13.3
Impact Factor
Theranostics 2026; 16(9):4702-4725. doi:10.7150/thno.126209 This issue Cite
Review
Nanobiomaterial-enabled boron delivery systems and innovative strategies for revolutionizing BNCT
1. Department of Radiation Oncology, Key Laboratory of Cancer Prevention and Intervention, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310058, Zhejiang, China.
2. Department of Pharmacy, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang, China.
3. Department of Pharmacy, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310009, China.
4. Center for Plastic & Reconstructive Surgery, Department of Dermatology, Zhejiang Provincial People's Hospital, Affiliated People's Hospital, Hangzhou Medical College, Hangzhou 310014, Zhejiang, China.
§ Qiyao Yang and Ruxuan Wang contributed equally to this work.
Received 2025-10-2; Accepted 2026-1-24; Published 2026-2-11
Abstract
Boron neutron capture therapy (BNCT) is a novel and emerging form of radiotherapy that combines the advantages of “heavy ion radiotherapy” and “biological targeting”. The therapeutic potential of BNCT is ultimately constrained by precise and efficient delivery of boron drugs. In this review, we systematically trace from conventional boron drugs to sophisticated nano-delivery platforms, emphasizing breakthroughs in carrier engineering, targeted delivery strategies, and multifunctional synergistic systems. A dedicated analysis of the biological foundations, such as cell cycle dynamics, tumor microenvironmental interactions, and immunostimulatory effects, provides a crucial framework for understanding mechanism-informed biomaterial design. By synthesizing current advances with an outlook on future challenges, this work aims to chart a course for translating innovative boron-loaded biomaterials from the laboratory into clinical reality, thereby unlocking the full promise of BNCT.
Keywords: boron neutron capture therapy (BNCT), drug delivery, nanotechnology, multifunctional nanostructures, tumor targeting, biological mechanism
1. Introduction
Cancer is one of the leading causes of death, with an estimated 20 million new cases and 9.7 million deaths worldwide in 2022, which will become a major stumbling block on the road to health for humans[1]. Radiotherapy is a kind of local and noninvasive treatment that kills tumors by radiation, which is relatively safer than chemotherapy and more applicable than surgery. However, its background radiation can inflict damage upon adjacent normal tissues. And the therapeutic effect is limited by the tumor's hypoxic environment.
In recent years, radiotherapy technology has undergone a progressive transition from traditional, single-modality approaches to more precise and comprehensive strategies[2]. Stereotactic body radiotherapy (SBRT) and particle radiotherapy have significantly enhanced treatment efficacy and safety[3]. Particles with higher linear energy transfer (LET) radiation cause DNA damage to cells that is harder to repair, yielding a higher relative biological effectiveness (RBE). Moreover, particle radiotherapy can partially circumvent the limitations imposed by tumor hypoxia, which makes it have a low oxygen enhancement ratio (OER)[4-8]. Consequently, particle radiation can efficiently destroy hypoxic tumors, which are resistant to conventional radiotherapy[9], and it has already been implemented in clinical practice[10-12].
Boron neutron capture therapy (BNCT) is emerging as a rising star among particle radiotherapy. Following the discovery of neutrons by the British physicist James Chadwick in 1932, the famous theory of the boron neutron capture reaction was proposed by Tylor and Goldhaber in 1935[13], which laid the groundwork for BNCT. In 1936, Gordon Locher first proposed the application of the boron neutron capture reaction to tumor therapy[14].
10B atoms possess a large thermal neutron capture cross section (3850 barns, 1 barn = 10-24 cm2) and can undergo fission reactions to produce alpha rays (4He) and lithium particle rays (7Li) after capturing uncharged thermal neutrons emitted from the outside. They are all high LET particle beams with significantly higher RBE than X-rays and γ-rays used in conventional tumor radiotherapy[15]. Moreover, the range of them in the tissue is only 5-9 μm, which limits the high destructive energy to a single cell[16].
The implementation of BNCT relies on the delivery of 10B-containing drugs to the tumor sites. The intervention of the boron drug offers the chance to connect radiotherapy with targeted therapy. Therefore, BNCT possesses the dual targeting advantages of the targeting contouring of radiotherapy and the potential targeting ability of drug delivery.
By combining "biological targeting" and "heavy ion radiotherapy", BNCT can reduce toxic effects and enhance the killing effect on tumor tissue, holding promise to revolutionize the paradigms of cancer treatment. Therefore, numerous clinical trials[17,18] related to BNCT have been conducted, and it has been applied in the treatment of various malignancies, including head and neck tumors, melanomas, glioblastomas, oral cancer, and recurrent tumors[19-26].
2. Boron Drugs
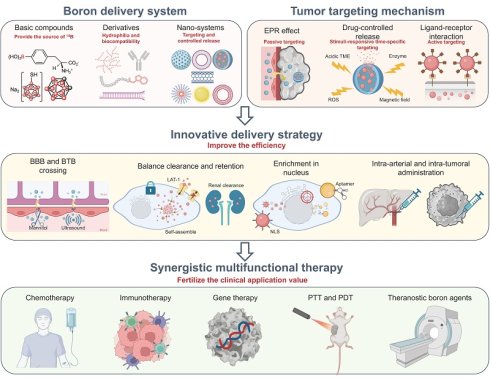
Apart from a stable, pure, and high-intensity thermal neutron source for BNCT development, a prerequisite for this dual-modality treatment to achieve maximum benefit is the precise delivery of the 10B into the tumor cells and maximizing the difference in boron content between normal and tumor cells.
The ideal boron drug must fulfill the following criteria: 1) achieving a tumor boron-10 concentration within the range of approximately 20-50 μg/g; 2) exhibiting selective boron concentration ratios in tumor versus normal tissue (T/N) and tumor versus blood (T/B) that are greater than 1, with a preference for 3 or higher; and 3) possessing minimal systemic cytotoxicity, rapid clearance from the bloodstream and normal tissues, while maintaining retention within the tumor during neutron irradiation. Therefore, boron drugs have undergone several generations of transformation (Figure 1) and are gradually evolving towards nanotechnology.
Schematic diagram of chemical structure of basic boron drugs and their derivatives. The boron element is represented by red spheres.
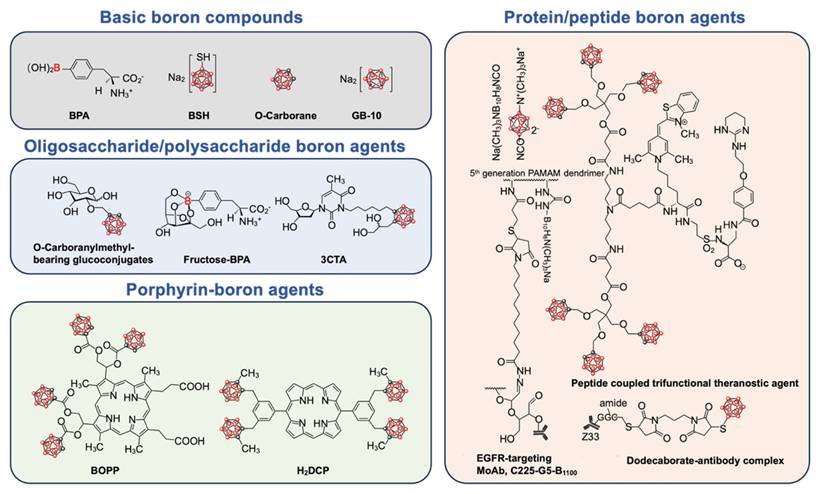
2.1. Basic boron drugs
Several boron drugs have been developed for possible use in BNCT, but only two, boronophenylalanine (BPA) and sodium borocaptate (BSH), have been clinically tested. Another boron drug with great application potential in BNCT is boron cluster, which has a high boron content. Many derivatives of these boron drugs have been developed in order to overcome their limitations.
2.1.1. Sodium borocaptate (BSH)
In 1967, Soloway and Hatanaka proposed a polyhedral borane anion named BSH with the chemical structural formula Na2B12H11SH[27]. Hatanaka used it for BNCT of glioblastoma multiforme (GBM) in 1968[28]. BSH is a hydrophilic small molecule with high boron content, capable of transporting 12 boron atoms per molecule. According to a European phase I clinical study (EPRTC11961), BSH had a brain tissue-to-blood boron ratio of 0.2 ± 0.02 and a tumor-to-blood boron ratio of 0.6 ± 0.2[29]. However, it lacks active targeting capability. Furthermore, due to its net charge, it is unable to cross the cell membrane, limiting its practical application.
2.1.2. Boronophenylalanine (BPA)
In 1968, Snyder et al. synthesized a neutral amino acid phenylalanine derivative called BPA[30]. A clinical trial of BPA in malignant melanoma was conducted in 1987 by Mishima et al.[31]. In patients with malignant melanoma, Fukuda et al. reported a mean skin-to-blood boron ratio of 1.31 ± 0.22 and a tumor-to-blood boron ratio of 3.40 ± 0.83 for BPA[32].
Research has found that BPA can be actively transported into cells via the L-type amino acid transporter system (LAT-1), which is highly expressed in tumor cells[33]. Therefore, BPA is generally preferred over BSH in clinical applications. However, BPA's water solubility is relatively low, at around 1.6 g/L, necessitating its formulation as a fructose complex (BPA-f) to improve its hydrophilicity. Furthermore, because BPA contains only one boron atom per molecule, it takes extremely high doses (500 mg/kg) to achieve the desired boron concentration in tumors. The hydrophobicity and low boron content restrict its tumor specificity[34].
2.1.3. Carboborane
Boron exhibits similarities to carbon in its ability to form covalent boron-hydrogen bonds and compounds with boron-boron interactions. To compensate for electron deficiencies, boranes tend to form polyhedral clusters with spherical structures. However, most boranes are unstable in aqueous environments. Carborane, the new compound formed by replacing two BH units with CH vertices, has exceptional biostability. Moreover, the two carbon atoms can serve as a foundation for organic modifications[35].
Carborane is structurally similar to the benzene ring in three dimensions, making it a unique pharmacophore. It has a high boron atomic content and a high neutron capture cross-section, making it an excellent candidate for BNCT[36]. As a result, carboranes have been extensively studied for modification with nanoparticles, antibodies, peptides, enzyme/receptor inhibitors, glycans, and other substances. Many chemists are still investigating various chemical modification processes for carboranes[37,38]. As a result, carborane will be an advantageous boron drug in BNCT's upcoming multifunctionalization process.
2.2. Derivatives of boron drugs
Existing boron drugs are limited by poor solubility, weak biocompatibility, and inadequate targeting capabilities. To address these limitations and expand their applications, researchers have modified boron drugs with various bioactive organic compounds, leading to the development of boron drug derivatives, including nucleoside/nucleotide boron drugs, protein/peptide boron drugs, oligosaccharide/polysaccharide boron drugs, and porphyrin-boron drugs.
