
200. Agita A, Alsagaff MT. Inflammation, immunity, and hypertension. Acta Medica Indones. 2017;49:158-65
201. Tse HM, Thayer TC, Steele C, Cuda CM, Morel L, Piganelli JD. et al. NADPH oxidase deficiency regulates th lineage commitment and modulates autoimmunity. J Immunol Baltim Md 1950. 2010;185:5247-58
202. Kim Y-H, Kumar A, Chang C-H, Pyaram K. Reactive oxygen species regulate the inflammatory function of NKT cells through promyelocytic leukemia zinc finger. J Immunol Baltim Md 1950. 2017;199:3478-87
203. Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70-81
204. Arakaki R, Yamada A, Kudo Y, Hayashi Y, Ishimaru N. Mechanism of activation-induced cell death of T cells and regulation of FasL expression. Crit Rev Immunol. 2014;34:301-14
205. Franchina DG, Dostert C, Brenner D. Reactive oxygen species: Involvement in T cell signaling and metabolism. Trends Immunol. 2018;39:489-502
206. Azzouz D, Palaniyar N. Mitochondrial ROS and base excision repair steps leading to DNA nick formation drive ultraviolet induced-NETosis. Front Immunol. 2023;14:1198716
207. Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J Immunol Baltim Md 1950. 2001;167:2538-46
208. Granfeldt D, Dahlgren C. An intact cytoskeleton is required for prolonged respiratory burst activity during neutrophil phagocytosis. Inflammation. 2001;25:165-9
209. Paclet M-H, Laurans S, Dupré-Crochet S. Regulation of neutrophil NADPH oxidase, NOX2: A crucial effector in neutrophil phenotype and function. Front Cell Dev Biol. 2022;10:945749
210. Degotte G, Frederich M, Francotte P, Franck T, Colson T, Serteyn D. et al. Targeting myeloperoxidase activity and neutrophil ROS production to modulate redox process: Effect of ellagic acid and analogues. Mol Basel Switz. 2023;28:4516
211. Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem. 2016;85:765-92
212. Scheel-Toellner D, Wang K, Craddock R, Webb PR, McGettrick HM, Assi LK. et al. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 2004;104:2557-64
213. Conus S, Perozzo R, Reinheckel T, Peters C, Scapozza L, Yousefi S. et al. Caspase-8 is activated by cathepsin D initiating neutrophil apoptosis during the resolution of inflammation. J Exp Med. 2008;205:685-98
214. Ryu J-C, Kim M-J, Kwon Y, Oh JH, Yoon SS, Shin SJ. et al. Neutrophil pyroptosis mediates pathology of P. aeruginosa lung infection in the absence of the NADPH oxidase NOX2. Mucosal Immunol. 2017;10:757-74
215. Morris G, Gevezova M, Sarafian V, Maes M. Redox regulation of the immune response. Cell Mol Immunol. 2022;19:1079-101
216. Tangye SG, Nguyen T, Deenick EK, Bryant VL, Ma CS. Inborn errors of human B cell development, differentiation, and function. J Exp Med. 2023;220:e20221105
217. Liu S, Huang B, Cao J, Wang Y, Xiao H, Zhu Y. et al. ROS fine-tunes the function and fate of immune cells. Int Immunopharmacol. 2023;119:110069
218. Vené R, Delfino L, Castellani P, Balza E, Bertolotti M, Sitia R. et al. Redox remodeling allows and controls B-cell activation and differentiation. Antioxid Redox Signal. 2010;13:1145-55
219. Yang H-Y, Kim J, Lee K-Y, Jang Y-S. Rac/ROS-related protein kinase C and phosphatidylinositol-3-kinase signaling are involved in a negative regulating cascade in B cell activation by antibody-mediated cross-linking of MHC class II molecules. Mol Immunol. 2010;47:706-12
220. Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767-77