These boron derivatives offer distinct advantages due to their varied organic compounds. For instance, nucleoside/nucleotide boron drugs combined with siRNA or ASOs can integrate BNCT with gene therapy. Protein/peptide boron drugs utilize functional proteins or peptides to achieve targeted delivery to tumors and realize some biological effects. Oligosaccharide/polysaccharide boron drugs enhance water solubility and biocompatibility, enabling tumor targeting through sugar transporters. Porphyrin-boron drugs exploit the photosensitizing properties of porphyrins to combine BNCT with photodynamic therapy (PDT). The specific functionalities of these boron drug derivatives will be discussed in detail in the following sections.
3. Boron-Containing Nano-System
Nanoparticles have gained significant attention in the field of drug delivery due to the enhanced permeability and retention (EPR) effect and their structural advantages. In BNCT, nanoparticles have been widely utilized to achieve passive-targeted delivery of boron drugs to tumors, enhance their water solubility and biocompatibility, increase boron loading, enable controlled release, and facilitate the functionalization of boron drugs. Therefore, in recent years, nano-delivery systems based on different materials have been extensively employed in BNCT[39]. In addition to a summary of boron delivery nano-systems, personalized delivery and treatment strategies based on the specialty of BNCT are presented in this review.
3.1. Increase tumor targeting delivery of boron (Initial development trend)
Many antineoplastic drugs cause significant physical and psychological harm to patients because they destroy both healthy and cancerous tissues non-selectively. So as to boron drugs, which can cause radiation damage to healthy tissues after neutron capture. From the perspectives of safety and efficacy, the initial development trend of the boron delivery system was to realize tumor-targeting delivery. Traditional mechanisms for tumor-targeted delivery primarily include the EPR effect of nano-systems, ligand-receptor interactions, and stimuli-responsive drug release mechanisms.
3.1.1. The EPR effect of nano-systems
In 1986, Matsumura and Maeda proposed the EPR effect, which has become a guiding principle for the development of nanomedicine. During tumor angiogenesis, the interstitial spaces between vascular endothelial cells can exceed 200 nm, in contrast to the tightly arranged endothelial cells in normal tissues[40,41]. This structural difference allows nanometer-sized particles to penetrate the tumor microenvironment more effectively, while limiting their access to normal tissue microenvironments. And the size of these particles is typically large enough to avoid renal clearance. While at the tumor site, impaired lymphatic drainage further hinders the clearance of particles, contributing to their retention within the tumor[42].
Nanotechnology has witnessed significant advancements in the field of drug delivery, which has also made substantial contributions to the delivery of boron drugs in BNCT. A variety of nanoscale structures have been successfully employed for this purpose, including nanoparticles[43-46], micelles[47-51], liposomes[52,53], boron nitride nanotubes[54], and dendritic polymers[55]. Nanoplatforms of different materials exhibit different advantages. Inorganic nanoparticles are readily accessible, amenable to large-scale preparation, and have better stability and higher boron-loading capacities. Their intrinsic superparamagnetism and fluorescence further enhance their theranostic capability. Conversely, organic nanoparticles display lower cytotoxicity and immunogenicity, and their surfaces can be conveniently functionalized with ligands to achieve enhanced targeting and membrane-penetrating efficiency. Consequently, both inorganic and organic materials possess unique advantages and have demonstrated promising performance in BNCT.
3.1.2. Ligand-receptor interactions
Active targeting, which is mediated by ligand-receptor interactions, can be used in addition to passive targeting. As a result, many target ligand-modified boron drugs have been developed for BNCT, which are listed in Table 1.
BPA is a specialized ligand used in BNCT, serving as both a source of boron and a targeting moiety for tumor cells due to the overexpression of its receptor, LAT-1, on tumor cells[56,57]. BPA-modified mesoporous silica nanoparticles have been utilized to enhance the boron loading capacity and the accuracy of targeted delivery[58]. Besides, boric acid derivatives have been identified as another promising boron-containing ligand for targeted drug delivery due to their selective binding affinity for salivary acid (SA)[59]. Ahram Kim et al. modified phenylboronic acid (PBA) on PLA-PEG polymers, achieving a potent anti-tumor effect with a dosage 100 times lower than that of BPA-fructose (BPA-f) (Figure 2)[59].
Boron-containing ligands represent a specialized subset of ligands used for boron delivery to tumors. Many classical ligands have also been employed for this purpose. For instance, the folate receptor (FR) is overexpressed in several types of human cancers, including ovarian, brain, renal, breast, myeloid, and lung malignant tumors[60,61]. Folate-modified nanostructures can be specifically internalized via endocytosis through FR[62]. Studies have reported folic acid modification of boron nitride nanoparticles[57], boron carbide nanoparticles[63], boron phosphate nanoparticles[62], iron-containing nanoparticles[64], liposomes[53], and PLGA nanoparticles[65].
Carbohydrates can also be used to increase the delivery accuracy of boron to tumor sites. Leveraging the Warburg effect, D-glucose derivatives containing o-carborane have been designed to target GLUT1 overexpressed in tumors[66,67]. Carbohydrates play a role in a variety of biological processes in addition to interacting with different cell-surface proteins to achieve targeted delivery. For instance, the interaction between galactose and asialoglycoprotein receptors (ASGP-Rs) on HepG2 cells can trigger the receptor-mediated endocytosis. Therefore, galactose-grafted carboranes can improve boron delivery to HepG2 cells and increase cell-killing efficacy by tenfold[68]. Besides, the high affinity of hyaluronic acid (HA) for CD44, which is overexpressed on tumor cells, was utilized to create a tumor-targeting hydrosoluble complex of HA and o-carborane for boron delivery. The complex's efficacy can surpass that of the BPA-fructose complex, which has widespread clinical use[69].
The antibody-drug conjugate (ADC) has gained significant momentum due to its targeted delivery, achieved by interacting with specific receptors. These coupling tactics have also been employed in BNCT. One early study has combined boron with cetuximab, a chimeric monoclonal antibody that targets EGFR, achieving not only the specific delivery of boron to gliomas but also exerting immunotherapeutic effects by blocking downstream signaling pathways[70-72]. Another study combined an anti-HER2 antibody (61 IgG) with boron-containing polyethylene glycol-coated gold nanoparticles, resulting in a significant increase in the accumulation of boron-containing nanoparticles in HER2-overexpressing tumors through phagocytosis[73].
However, the large molecular weight and immunogenicity of antibodies restrict their application. Therefore, a small molecule peptide-drug conjugate (PDC) has emerged as a promising alternative to mitigate the drawbacks. These peptides can be categorized into two main types: cell-penetrating peptides (CPPs) and cell-targeting peptides (CTPs). RGD, one of the CTPs, as a tumor-homing peptide can be utilized to modify boron drugs directly, as well as grafted onto a boron nano-delivery system. RGD peptides specifically recognize and bind to integrin receptors (such as αvβ3 and αvβ5) that are overexpressed on tumor cells (breast cancer, glioma, pancreatic tumors, etc.) and tumor-associated endothelial cells[74]. Various RGD-modified nano-systems have demonstrated enhanced tumor penetration and accumulation[73-75]. In our team's iRGD-modified polymer study, the nanostructures achieved a T/B ratio of 14.11 and a T/N ratio of 19.49 in vivo[75].
Ligand modification represents the most direct approach for nano-systems to target tumors, although it is not the only one. There are more nano-delivery systems with advanced targeting capabilities being developed and utilized.
3.1.3. Stimuli-responsive drug-controlled release
Conventional nano-formulations tend to release drugs continuously as they travel to their target sites, either through active uptake or passive diffusion. This uncontrolled release can result in unintended exposure of healthy tissues to the drug, potentially causing toxic side effects.
Schematic illustrations of PBA modified polymers[59], copyright 2020 Elsevier. A. Chemical structure and molecular design of the NanoPBA. B. Different mechanisms of cellular uptake facilitation between BPA-fructose complex and NanoPBA. C. Passive targeting of the tumors via the EPR effect. D. Facilitated endocytosis of the NanoPBA and subsequent nuclear fission in response to the thermal neutron irradiation.
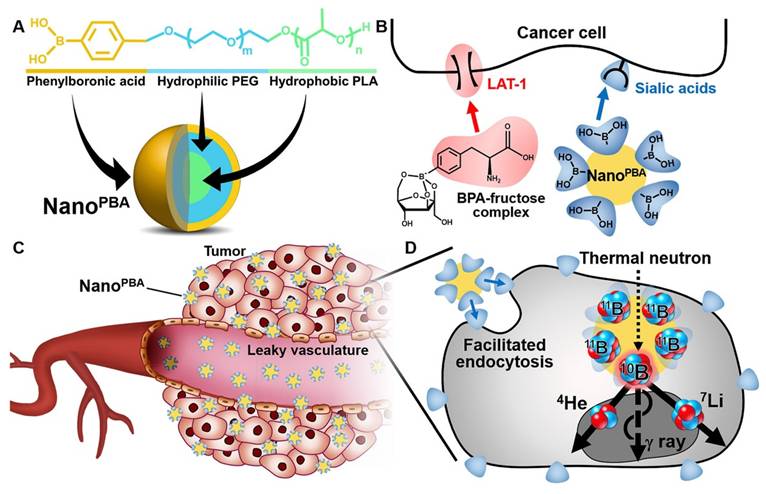
While ligand-modified nanostructures achieve spatial targeting of tumors, responsive nanostructures enable intelligent drug release to enhance cellular uptake in a controlled manner, both temporally and spatially. Stimuli-responsive nanostructure design mainly relies on internal and external stimuli. Internal stimuli mainly refer to the tumor microenvironment biomarkers, including acidic or hypoxic conditions, reactive oxygen species (ROS), specific enzymes, and glutathione (GSH). External stimuli, including near-infrared light (NIR) and magnetic fields, are commonly used to achieve controlled drug release[76]. Responsive boron-containing nano-systems used in BNCT include GSH-responsive nano-micelles[47], thermo-responsive polymer micelles[49], as well as chitosan nanoparticles[77], acid-responsive[50], and enzyme-responsive nanostructures[78], as showed in Table 2.
For instance, Hao Quan et al. developed a micelle with a dual-responsive shell and core structure for precise boron delivery. The micelle was formed by HA shelled around the surface of positively charged o-carborane tagged by the cell-penetrating peptide TAT. The micelles can target tumor sites due to the EPR effect and HA's ability to bind to CD44. When HA enters the acidic tumor microenvironment, it is rapidly degraded by hyaluronidase (HAase), which is highly expressed in the tumor microenvironment. This degradation exposes the TAT, thereby enhancing the tumor cell membrane permeability of the micelles and facilitating responsive boron uptake[50].
Summary of boron nano-delivery systems based on ligand-receptor interactions
| Target Point | Target Head | Drug Carrier | Boron Drug | Caner Species | T/B | T/N | Ref. |
|---|---|---|---|---|---|---|---|
| Folate receptor α | Folate | Boron nitride | Boron nitride | Glioma | / | / | [79] |
| Boron carbide | Boron carbide | Cervical cancer | / | / | [63] | ||
| Boron phosphate (BPO4) | Boric acid | Colon cancer; osteosarcoma | / | / | [62] | ||
| Iron nanoparticles | Boric acid | Pancreatic cancer; cervical cancer; lung cancer | / | / | [64] | ||
| Liposomes | Borane | Glioblastoma | / | / | [53] | ||
| Polymers | Boric acid | Ovarian cancer | / | / | [65] | ||
| HER2 | Anti-HER2 antibody | Boron nitride | Boron nitride | Ovarian cancer | 390/24 h | 170/24 h | [80] |
| Liposomes | Carborane | Ovarian cancer | / | 11/24 h | [81] | ||
| 61IgG | Silica nanoparticles | Boron cage-SH | Gastric cancer | / | 41.05/12 h | [73] | |
| EGFR | Anti-EGFR antibody | BPO4 | Boric acid | Head and neck cancer | 4.27/6 h | / | [82] |
| GdB6 | Boric acid | Head and neck cancer | 4.18/24 h | / | [83] | ||
| Liposomes | Boric acid | Glioma | / | 5.6/24 h | [84] | ||
| Cetuximab | Liposomes | BSH | Glioma | / | / | [85] | |
| Erlotinib | Liposomes | Carborane | Glioma | / | / | [86] | |
| PSMA | PSMACR ligands | Boron carbon oxynitride (BCNO) | Boric acid | Astrocytoma | / | / | [87] |
| ACUPA | Polymers | Carborane | Prostatic cancer | 25/96 h | 10/96 h | [88] | |
| Integrin receptor αvβ3 | cRGD | Gold nanoparticles (AuNPs) | BSH | Glioma | >50/6 h | >400/6 h | [89] |
| Polymers | BSH | Melanoma | / | / | [90] | ||
| Copolymers | Benzoborazole pendant | Breast cancer | 5.2/24 h | 18.1/24 h | [91] | ||
| Chitosan nanoparticles | Carborane | Liver cancer | 4.38/12 h | / | [92] | ||
| iRGD | Liposomes | Carborane | Glioma | / | / | [93] | |
| Polymers | BSH | Lung cancer | 14.11/24 h | 19.49/24 h | [75] | ||
| RGD-K | Silica nanoparticles | Boron | Astrocytoma | 2.8/24 h | 1.64/24 h in brain | [94] | |
| LAT-1 | BPA | Silica nanoparticles | BPA | Ovarian cancer | / | / | [58] |
| Boronotryptophan derivatives | Polymers | Boronotryptophan derivatives | Glioma | / | 170/50 min in U87; 8/1 h in LN229 | [95] | |
| Glucose-regulatory protein receptor | SP94 Peptide | Silica nanoparticles | Boric acid | Hepatic cancer | 5.92/4 h | 4.41/4 h | [96] |
| ASGPR | Trivalent galactosyl | Silica nanoparticles | Carborane | Hepatic cancer | / | / | [96] |
| CD44 | Hyaluronic acid | Nanogels | BSH | Glioma | / | 6/48 h in muscle; 2/48 h in brain | [97] |
| Nanomicelles | Carborane | Breast cancer | 3.58/12 h | / | [50] | ||
| Sialic acid | PBA | Polymers | BPA | Pancreatic cancer | 4.1/6 h | 6.7/6 h | [98] |
| Polymers | PBA | Melanoma | / | / | [59] | ||
| Copolymers | Fluorophenyl boronic acid | Cervical cancer; colon cancer | / | / | [99] | ||
| BPA | Chitosan nanoparticles | BPA | Glioma | / | / | [77] |
Summary of boron nano-delivery systems based on stimuli-responsive release
| Responsive Mechanism | Drug Carrier | Boron Drug | Caner Species | T/B | T/N | Ref. |
|---|---|---|---|---|---|---|
| H2O2 and GSH responsive | Boron nitride | Boron nitride | Breast cancer | 5.5/12 h | 41.5/12 h | [100] |
| MMP2/9 enzyme responsive | Silica nanoparticles | BSH | Chondrosarcomas | / | / | [78] |
| Acid responsive | Silica nanoparticles | Boric acid | Fibrosarcoma; melanoma | / | / | [101] |
| GSH responsive | Nanomicelles | BSH | Melanoma; breast cancer | / | / | [47] |
| Copolymers | Benzoborazole pendant | Breast cancer | 5.2/24 h | 18.1/24 h | [91] | |
| HAase and acid responsive | Nanomicelles | Carborane | Breast cancer | 3.58/12 h | / | [50] |
| Light-responsive | Copolymers | BSH | Colon cancer | 3/48 h | 10/48 h | [102] |
| Thermo-responsive | Chitosan nanoparticles | BPA | Glioma | / | / | [77] |
Besides, several types of magnetic nanoparticles have been designed for their potential application in BNCT. While magnetic targeting experiments have not yet been conducted, these nanoparticles have the potential to utilize magnetophoretic forces to accumulate boron drugs specifically at the tumor site, establishing a foundation for magnetically targeted delivery of boron nanoparticles.
3.2. Increase boron loading capacity and safety (Novel development trend)
Several tumor-targeting design concepts are illustrated above, which successfully increase the boron content at the tumor site. However, nano-systems still have major problems translating to clinical practice. On the one hand, inefficient boron delivery to the tumor will significantly impact the clinical treatment outcome. The emergence of boronizing nanocarriers doubles the boron load several times. On the other hand, biotoxicity and immunogenicity are real concerns that most nanoparticle platforms cannot be rendered completely non-toxic. Many biocompatible drug carriers, such as liposomes and micelles, have been designed to safely deliver boron. Biomimetic nanocarriers are another novel option for lowering biotoxicity and immunogenicity.
3.2.1. Boronizing nanocarriers
The efficient delivery of boron is crucial for BNCT. However, whether using boron drugs or traditional boron-delivery nano-systems, the boron content in each dosing unit is limited. And after clearance by systemic circulation, the amount of boron that ultimately reaches the tumor site is significantly reduced. To meet the boron content requirements for BNCT, the dosage of boron drugs must be increased, raising the risk of toxicity associated with high drug concentrations in the body. In recent years, researchers have proposed novel approaches for directly integrating boron into nanocarrier materials, significantly increasing boron's delivery capacity and therapeutic effect.
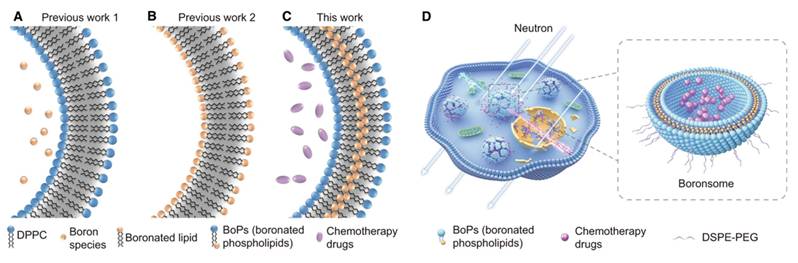
For instance, researchers have developed a boron-containing liposome (boronsome) by directly incorporating boron into the phospholipid bilayer. This design not only increases the boron content but also enhances the stability and biocompatibility of the nanovesicles. The results show that boronsome exhibit high selective accumulation and prolonged retention in the tumor, thereby improving the efficacy of BNCT(Figure 3)[103]. In another study, lipoic acid-boronophenylalanine (LA-BPA) derivatives were used to develop multifunctional nanovesicles. By targeting SA on the surface of tumor cells through phenylboronic acid groups, these nanovesicles achieved the targeted delivery of boron to tumor cells. The boron concentration in the tumor reached 93.3 ppm, which is significantly higher than that in normal tissues. This design not only enhances the boron delivery capacity but also employs a GSH-responsive release mechanism to facilitate efficient drug release within tumor cells[98].
Both of these nanocarrier systems demonstrate high boron content, excellent tumor targeting, and biocompatibility. These innovative approaches not only provide new strategies for the clinical application of BNCT but also offer important technological support for multimodal cancer treatment and precision medicine.
3.2.2. Biomimetic nanocarriers loaded with boron
Traditional inorganic and organic nanocarriers face major challenges in clinical translation due to their immunogenicity and toxicity, which lead to extensive clearance by the reticuloendothelial system upon administration. In contrast, biomimetic nanocarriers have emerged as a promising alternative, offering enhanced biocompatibility and biological functions analogous to those of cells, which have garnered significant attention for drug delivery systems. Currently, extracellular vesicle (EV)-based nano-systems and cell membrane encapsulation are the most prevalent techniques in biomimetic cellular nanocarrier applications[104]. Furthermore, endogenous proteins have also been identified as a viable type of biomimetic nanocarrier[105]. The biomimetic nanocarriers loaded with boron have been listed in Table 3. However, as a rising star in the field of drug delivery, biomimetic nanocarriers remain underexplored in BNCT.
Schematic illustration of boronated liposomes for BNCT[103], copyright 2022 Springer Nature. A-C Formulations of previous boronated liposomes (A, B) and boronated liposomes (C). D Boronsomes emit high-energy particles when irradiated by neutrons and release chemotherapy drugs sequentially to kill cancer cells.
Summary of biomimetic nanostructures
| Drug Carrier | Boron Drug | Target Head | Target Point | Responsive Mechanism | Cancer Species | T/B | T/N | Multifuction | Other | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Protein/peptide nanotubes | Carborane | / | / | / | Breast cancer | / | / | / | Perinuclear localization | [106] |
| BSH | / | / | / | Glioma | / | 40/24 h | / | / | [107] | |
| Virus | BSH | / | / | / | Liver cancer | / | / | Immunotherapy with virus | Abscopal effect | [108] |
| Extracellular vesicles | BSH | / | / | / | Glioblastoma | / | / | / | / | [109] |
| Carborane | / | / | / | Colon cancer | 63/24 h | 420/24 h | / | / | [110] | |
| Erythrocyte membrane | Boron nitride | / | / | / | / | / | / | / | / | [111] |
| Cancer cell membrane | Boron nitride | / | / | / | / | / | / | Chemotherapy with DOX | / | [112] |
| HSA | Boric acid | HSA | SPARC | / | Prostatic cancer; melanoma | / | 5.94/3 h in prostatic cancer; 6.53/3 h in Melanoma | Fluorescence visualization with boron-containing carbon dots | / | [113] |
| Dodecarborate | / | / | / | Glioma | / | / | Chemotherapy with gemcitabine | / | [114] | |
| BSA | BSH | Hyaluronic acid | CD44 | / | Breast cancer; lung cancer; liver cancer | / | / | / | / | [115] |
| Dodecaborate | / | / | / | Oral cancer | 1.6/19 h | 4.7/19 h | / | / | [116] |
3.2.2.1. Extracellular vehicles (EVs)
Extracellular vehicles (EVs), composed of lipid bilayers containing transmembrane proteins and soluble cytoplasmic components, are well-established mediators of intercellular communication. Exosomes are the most extensively studied EVs. They are cup-shaped vesicles with diameters raging from 30 to 100 nm. Their nanoscale size and ability to selectively target tissues make them ideal vehicles for delivering therapeutic agents. In addition, some exosomes have been shown to exhibit immunomodulatory effects[117]. Exosomes from HeLa cells have been studied as boron drug carriers in BNCT. The surface of EV was modified with hexadecyl oligoarginine (R16) peptide to enhance actin-dependent megacellular uptake. In vitro studies have showed that EV-encapsulated boron nano-systems exhibit higher anticancer activity than unencapsulated systems[109]. However, in vivo evaluation of these nanocarriers remains to be conducted.