221. Shimizu Y, Hendershot LM. Oxidative folding: Cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal. 2009;11:2317-31
222. Kim E-K, Seo H-S, Chae M-J, Jeon IS, Song BY, Park YJ. et al. Enhanced antitumor immunotherapeutic effect of B-cell-based vaccine transduced with modified adenoviral vector containing type 35 fiber structures. Gene Ther. 2014;21:106-14
223. Bertolotti M, Sitia R, Rubartelli A. On the redox control of B lymphocyte differentiation and function. Antioxid Redox Signal. 2012;16:1139-49
224. Wang L, Kuang Z, Zhang D, Gao Y, Ying M, Wang T. Reactive oxygen species in immune cells: A new antitumor target. Biomed Pharmacother Biomedecine Pharmacother. 2021;133:110978
225. Yan W, Chen W, Huang L. Reactive oxygen species play a central role in the activity of cationic liposome-based cancer vaccine. J Control Release Off J Control Release Soc. 2008;130:22-8
226. Cheong T-C, Shin EP, Kwon E-K, Choi J-H, Wang K-K, Sharma P. et al. Functional manipulation of dendritic cells by photoswitchable generation of intracellular reactive oxygen species. ACS Chem Biol. 2015;10:757-65
227. Oberkampf M, Guillerey C, Mouriès J, Rosenbaum P, Fayolle C, Bobard A. et al. Mitochondrial reactive oxygen species regulate the induction of CD8+ T cells by plasmacytoid dendritic cells. Nat Commun. 2018;9:2241
228. Li J, Ding H, Meng Y, Li G, Fu Q, Guo Q. et al. Taurine metabolism aggravates the progression of lupus by promoting the function of plasmacytoid dendritic cells. Arthritis Rheumatol Hoboken Nj. 2020;72:2106-17
229. Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD. et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res. 2019;7:335-46
230. Fu J, Li Y, Zhang Y, Liang Y, Zheng Y, Li Z. et al. An engineered pseudo-macrophage for rapid treatment of bacteria-infected osteomyelitis via microwave-excited anti-infection and immunoregulation. Adv Mater Deerfield Beach Fla. 2021;33:e2102926
231. Cai X, Li B, Zhang Y, Han J, Han Y. Piezopotential and vacancies co-enhanced sonodynamic response of a ZnO-TiO2- heterojunction array for osteointegration in MRSA-infected osteomyelitis. Chem Eng J. 2024;479:147804
232. Zhang Y, Cheng Y, Zhao Z, Jiang S, Zhang Y, Li J. et al. Enhanced chemoradiotherapy for MRSA-infected osteomyelitis using immunomodulatory polymer-reinforced nanotherapeutics. Adv Mater. 2024;36:2304991
233. Nauth A, Schemitsch E, Norris B, Nollin Z, Watson JT. Critical-size bone defects: Is there a consensus for diagnosis and treatment? J Orthop Trauma. 2018;32(Suppl 1):S7-11
234. Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res Off J Am Soc Bone Miner Res. 2008;23:915-27
235. Mussbacher M, Derler M, Basílio J, Schmid JA. NF-κB in monocytes and macrophages - an inflammatory master regulator in multitalented immune cells. Front Immunol. 2023;14:1134661
236. Trouillet-Assant S, Gallet M, Nauroy P, Rasigade JP, Flammier S, Parroche P. et al. Dual impact of live staphylococcus aureus on the osteoclast lineage, leading to increased bone resorption. J Infect Dis. 2015;211:571-81
237. Somayaji SN, Ritchie S, Sahraei M, Marriott I, Hudson MC. Staphylococcus aureus induces expression of receptor activator of NF-kappaB ligand and prostaglandin E2 in infected murine osteoblasts. Infect Immun. 2008;76:5120-6
238. Min Z, Zou Y, Meng Y, Liu X, Li H, Liu H. et al. Harnessing redox: Biocomposites modulate macrophage-stem cell dynamics in osteo-inflammation. Tissue Eng Part B Rev. 2025.