3.2.2.2. Cell membrane-encapsulated nanoparticles
Cell membrane-encapsulated nanoparticles are engineered by enveloping the synthesized nanoparticles with a natural cell membrane. The cell membrane, as a communication tool with the external environment, is crucial for cellular function. By mimicking the biological functions of the cell membrane, cell membrane-encapsulated nano-systems can achieve prolonged circulation in vivo, tumor homing, cancer cell targeting, vaccine delivery, and other related functions, thereby significantly enhancing nanoparticle stability[104]. Currently, cell membranes derived from erythrocytes, leukocytes, tumor cells, and bacterial bilayer membranes are primarily used to encapsulate nanoparticles. Researchers have prepared erythrocyte membrane-coated boron nitride nanoparticles (BN@RBCM), which significantly reduced the systemic toxicity of BN[111]. Additionally, a recent study has explored the encapsulation of boron nitride nanoparticles (BN) and doxorubicin (DOX) within a cancer cell membrane (CCM), forming a biomimetic nano-system denoted as CM@BN/DOX. The CCM-coated nanoparticles demonstrated good biocompatibility, strong targeting ability, and enhanced homogeneous tumor eradication[112].
3.2.2.3. Endogenous proteins derived nano-system
Endogenous proteins-derived nano-system is the third type of biomimetic structure, with albumin and ferritin being the most extensively utilized due to their low immunotoxicity. Classical albumin-paclitaxel nanoparticles have been clinically approved for tumor therapy, making these nanocarriers highly promising for various applications[105]. Currently, structural designs such as boron-albumin conjugated nanoparticles[113,115,116,118] have been developed, which significantly enhance the stability and biocompatibility of boron drugs. Additionally, an archaea-derived protein has been engineered into a nanotube structure known as RHCC-NT, which is capable of stably encapsulating carbon borane within its lumen. In in vivo experiments, endotoxin-purified protein nanotubes elicit minimal immune response and are efficiently internalized by cells, accumulating in the perinuclear cytoplasm, resulting in a strong and effective neutron therapy-induced cytotoxic effect[106].
4. Innovative Delivery Strategies Overcoming BNCT Bottlenecks
Boron-delivery systems continue to confront critical bottlenecks. Insufficient boron content in the tumor, as well as unfavorably low tumor-to-normal tissue and tumor-to-blood boron ratios, reduces the therapeutic effect. Tumor heterogeneity hinders the accumulation of nanoparticles, and dense solid tumors cannot be uniformly penetrated by relying solely on the EPR effect. The long-term biodistribution and metabolic pathways of nano-boron drugs are still inadequately studied, and potential biosafety risks require comprehensive preclinical evaluation. Although boron delivery systems have been supported by the latest research results in the field of pharmacy in terms of materials and structures, as outlined above, a growing number of researchers are now pursuing new delivery strategies that can appropriately correct the above deficiencies and achieve more clinically transformative breakthroughs.
4.1. Cross the blood-brain barrier (BBB) and blood-tumor barrier (BTB)
Given the high application status of radiotherapy in the treatment of cerebral tumors, BNCT also holds significant promise. However, the prerequisite for effective BNCT is the delivery of boron drugs across the BBB and blood-tumor barrier BTB, which remains a considerable challenge. Currently, two innovative studies have addressed this challenge by developing novel strategies to enhance the delivery of boron drugs to brain tumor sites.
In one study, Ching-Hsiang Fan et al. employed microbubbles as a delivery system. These microbubbles interact with the ultrasound energy generated by transcranial focused ultrasound (FUS) to enhance acoustic effects and microfluidic dynamics. This interaction generates mechanical stress that activates transient tight junction deformations, thereby noninvasively and reversibly opening the BBB/BTB to facilitate enhanced drug delivery to brain tissue. The study achieved a high tumor-to-normal brain ratio (T/N ratio) of 4.4, setting the stage for the realization of BNCT in brain tumors[119].
In another study, the authors exploited the transient disruption of the BBB by intravenous mannitol. Mannitol administered intravenously 10 minutes before the administration of the designed boron-containing nanoparticles causes a temporary disruption of the BBB that lasts 10-60 minutes, ensuring that the nanoparticles penetrate the brain[94].
4.2. Balance the drug clearance and retention
The development of effective boron drug delivery systems faces a dual challenge: boron drugs must be rapidly cleared from the bloodstream and normal tissues to minimize systemic toxicity, while simultaneously achieving sufficient accumulation in tumors during irradiation. These requirements are frequently contradictory, as the prolonged circulation times required for tumor accumulation can cause off-target accumulation in organs such as the liver and spleen, resulting in negative side effects. To address these challenges, new delivery strategies have been developed to enhance tumor retention while ensuring rapid clearance from non-target tissues.
4.2.1. Transform of nano-scale
Irene V.J. Feiner et al. designed an ultra-small boron carbon dot nanoparticle. Trastuzumab, a monoclonal antibody with high tumor-targeting specificity, was functionalized with trans-cyclooctene (TCO) and administered. Subsequently, tetrazine (tz)-modified ultra-small boron carbon dot nanoparticles were introduced. These dot nanoparticles were enriched in tumor tissues through the orthogonal reaction of tz and TCO. Because of their small size, nanoparticles not captured by trastuzumab were quickly cleared from the body, resulting in rapid clearance of boron drugs while retaining them in tumors[120].
Liping Li et al. made a smart boron nanosensitizer (BATBN) comprising an ultrasmall boron quantum dots (BQD) core conjugated with CPPs (Figure 4). The small size of the BQD core and CPPs facilitates the deep tumor penetration. Furin and GSH in TME induce the cleavage of CPPs, exposing disulfide bonds that trigger the self-assembly of BQDs. This process results in an increase in nanoparticle size, thereby optimizing boron retention within the tumor[121].
4.2.2. Boron drugs' antidote
In another innovative approach, phase-transformation lysozyme (PTL) was utilized to encapsulate boron carbide nanoparticles (BNNPs) for drug delivery. This method protects the BNNPs from hydrolysis during circulation, thereby prolonging their circulation time and enhancing boron accumulation in tumors. Furthermore, to mitigate off-target accumulation, vitamin C was administered intravenously to detoxify BNNPs that had accumulated in major organs, such as the liver and spleen. This strategy facilitated the rapid removal of BNNPs from these organs, thereby minimizing their toxic effects[122].
Schematic illustration of biomarker-activated size-transformed boron nanosensitizers for enhanced BNCT[121], copyright 2024 Wiley-VCH GmbH. (A) Molecular mechanisms of the size transformation of small-sized boron nanomedicine into larger particle size nanoassembly. Red arrows indicate the sites of furin/GSH cleavage. (B) Schematic illustration of the intratumoral boron accumulation behavior of BATBN and size-fixed BQDs, and their BNCT efficiency. (C) The intratumoral boron concentration and retention time were significantly enhanced by BATBNs compared to BQDs.
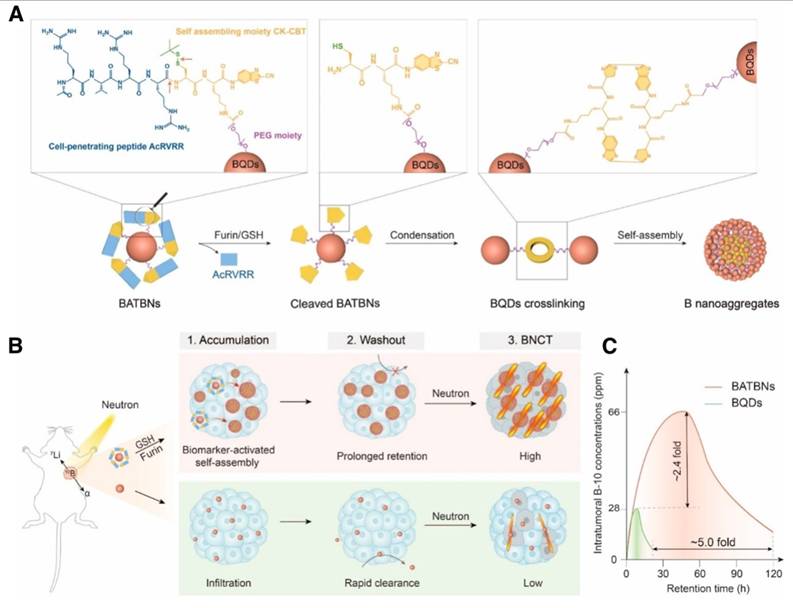
4.2.3. Block the reverse transport of LAT-1
BPA is a widely used boron delivery agent that targets LAT-1 for tumor targeting in BNCT. However, BPA's short retention time in tumors limits its effectiveness in BNCT. Reportedly, this limitation is related to the reverse transport mechanism of amino acid transport proteins, including LAT-1, which exchanges intracellular BPA for extracellular amino acids such as tyrosine. This exchange is unfavorable for BPA accumulation within tumor cells[57]. One study utilized polyvinyl alcohol (PVA) to form a borate complex with BPA. This complex not only facilitated the internalization of BPA into the cell via LAT-1-mediated transport but also resulted in the accumulation of BPA in lysosomes, slowing down the reverse transport mediated by LAT-1 and leading to the long-term retention of BPA in tumor cells[123,124]. Additionally, the authors exploited the partial cationic properties of the PEG-PLys backbone to promote renal clearance and enhance the tumor-to-blood (T/B) ratio, thereby further improving the therapeutic efficacy of BPA in BNCT.
Furthermore, studies have shown that both D-BPA and L-BPA can be absorbed by tumor cells via the LAT-1 transporter. However, L-BPA has a higher transport efficiency than D-BPA, making it easier to efflux through LAT-1's reverse transport. As a result, the authors created a PVA-D-BPA complex that significantly slows the reverse transport of LAT1. This strategy effectively increases the intracellular retention of BPA within tumor cells[125].
4.3. Increase the enrichment of boron in the nucleus
The particle rays generated by BNCT through fission reactions exert cytotoxic effects within a range of approximately 10 μm, with their energy decaying exponentially as the distance increases[126]. Therefore, reducing the distance between 10B and the nucleus (ideally within 5 μm) would enhance the efficacy of α-particle-induced DNA double-strand breaks, thereby augmenting the tumoricidal capability of BNCT[59]. Therefore, the nucleus-positioning boron delivery system is highly anticipated. However, many nanocarriers remain extracellular or cytosolic and fail to achieve precise perinuclear or nuclear localization due to lysosomal clearance and their large diameter, which may reduce the local efficacy of high-LET particle damage and contribute to clinical recurrence and metastasis. An accurate and efficient nuclear-targeted delivery strategy still remains to be solved.
Several studies have demonstrated that various nano-systems can localize around or be internalized by the cell nucleus. These nano-systems include mesoporous silica nanoparticles[58], boronated porphyrin-containing polymeric nanoparticles[48], phenylboronic acid-modified polymeric nanoparticles[59], RHCC protein helical nanotubes[106], and multifunctional liposomes containing DOX-grafted carbaboranes[93]. Achieving precise delivery of boron drugs to the tumor cell nucleus could potentially reduce the required dose while maintaining therapeutic efficacy, thereby minimizing the toxic side effects associated with the systemic boron burden. For example, using DOX's ability to transverse the nucleus, carborane was introduced into the nucleus, resulting in boron aggregation[93].
The primary strategies for achieving nuclear delivery of drugs include the use of nuclear localization signal (NLS) peptides and aptamers. The NLS is a specific protein domain that functions as a signal fragment to interact with nuclear import receptors, thereby mediating the transport of proteins from the cytoplasm to the nucleus. Aptamers, on the other hand, are short single-stranded DNA or RNA molecules that can bind to molecular targets with high affinity and specificity. Among these, the AS1411 aptamer serves as a ligand for nucleolin, facilitating nuclear transport[127]. However, since the NLS was modified on the boronated porphyrin in 2005[128], no research has emerged regarding the use of NLS for the nuclear-targeted delivery of boron in the past two decades. And there are no reports about aptamers being used in boron's nuclear delivery. Besides, the majority of boron delivery systems that exhibit nuclear or perinuclear localization have merely observed this phenomenon without elucidating the underlying nuclear targeting mechanisms. Consequently, the reproducibility of these systems remains a matter of concern. Therefore, the development of boron delivery systems that specifically target the cell nucleus represents a valuable direction for future research.
4.4. Intra-arterial and intra-tumoral administration of boron drugs
Intra-tumoral administration is a direct method for delivering effective components to tumor sites, thereby achieving a high local drug concentration. Intra-arterial administration infuses the anti-tumor agents into the feeding artery of the tumor, which has been well-established in the treatment of liver cancer. These two administrations enhance drug bioavailability at the target site while simultaneously reducing systemic exposure to the therapeutic agents.
Intra-arterial administration of boron drugs has already been explored in BNCT of head and neck cancers[129,130], brain tumors[131], and liver cancer[132,133]. Early in 2006, researchers compared the intra-arterial (IA) and the intravenous (IV) delivery of boron drugs for recurrent head-and-neck tumors. The IA route achieved higher boron concentrations in the tumor, with one patient achieving a complete response (CR) and three showing a partial response (PR), compared to two PR, three stable cases, and one progressive disease (PD) in the IV group[129]. Hironobu Yanagie et al. used a 10B-shielded water-in-oil-in-water emulsion for intra-arterial injection in a liver cancer model, reporting boron concentrations of 61.7 ppm in the tumor, 4.3 ppm in normal liver tissue, and 0.1 ppm in blood[132,133]. These findings demonstrate that intra-arterial delivery can effectively concentrate boron drugs in tumors, enhancing efficacy and reducing toxicity.
Intra-tumoral administration has not yet been trialed in BNCT; however, it has been used for a broad range of agents in cancer immunotherapy, with encouraging clinical trial results[134,135]. Given its ability to achieve high local drug concentrations within the target tumor, intra-tumoral administration holds significant potential for enhancing the efficacy of BNCT.
Nevertheless, both intra-arterial and intra-tumoral drug administration remain underdeveloped in clinical practice, primarily due to their high technical thresholds and complex operational procedures. Intra-arterial administration requires the expertise of specialized surgeons, while intra-tumoral administration demands precise image guidance. Moreover, the anatomical location and depth of tumor growth influence the specific operational methods of drug delivery. Additionally, intra-tumoral administration is affected by tumor heterogeneity and the high interstitial fluid pressure within the tumor microenvironment. Therefore, the development of intra-arterial and intra-tumoral administration in BNCT in the future will require the collaborative efforts of radiation oncologists, surgeons, radiologists, and other healthcare professionals.
5. Synergistic Multifunctional Boron Delivery System
In addition to the vertical development of boron delivery systems to become more accurate, more abundant, and safer through the above means, researchers have increasingly focused on the horizontal development of multifunctional delivery systems that integrate BNCT with other therapeutic or diagnostic modalities. To meet the demand for a comprehensive treatment model in the clinic, a boron delivery system that combines chemotherapy, immunotherapy, gene therapy, photodynamic therapy (PDT), photothermal therapy (PTT), and other therapeutic approaches have begun to emerge. Furthermore, to achieve simpler, more reliable, and clinically applicable methods for the determination of boron content in tumors, the development of visualized theranostic boron delivery systems has also become a key focus.
5.1. Combination with chemotherapy
Various studies have used nanostructures to co-deliver boron and chemotherapeutic drugs, taking advantage of their favorable drug-carrying capabilities. Consequently, a combined BNCT and chemotherapy treatment approach has been successfully achieved.
Our team has successfully conjugated the chemotherapeutic agents DOX[93] and paclitaxel (PTX)[92] with carborane, respectively, to construct a multifunctional nano-system that integrates chemotherapy and BNCT. The RGD peptide was used to achieve tumor-targeting delivery, thereby enhancing the therapeutic efficacy of BNCT. Furthermore, BPA has been used as a boron source to form core-shell nanostructures for the delivery of methotrexate, a poorly water-soluble folate-based antimetabolite anticancer medication, in the treatment of glioblastoma multiforme[77].
Some studies have demonstrated that neutron irradiation can also serve as a stimulus for drug-responsive release. Liping Li et al. designed a boron nanosheet DOX@BNNS with surface-loaded DOX for the treatment of triple-negative breast cancer. The study found that the nanosheet exhibited strong tumor-targeting capability. Additionally, the research revealed that neutron irradiation and the tumor's acidic environment can trigger the controlled release of DOX in the nano-system. This led to a significant improvement in the therapeutic efficacy of triple-negative breast cancer in vitro and in vivo and reduced the systemic toxicity associated with chemotherapeutic drugs[136].
5.2. Combination with immunotherapy
Cancer immunotherapy has emerged as an advanced therapeutic modality, garnering significant attention for its ability to harness the immune system to combat cancer. Recently, the integration of immunotherapy with radiotherapy has become a focal point of research, with numerous global clinical trials underway in specific cancer types. However, the development of nano-delivery systems that combine immunotherapy with BNCT remains in its nascent stages, with only a limited number of studies having been conducted thus far.
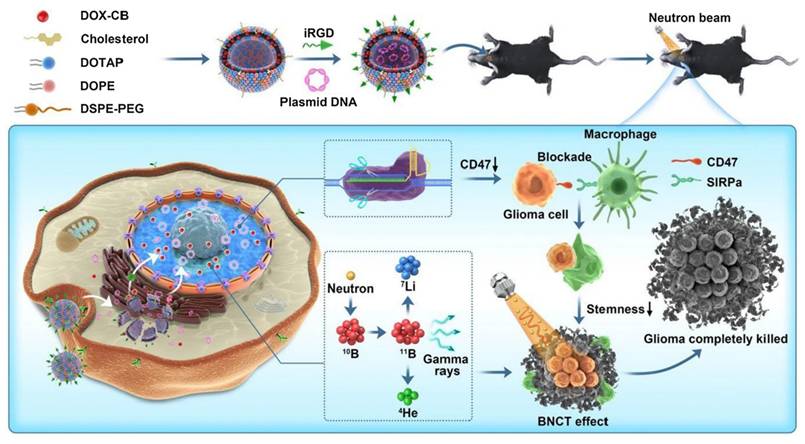
Our team investigated a triple therapy nano-delivery system combining immunotherapy, chemotherapy, and BNCT, in which integrin-related protein CD47 was selected as the target for combination immunotherapy (Figure 5). CD47-blocking immunotherapy can effectively activate macrophage-mediated phagocytosis, promote adaptive immunity, and reduce the risk of recurrence. In our study, we employed cationic liposomes to deliver a CD47 gene-targeted CRISPR-Cas9 knockout plasmid for immunotherapy. In an in vitro study, CD47 expression in transfected GL261 cells was significantly reduced, leading to a marked enhancement of macrophage phagocytosis. In in vivo studies, this nano-system significantly improved anti-tumor efficacy, reduced the recurrence rate, and prolonged survival time[93].
A new immunotherapeutic strategy utilizing inactivated Japanese enveloped hemagglutinating virus (HVJ-E) as a gene delivery vector has garnered considerable attention. Previous studies have demonstrated that the membrane fusion ability of HVJ-E activates anti-tumor immunity by inducing the expression of interleukin (IL)-6 and chemokine (CXCL)-10 in dendritic cells, thereby facilitating the elimination of cancer cells. The viral RNA genomic fragment of HVJ-E activates the retinoic acid-inducible gene I (RIG-I) signaling pathway and upregulates tumor necrosis factor-associated apoptosis-inducing ligand (TRAIL) and Noxa. However, as a vector, the virus is prone to membrane fusion with erythrocytes, leading to hemolysis. Therefore, a novel boron-containing biocompatible polymer modified on the HVJ-E surface was developed to significantly and synergistically enhance boron accumulation in tumor cells while providing immunotherapeutic effects[108].
Shaohui Deng et al. developed a PD-L1 siRNA-loaded boron nanoparticle by forming phenylboronic ester bonds with the cis-diols of ribose groups at the ends of the siRNA. The nano-system can activate T-cell immunity while also suppressing DNA repair by inhibiting the expression of PD-L1 in tumor cells, resulting in more significant tumor immunogenic cell death. The results showed that the nano-system not only significantly inhibited the progression of primary and metastatic tumors but also demonstrated a strong abscopal effect[91].
Schematic illustration of therapeutic mechanism of DOX-CB@lipo-pDNA-iRGD[93], copyright 2022 Springer Nature.
5.3. Combination with gene therapy
Boron drugs conjugated to nucleotides exhibit favorable bioavailability, and various mature synthetic methods for nucleotide-derived boron drugs have been developed[137]. Besides, the functional properties of nucleic acids can be used to achieve the dual effect of BNCT and gene therapy. For example, boron clusters incorporated in siRNA double strands enhance the resistance of siRNA to degradation without impairing its silencing activity[138]. Similarly, antisense oligonucleotides (ASOs) modified with boron clusters have been designed to regulate gene expression in disease. Researchers have designed a boron cluster-containing ASO that is able to inhibit the expression of EGFR and can be applied in BNCT concurrently[139,140]. Boron clusters have also been shown to increase RNase H activity in ASO silencing strategies, potentially enhancing ASO efficacy[141].