239. Yang Y, Yao Z, Sun Y, Nie Y, Zhang Y, Li Z. et al. 3D-printed manganese dioxide incorporated scaffold promotes osteogenic-angiogenic coupling for refractory bone defect by remodeling osteo-regenerative microenvironment. Bioact Mater. 2025;44:354-70
240. Liao X, Shen M, Li T, Feng L, Lin Z, Shi G. et al. Combined molybdenum gelatine methacrylate injectable nano-hydrogel effective against diabetic bone regeneration. Int J Nanomedicine. 2023;18:5925-42
241. Yu B-F, Wang Z, Chen X-X, Zeng Q, Dai C-C, Wei J. Continuous dynamic identification of key genes and molecular signaling pathways of periosteum in guided bone self-generation in swine model. J Orthop Surg. 2023;18:53
242. Vullien A, Röttinger É, Vervoort M, Gazave E. [a trio of mechanisms involved in regeneration initiation in animals]. Med Sci MS. 2021;37:349-58
243. Zhang Y, Dai J, Hang R, Yao X, Bai L, Wang H. et al. Tailoring surface stiffness to modulate senescent macrophage immunomodulation: Implications for osteo-/angio-genesis in aged bone regeneration. Biomater Adv. 2024;165:214010
244. Sheppard AJ, Barfield AM, Barton S, Dong Y. Understanding reactive oxygen species in bone regeneration: A glance at potential therapeutics and bioengineering applications. Front Bioeng Biotechnol. 2022;10:836764
245. Wu S, Shuai Y, Qian G, Peng S, Liu Z, Shuai C. et al. A spatiotemporal drug release scaffold with antibiosis and bone regeneration for osteomyelitis. J Adv Res. 2023;54:239-49
246. Ma L, Zhang X, Wang H, Feng X, Lei J, He Y. et al. Two-dimensional Nb2C-based nanoplatform augmented sonodynamic antibacterial therapy and bone regeneration. Sci China Mater. 2023;66:2913-24
247. Ma L, Cheng Y, Feng X, Zhang X, Lei J, Wang H. et al. A janus-ROS healing system promoting infectious bone regeneration via sono-epigenetic modulation. Adv Mater Deerfield Beach Fla. 2024;36:e2307846
248. Egbuna C, Parmar VK, Jeevanandam J, Ezzat SM, Patrick-Iwuanyanwu KC, Adetunji CO. et al. Toxicity of nanoparticles in biomedical application: Nanotoxicology. J Toxicol. 2021;2021:9954443
249. Yu M, Xu J, Zheng J. Renal clearable luminescent gold nanoparticles: From the bench to the clinic. Angew Chem Int Ed Engl. 2019;58:4112-28
250. Ji Z, Yin Z. Machine learning-driven prediction of reactive oxygen species dynamics for assessing nanomaterials' cytotoxicity. Biomimetics. 2025;10:718
251. Phan HT, Haes AJ. What does nanoparticle stability mean? J Phys Chem C Nanomater Interfaces. 2019;123:16495-507
252. Lazareva P, Chulanov V, Kostyushev D, Abakumov M. In vivo behavior of biomimetic nanoparticles: Strategies for clearance avoidance, targeting, and functional delivery. Molecules. 2025;30:4487
253. Sultana S, Alzahrani N, Alzahrani R, Alshamrani W, Aloufi W, Ali A. et al. Stability issues and approaches to stabilised nanoparticles-based drug delivery system. J Drug Target. 2020;28:468-86
254. Mazumdar H, Khondakar KR, Das S, Halder A, Kaushik A. Artificial intelligence for personalized nanomedicine; from material selection to patient outcomes. Expert Opin Drug Deliv. 2025;22:85-108
255. Morte-Romea E, Pesini C, Pellejero-Sagastizábal G, Letona-Giménez S, Martínez-Lostao L, Aranda SL. et al. CAR immunotherapy for the treatment of infectious diseases: A systematic review. Front Immunol. 2024;15:1289303
256. Kolanu ND. CRISPR-Cas9 gene editing: Curing genetic diseases by inherited epigenetic modifications. Glob Med Genet. 2024;11:113-22
257. Pickar-Oliver A, Gersbach CA. The next generation of CRISPR-cas technologies and applications. Nat Rev Mol Cell Biol. 2019;20:490-507
258. Henriksen NL, Gottlieb H, Bue M, Vittrup S, Jensen LK. In vivo models of infection: Large animals - Mini review on human-scale one-stage revision in a porcine osteomyelitis model. Injury. 2024;55(Suppl 6):111842
259. Mansoorifar A, Gordon R, Bergan R, Bertassoni LE. Bone-on-a-chip: microfluidic technologies and microphysiologic models of bone tissue. Adv Funct Mater. 2021;31:2006796
260. Timofticiuc IA, Grigore AG, Tomescu ET, Vlaicu TM, Dragosloveanu S, Scheau AE, Caruntu A, Dragosloveanu CDM, Badarau IA, Didilescu AC, Caruntu C, Scheau C. Advances in 3D-Printed Drug Delivery and Screening Platforms for Bone Disease Therapy. Pharmaceutics. 2025;17:1372
261. Liò P, Paoletti N, Moni MA, Atwell K, Merelli E, Viceconti M. Modelling osteomyelitis. BMC Bioinformatics. 2012;13:S12
Author contact
![]() Corresponding authors: Ting Wang, Email: tingwangedu.cn; Jinsheng Shi, Email: shijinshengedu.cn; Xiaoying Kong, Email addresses: kongxiaoyingedu.cn.
Corresponding authors: Ting Wang, Email: tingwangedu.cn; Jinsheng Shi, Email: shijinshengedu.cn; Xiaoying Kong, Email addresses: kongxiaoyingedu.cn.
 Global reach, higher impact
Global reach, higher impact