However, the introduction of boron clusters could influence key properties of functional nucleic acids, such as lipid solubility, cell penetration, enzyme resistance, thermal stability, and binding affinity with complementary DNA and RNA. The effect of boron cluster introduction on the oligonucleotide depends on the specific site of incorporation, making this an important consideration in the design of nucleotide-complexed boron drugs.
5.4. Combination with PTT and PDT
The effectiveness of PTT is based on the photoabsorbent, which converts absorbed light energy into heat, thereby inducing thermal ablation of adjacent tumor cells. Research has demonstrated that PTT can enhance blood perfusion within tumor tissues, alleviating hypoxia and consequently augmenting radiosensitivity to a certain extent. And it can facilitate the further enrichment of boron drugs at the tumor site, which is advantageous for BNCT. Therefore, the integration of PTT and BNCT using nanomaterials represents a promising and synergistic therapeutic modality. In recent years, various nanomaterials, including gold nanostructures, carbon-based nanostructures, and copper-sulfur nanostructures, have been studied as photo-absorbers in PTT. Among these, gold nanoparticles garnered significant attention due to their low toxicity, high biocompatibility, and versatility in functional modifications, making them particularly suitable for use in PTT/BNCT co-delivery systems, and they have demonstrated promising results in this context[142,143]. Additionally, boron carbide nanoparticles, which inherently contain boron, have been reported as effective PTT nano-potentiators. These nanostructures enable the delivery of bimodal therapy within a single nanomaterial, thereby enhancing the therapeutic efficacy of combined PTT and BNCT[144].
PDT is a cancer treatment using the biocompatible triple-state photosensitizers (PS) activated by light of a specific wavelength. The excited PS will produce either single-linear oxygen species that lead to direct cellular damage (Type II PDT) or ROS that lead to indirect cell death (Type I PDT). Common photosensitizers used in PDT include BODIPY, porphyrins, dihydroporphyrinols, phthalocyanines, and metal complexes. Specifically, dihydroporphyrinol[145], porphyrin[146], and Ru(II) or Ir(III) polypyridyl complexes[147]have been explored as photosensitizers in combination with boron drugs to create a combined therapeutic nano-delivery system for PDT and BNCT. For instance, a boronated porphyrin, Octa-anionic 5,10,15,20-tetra[3,5-(nido-carboranylmethyl)phenyl] porphyrin (H2OCP), which incorporates eight boron clusters on a porphyrin ring, has been designed. H2OCP accumulates intracellularly to a greater extent than BPA and BSH, making it a superior delivery system for BNCT. Moreover, it is a highly efficient photosensitizer with cytotoxic effects upon photoactivation[148].
5.5. Theranostic boron drugs
The timing of neutron irradiation is determined by the ratios of boron concentration (T/B and T/N), which are typically estimated by measuring boron levels in the blood. However, this method is often inaccurate due to individual heterogeneity. Therefore, researchers have designed theranostic boron drugs that can accurately determine boron concentration, enabling the performance of neutron irradiation within the optimal time window. Currently, fluorescence, MRI, and PET imaging are the most commonly used methods.
Strategies for visualizing fluorescence range from using autofluorescent materials as boron drug carriers[87,101,145,149] to grafting fluorescent chemical groups, such as Cy3, Cy5, and Cy7, onto boron carriers for imaging[150,151]. Fluorescent materials used as boron drug carriers include boron carbon oxynitride (BNCO), fluorescent gold nanoclusters, zinc gallate-based (ZnGa2O4:Cr3+), and dihydroporphyrin, etc. BNCO is an environmentally friendly phosphor which is non-toxic and non-rare-earth. Its photoluminescent properties make it suitable for imaging. Fluorescent gold nanoclusters (Au NCs) have emerged as an alternative to traditional fluorescent materials in biosensing, imaging, and optoelectronic display[152]. Persistent luminescent nanoparticles (PLNPs) based on zinc gallate groups (ZnGa2O4:Cr3+) have significant potential for bioimaging due to their ability to eliminate tissue autofluorescence and improve the signal-to-noise ratio. They can exhibit long-lasting phosphorescence in the near-infrared, which is suitable for in vivo bioimaging[153]. However, optical imaging is less applicable and is only used in animal studies. In the clinic, PET or MRI imaging is often used.
MRI is a widely used clinical imaging modality that has desirable intrinsic contrast in low-density tissue and better high spatial resolution. Unlike PET, which requires a radioactive tracer, MRI does not rely on ionizing or radioactive tracers, which can cause radioactive damage to tissues. MRI contrast agents, such as magnetic nanoparticles, can help distinguish between healthy tissues and lesions. In MRI, the two primary types of contrast agents are ferrous-containing magnetic materials and gadolinium (Gd) chelates. Among these, ferromagnetic nanoparticles have emerged as a research hotspot. Specifically, various ferromagnetic nanoparticles have been explored for the MRI localization of boron content, including manganese ferrite nanoparticles[143], iron oxide nanoparticles[64,154,155], and iron-boron composite nanoparticles[156]. Gd conjugate is a commonly used MRI contrast agent. Tissues with higher Gd uptake produce better image contrast in T1-weighted imaging. Meanwhile, 157Gd, similar to 10B, has a high thermal neutron capture cross-section and is a promising candidate for neutron capture therapy. A study was conducted on Gd atoms and boron cages co-encapsulated with gold nanoparticles in PLGA nanoparticles to visually examine the in vivo pharmacokinetics and tumor distribution. The nano-systems were found to be enriched at the tumor site, with a T/N ratio of 4.17[157]. In addition, MRI imaging has been performed with Gd-DTPA grafted into the mesopores of mesoporous silica nanoparticles[78] or covered with silica coating[94]. Our team recently assembled Gd-DOTA and BPA-F into lipid nanocarriers, which enabled MRI visualization capability[158].
PET is more sensitive than MRI[159], but it has the disadvantage of requiring radiotracers for visualization, such as 64Cu and 18F. 64Cu is frequently bound to boron nitride, polymer, gold, or boronated porphyrin nanoparticles[48,122,142,146,160] to be delivered to the tumor site along with boron, while 18F is chelated onto BPA for PET imaging[161]. The visualization of boron amounts in nano-systems has a useful role in providing delivery systems for BNCT and alternative diagnostic techniques that could facilitate future clinical trials.
Jae Hun Ahn et al. synthesized a boronic-acid-conjugated porphyrin and radiolabeled it with 64Cu, which has been proven to be a helpful boron-loaded theranostic agent for precise tumor imaging and BNCT[162]. Zhibo Liu et al. designed a bis-boron amino acid (BBPA) by replacing the carboxyl group of BPA with a trifluoroborate group (-BF3-). Radiological imaging is achieved through 18F-19F isotopic exchange at the B-F bond, allowing BBPA to accurately reflect the boron drug's in vivo distribution using PET imaging. Moreover, the additional boron atom introduced into the molecular structure of BBPA enables it to deliver twice the amount of boron compared to BPA[163].
MRIs do not rely on the radioactive tracers, which are essential for PET-CT. But PET-CT is more sensitive than MRI. And in the application of BNCT visualization, the chemical bonding between 18F and BPA is tighter than the physical encapsulation of Gd or Fe with boron drugs, reflecting a closer relationship between contrast agent concentration and boron drug concentration. However, MRI is more accessible to patients and can be performed multiple times, allowing for more convenient real-time monitoring of boron drug accumulation in tumors.
The primary objective of the versatile boron drug delivery systems in BNCT is to facilitate combination therapy. While the majority of the studies reviewed above have demonstrated significant and effective outcomes, the true realization of combination therapy hinges on the widespread and mature clinical application of BNCT in the future. Despite showing promising tumor clearance in laboratory settings, the clinical scenario is considerably more complex. Therefore, there remains substantial scope for further research into the combination of BNCT with other therapeutic modalities.
Interdisciplinary cooperation is a crucial direction for the future development of BNCT. In the clinic, tumor treatment often requires a combination of multiple treatment methods tailored to the patient's specific condition. A variety of pre-clinical investigations have shown that interdisciplinary cooperation can enhance the anti-tumor effect. However, its clinical translation remains significantly hindered. On the one hand, BNCT itself is still restricted to early-phase clinical trials. On the other hand, the precise control of individual drug doses within a shared nano-platform remains technically challenging, and the safety remains to be defined. However, since it is a trend, the future will likely emerge with simpler, more standardized, and more definitive nano-delivery systems in BNCT.
Summary of multifunctional boron nano-delivery systems
| Multifunction | Function element | Drug Carrier | Boron Drug | Caner Species | T/B | T/N | Ref. |
|---|---|---|---|---|---|---|---|
| Chemotherapy combination | DOX | Boron nitride | Boron nitride | Breast cancer | 2.4/24 h | / | [149] |
| Liposomes | Carborane | Glioma | / | / | [151] | ||
| Polymers | PBA | Pancreatic cancer | 4.1/6 h | 6.7/6 h | [150] | ||
| PARP inhibitors | Liposomes | Carborane | Breast cancer | 4/12 h | 37/12 h | [97] | |
| Endostar | Polymers | BSH | Lung cancer | 14.11/24 h | 19.49/24 h | [48] | |
| Curcumin | Polymers | Boric acid | Ovarian cancer | / | / | [83] | |
| Methotrexate | Chitosan nanoparticles | BPA | Glioma | / | / | [89] | |
| PTX | Chitosan nanoparticles | Carborane | Liver cancer | 4.38/12 h | / | [166] | |
| Immunotherapy combination | anti-PD-L1 | Nanomicelles | PBA | Melanoma | 6.56/24 h | 8.2/24 h | [157] |
| PD-L1 siRNA | Copolymers | Benzoborazole pendant | Breast cancer | 5.2/24 h | 18.1/24 h | [94] | |
| CD47 | Liposomes | Carborane | Glioma | / | / | [78] | |
| PTT and PDT combination | PTT with boron carbide | Boron carbide | Boron carbide | Colon cancer | 4.4/48 h | / | [64] |
| PTT with AuNPs | AuNPs | Carborane derivatives | Gastric cancer | / | 5.4/24 h | [158] | |
| PDT with chlorin | Iron nanoparticles | Bis (dicarbollide) | Glioblastoma | / | / | [65] | |
| Fluorescence visualization | BCNO | BCNO | Boric acid | Astrocytoma | / | / | [143] |
| BCNO | Boric acid | Breast cancer | 3.5/24 h | / | [156] | ||
| ZnGa2O4 | Silica nanoparticles | Boric acid | Fibrosarcoma; melanoma | / | / | [160] | |
| Fluorescent gold nanoclusters | AuNPs | Carborane derivatives | Cervical cancer | / | / | [142] | |
| Cy3 | Silica nanoparticles | Carborane | Hepatic cancer | / | / | [103] | |
| Cy7/Cy5 | AuNPs | Dodecaborate | Glioma | / | / | [146] | |
| aza-BODIPYs | Nanogels | BSH | Glioma | / | 6/48 h in muscle; 2/48 h in brain | [95] | |
| Boronated porphyrin; PET visualization with 64Cu | Polymers | Boronated porphyrin | Melanoma; breast cancer | 2.5/24 h | 6.54/24 h | [73] | |
| CT visualization | CT/MRI visualization with GdB6 | GdB6 | Boric acid | Head and neck cancer | 4.18/24 h | / | [100] |
| AuNPs | AuNPs | BSH | Glioma | >50/6 h | >400/6 h | [83] | |
| AuNPs | Carborane | Osteosarcoma | / | / | [149] | ||
| MRI visualization | Gd | AuNPs | Thiol Boron cage-SH | Gastric cancer | / | 4.17/24 h | [151] |
| Silica nanoparticles | Boron | Astrocytoma | 2.8/24 h | 1.64/24 h in brain | [150] | ||
| Silica nanoparticles | BSH | Chondrosarcomas | / | / | [97] | ||
| Iron nanoparticles | Boric acid | Pancreatic cancer; cervical cancer; lung cancer | / | / | [48] | ||
| Liposomes | BPA-F | Glioblastoma | 4.29/6 h | 7.82/6 h | [83] | ||
| Polymers | Boric acid | Ovarian cancer | / | / | [89] | ||
| MnFe2O4 | Iron nanoparticles | Boric acid | Glioma | / | / | [166] | |
| Fe-B | Iron nanoparticles | Fe-B | Breast cancer; melanoma | / | / | [157] | |
| PET visualization | 64Cu | Boron nitride | Boron nitride | / | / | / | [94] |
| AuNPs | Carborane derivatives | Gastric cancer | / | 5.4/24 h | [78] | ||
| Liposomes | Carborane | Breast cancer | 4/12 h | 37/12 h | [64] | ||
| Copolymers | Carborane | Breast cancer | 7.46/24 h | 25.2/24 h | [158] | ||
| 18F | Polymers | Boronotryptophan derivatives | Glioma | 170/50 min in U87; 8/1 h in LN229 | [65] | ||
| SPECT visualization | I-123 | AuNPs | Boron cage-SH | Gastric cancer | / | 41.05/12 h | [143] |
| Chemodynamic therapy combination | Fe3+ (Fenton translation) | Boron nitride | Boron nitride | Breast cancer | 5.5/12 h | 41.5/12 h | [156] |
| GdNCT | Gd | GdB6 | Boric acid | Head and neck cancer | 4.18/24 h | / | [160] |
6. Future Perspectives of BNCT
Most studies use combinations of off-the-shelf boron drugs and nanostructures in BNCT. However, the drug delivery bottleneck of BNCT has not been solved. To successfully promote BNCT for clinical use, collaboration among doctors, physicists, biologists, chemists, material scientists, and pharmacists is necessary.
From a physics perspective, a stable neutron source and the methods to control and determine the radiation dose are necessary. From a chemistry perspective, research is needed to develop a new structure for the boron drug and to modify the boron drug group to establish a chemical foundation. From a materials science perspective, materials that exhibit high biocompatibility, low toxicity, and high boron loading with tumor-affinity are desirable. From a pharmacist's perspective, clear pharmacokinetic studies of boron drugs, nanoparticles, and novel drug formulations or drug delivery systems are needed. From a biological perspective, exploring the biological pathways and mechanisms associated with BNCT is crucial for the subsequent development of this treatment, including its radiosensitivity, death pathways, changes in the immune environment, and abscopal effects.
The structures, nano-systems, and delivery strategies of boron drugs have been discussed above. However, the biological mechanisms of BNCT have not been thoroughly explored, which is beneficial for the future development of boron drug delivery systems. This section will primarily elucidate the incomplete mechanisms of BNCT, providing insights for the design of boron delivery systems (Figure 6).
6.1. Cell cycle
The cell cycle can influence the radiosensitivity of cells, with the general order being G2/M > G1 > S[167]. This is related to the differences in DNA content and the DNA damage repair systems. Cells in the G2 phase have twice the DNA content of those in the G1 phase, which increases the initial yield of DNA double-strand breaks (DSBs) and, consequently, the probability of cell death. Additionally, the more accurate homologous recombination (HR) repair in the S phase reduces the radiosensitivity of cells during this phase.
The relationship between BNCT and the cell cycle can be summarized in two aspects:
1. BNCT generates high-LET radiation, which is more likely to arrest the cell cycle in the S or G2 phase compared to low-LET radiation. Studies have shown that BNCT can damage glioma stem/progenitor cells (GSPCs) that are resistant to X-rays and γ-rays, inducing cell cycle arrest at the G2/M phase and subsequently triggering apoptosis via the mitochondrial pathway[168].
Summary of biological mechanisms underlying BNCT. A. The relationship of the cell cycle and radiosensitivity of BNCT. Checkpoint block will increase the uptake of boron and radiosensitivity (a). BNCT arrests the cell cycle at the G2/M to reduce the heterogeneity (b). B. Death mechanism of radiotherapy. Radiotherapy can induce indirect and direct damage to DNA. C. Tumor microenvironment influences the efficiency of radiotherapy. Anti-hypoxia (a) and anti-abnormal angiogenesis (b) can enhance the uptake of boron drugs and stimulate the immune response after radiotherapy. D. The immune response caused by BNCT. Immune checkpoint inhibitors will likely increase the immune response of BNCT (a); Immunologic adjuvants can increase the abscopal effect (b); The RT-EVs from BNCT with more damaged DNAs have greater anti-tumor immune response (c).
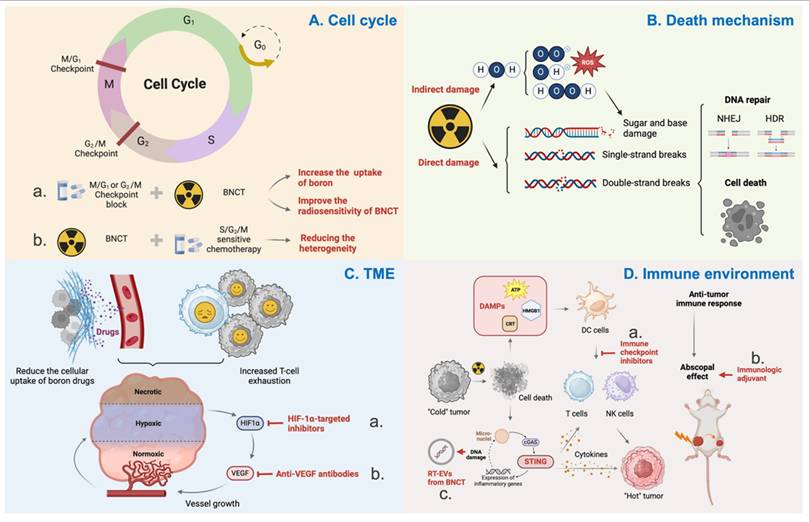
2. The cell cycle also affects the uptake of boron by cells. Cells in the S/G2/M phases can take up more BPA than those in the G0/G1/S phases, and this difference becomes more pronounced with increasing BPA concentration. Research suggests that this phenomenon may be related to L-type amino acid transporters[169].
Considering the heterogeneity of tumor cells and the aforementioned theoretical basis, combining cell cycle-related chemotherapeutic agents to arrest cells in the S/G2/M phases can enhance the uptake of boron drugs and radiosensitivity in tumor cells[170]. Additionally, balancing the uptake of boron drugs across different cell cycle phases can also homogenize the cytotoxic effects of BNCT at the cellular level. Studies have already demonstrated that PVA-BPA can reduce the cell cycle dependency of boron uptake[171].
6.2. Cell death mechanism
Radiotherapy kills cancer cells by damaging DNA, including ionization-induced sugar and base damage, single-strand breaks (SSBs), and DSBs[172]. In addition to the direct effects on DNA, charged particles can ionize water molecules surrounding DNA, generating highly reactive free radicals that cause indirect damage. For high-LET radiation, such as α particles and Li particles, direct damage predominates. In contrast, for low-LET radiation, the ratio of indirect to direct effects is approximately 3:1. Upon sensing DNA damage, cells initiate the DNA damage response (DDR) to maintain genomic integrity. The DDR is a complex network composed of multiple signaling pathways, ultimately inducing cell cycle arrest, DNA repair, and apoptosis[173,174]. The complex mixed radiation field is a characteristic of BNCT, which currently obscures the specific molecular pathways of tumor cell killing, the modes of tumor cell death, and the DNA repair patterns. Further exploration will help identify BNCT-specific radiosensitizers and, through the exploration of molecular pathways, uncover potential biological targets that may elevate the therapeutic efficacy of BNCT to a higher level.
6.3. Tumor microenvironment (TME)
Owing to the high oxygen consumption of tumor cells and the dysfunctional interstitial microvasculature, hypoxia is a common feature of the tumor microenvironment[175]. This hypoxic environment significantly limits the efficacy of conventional radiotherapy. BNCT-generated high-LET particle beams with low OER are less affected by hypoxia, thereby offering the advantage of overcoming the hypoxic conditions. However, it is regrettable that chronic hypoxia can reduce the cellular uptake of boron drugs and thus affect the efficacy of BNCT[176,177]. Moreover, hypoxia is often associated with increased T-cell exhaustion, which in turn suppresses the tumor immune response following BNCT. Studies have confirmed that hypoxia-inducible factor 1α (HIF-1α) can inhibit the expression of LAT-1 in hypoxic cells, thereby affecting the uptake of BPA[177]. BNCT's antitumor effects can be sensitized following treatment with HIF-1α-targeted inhibitors[178]. Therefore, overcoming hypoxia is also an excellent means of enhancing the efficacy of BNCT.
Hypoxia can induce the formation of abnormal blood vessels, and these malformed neo vessels, in turn, exacerbate hypoxia in TME. Additionally, the abnormal vascular morphology and reduced vascular density can decrease the perfusion and accumulation of boron drugs in tumors. Therefore, remodeling the tumor vasculature is a useful strategy to significantly improve the efficacy of BNCT. Currently, strategies for normalizing tumor vasculature mainly focus on blocking the expression of angiogenic factors. Bevacizumab, an anti-VEGF antibody, can normalize tumor blood perfusion and oxygenation during radiation therapy. It can also prevent radiation necrosis (RN) and symptomatic pseudoprogression (psPD) after reirradiation[179]. Additionally, some researchers have utilized vascular-targeting drugs to disrupt the abnormal vascular network, thereby facilitating the passive diffusion of boron drugs to quiescent cells and enhancing the effects of BNCT. Increased infiltration of immune cells into tumor tissues has also been observed following treatment[180].
6.4. Tumor immune environment
Firstly, radiotherapy can promote immunogenic cell death, releasing damage-associated molecular patterns (DAMPs), which facilitate the priming and activation of immune cells. Secondly, activation of the STING inflammatory signaling pathway in cancer cells leads to the release of pro-inflammatory cytokines, resulting in the remodeling of the inflammatory microenvironment and the induction of the abscopal effect. Thirdly, the cytokines, stromal, immune, and vascular changes induced by radiotherapy reshape the tumor microenvironment, transforming "cold" tumors with limited immune cell infiltration into "hot" tumors infiltrated by lymphocytes. However, radiation therapy can also induce immune suppression by increasing inhibitory immune cells, including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), as well as the production of immunosuppressive cytokines[181,182]. Nevertheless, the relationship between radiotherapy and the immune system remains under continuous exploration, and the complex radiation sources of BNCT differ significantly from those of conventional radiotherapy, making its impact on the immune system largely unexplored[175].
In the author's view, exploring the relationship between the immune environment and radiotherapy will promote the future development of BNCT in two key aspects: Firstly, immune checkpoint blockade (ICB) therapy is currently the most promising approach to immunotherapy. Preclinical and clinical evidence suggest that immune checkpoint inhibitors (ICIs) can be combined with radiotherapy to enhance therapeutic efficacy. The co-administration of ICIs and boron drugs targeting tumors may reduce immune-related adverse events in clinical practice, while potentially igniting the spark of an immune attack on tumor cells following BNCT. Secondly, since the abscopal effect was first described in 1953, it has been considered a promising frontier in cancer therapy, particularly for metastatic disease. The abscopal effect is primarily mediated through radiation-induced immunogenic cell death, which releases DAMPs and activates the immune system[183]. Studies have already observed the abscopal effect in xenograft mice when combining BNCT with immunotherapy[91,108,184,185]. The high-LET radiation generated by BNCT mainly induces direct DNA damage, with double-strand DNA damage activating innate immune signaling pathways. This suggests that BNCT has the potential to contribute to an abscopal effect. The integration of immunotherapy with advanced radiation techniques such as BNCT holds the potential to revolutionize treatment strategies.
Additionally, BNCT can also help with immunotherapy. The high-LET properties of alpha particles from BNCT can cause more DNA breaks and DNA oxidation, which resist nuclease degradation and accumulate in radiated tumor cell-derived extracellular vesicles (RT-EVs). It enhances antigen presentation, migration capacity, and cytokine secretion after being internalized by dendritic cells (DCs). These EV-educated DCs not only effectively inhibited primary tumor growth and metastasis formation but also elicited long-term immune memory[186].
7. Conclusion
The authors contend that cost-effective boron delivery systems, characterized by their simple structure and high efficacy, are likely to hold a dominant position as BNCT progressively transitions into clinical practice. Small-molecule boron drugs may well carve out a bright future in this context. However, with the in-depth exploration of BNCT mechanisms, there is a significant potential for developing BNCT combination therapies that achieve synergistic effects far greater than the sum of their individual contributions. Therefore, boron nano-systems retain an indispensable value in this regard. Additionally, innovative delivery strategies that can effectively and simply address the bottlenecks encountered by delivery structures from an alternative perspective are essential and also need more brainpower.
Abbreviations
Boron neutron capture therapy (BNCT); Stereotactic body radiotherapy (SBRT); Linear energy transfer (LET); Relative biological effectiveness (RBE); Oxygen enhancement ratio (OER); Boronophenylalanine (BPA); Sodium borocaptate (BSH); Glioblastoma multiforme (GBM); Photodynamic therapy (PDT); Enhanced permeability and retention (EPR); Salivary acid (SA); Phenylboronic acid (PBA); BPA-fructose (BPA-f); Folate receptor (FR); Asialoglycoprotein receptors (ASGP-Rs); Hyaluronic acid (HA); Antibody-drug conjugate (ADC); Peptide-drug conjugate (PDC); Cell-penetrating peptides (CPPs); Cell-targeting peptides (CTPs); Reactive oxygen species (ROS); Glutathione (GSH); Near-infrared light (NIR); Hyaluronidase (HAase); Boron carbon oxynitride (BCNO); Gold nanoparticles (AuNPs); Boron-containing liposome (boronsome); Lipoic acid-boronophenylalanine (LA-BPA); Extracellular vesicle (EV); Boron nitride nanoparticle (BN); Doxorubicin (DOX); Cancer cell membrane (CCM); Blood-brain barrier (BBB); Blood-tumor barrier (BTB); Focused ultrasound (FUS); Trans-cyclooctene (TCO); Tetrazine (tz); Boron quantum dots (BQD); Phase-transformation lysozyme (PTL); Boron carbide nanoparticles (BNNPs); Polyvinyl alcohol (PVA); Nuclear localization signal (NLS); Intra-arterial (IA); Intravenous (IV); Complete response (CR); Partial response (PR); Progressive disease (PD); Photodynamic therapy (PDT); Photothermal therapy (PTT); Paclitaxel (PTX); Interleukin (IL); Antisense oligonucleotides (ASOs); Photosensitizers (PS); DNA double-strand breaks (DSBs); Homologous recombination (HR); Single-strand breaks (SSBs); DNA damage response (DDR); Tumor microenvironment (TME); Hypoxia-inducible factor 1α (HIF-1α); Radiation necrosis (RN); Symptomatic pseudoprogression (psPD); Damage-associated molecular patterns (DAMPs); Myeloid-derived suppressor cells (MDSCs); Immune checkpoint blockade (ICB); Immune checkpoint inhibitors (ICIs); Radiated tumor cell-derived extracellular vesicles (RT-EVs); Dendritic cells (DCs).
Acknowledgements
All original images in this article were created through www.biorender.com. This work was supported by grants from the National Natural Science Foundation of China (No.12405397, 82473234 and 82204283).
Authorship contribution statement
Qiyao Yang: Conceptualization, Writing - original draft, Writing - review & editing. Ruxuan Wang: Writing - original draft, Writing - review & editing. Lingling Huang: Writing - review & editing. Qiong Bian: Supervision, Writing - review & editing. Qichun Wei: Conceptualization, Supervision, Writing - review & editing.
Generative AI statement
Generative AI DeepSeek V3.2 were used for language assistance during writing but not for any content generation.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Mathieu L, Sung H, Ferlay J, Siegel R, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2024 74
2. Macia I Garau M. Radiobiology of stereotactic body radiation therapy (SBRT). Reports of Practical Oncology and Radiotherapy : Journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology. 2017 22
3. Matsumoto Y, Fukumitsu N, Ishikawa H, Nakai K, Sakurai H. A Critical Review of Radiation Therapy: From Particle Beam Therapy (Proton, Carbon, and BNCT) to Beyond. Journal of Personalized Medicine. 2021 11
4. Gray L, Green F, Hawes C. Effect of nitric oxide on the radiosensitivity of tumour cells. Nature. 1958 182
5. Koichi A, Yuki K. Biological characteristics of carbon-ion therapy. International Journal of Radiation Biology. 2009 85
6. Hirayama R, Ito A, Tomita M, Tsukada T, Yatagai F, Noguchi M. et al. Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiation Research. 2009 171
7. Aoki-Nakano M, Furusawa Y. Misrepair of DNA double-strand breaks after exposure to heavy-ion beams causes a peak in the LET-RBE relationship with respect to cell killing in DT40 cells. Journal of Radiation Research. 2013 54
8. Wenzl T, Wilkens Jj. Theoretical analysis of the dose dependence of the oxygen enhancement ratio and its relevance for clinical applications. Radiation Oncology (London, England). 2011 6
9. Furusawa Y, Fukutsu K, Aoki M, Itsukaichi H, Eguchi-Kasai K, Ohara H. et al. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He-, (12)C- and (20)Ne-ion beams. Radiation Research. 2000 154
10. Wang J, Wei C, Tucker Sl, Myles B, Palmer M, Hofstetter Wl. et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. International Journal of Radiation Oncology, Biology, Physics. 2013 86
11. Wang X, Palaskas Nl, Yusuf Sw, Abe Ji, Lopez-Mattei J, Banchs J. et al. Incidence and Onset of Severe Cardiac Events After Radiotherapy for Esophageal Cancer. Journal of Thoracic Oncology : Official Publication of the International Association for the Study of Lung Cancer. 2020 15
12. Xi M, Xu C, Liao Z, Chang Jy, Gomez Dr, Jeter M. et al. Comparative Outcomes After Definitive Chemoradiotherapy Using Proton Beam Therapy Versus Intensity Modulated Radiation Therapy for Esophageal Cancer: A Retrospective, Single-Institutional Analysis. International Journal of Radiation Oncology, Biology, Physics. 2017 99
13. Taylor HJ, Goldhaber M. Detection of Nuclear Disintegration in a Photographic Emulsion. Nature. 1935;135:341-341
14. Locher L. Biological effects and therapeutic possibilities of neutrons. American Journal of Roentgenology. 1936;36:1-13
15. Suzuki M. Boron neutron capture therapy (BNCT): a unique role in radiotherapy with a view to entering the accelerator-based BNCT era. International Journal of Clinical Oncology. 2020 25
16. Cong Y, Abulimiti M, Matsumoto Y, Jin J. Current research trends and hotspots of boron neutron capture therapy: a bibliometric and visualization analysis. Frontiers in Oncology. 2024 14
17. Yang R, Shi M, Zhang Y, Ma H, Chen X, Xu X. Achievements, challenges, and perspectives in the design of boron delivery agents for neutron capture therapy. Coordination Chemistry Reviews. 2026;553:217555
18. Li X, Liu Z, Liu K, Wang C, Liu Z, Xu X. Advances in Clinical Trials of Boron Neutron Capture Therapy. Research (Wash D C). 2026;9:0988
19. Miyaguchi J, Shiga K, Katagiri K, Saito D, Tsuchida K, Kusaka T. et al. Efficacy of boron neutron capture therapy (BNCT) for patients with oral cancer. Oral Oncol. 2025;163:107228
20. Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J. et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. International Journal of Radiation Oncology, Biology, Physics. 2012 82
21. Wang Lw, Chen Yw, Ho Cy, Hsueh Liu Yw, Chou Fi, Liu Yh. et al. Fractionated Boron Neutron Capture Therapy in Locally Recurrent Head and Neck Cancer: A Prospective Phase I/II Trial. International Journal of Radiation Oncology, Biology, Physics. 2016 95
22. Kawabata S, Suzuki M, Hirose K, Tanaka H, Kato T, Goto H. et al. Accelerator-based BNCT for patients with recurrent glioblastoma: a multicenter phase II study. Neuro-Oncology Advances. 2021 3
23. Shen S, Wang S, Zhou D, Wu X, Gao M, Wu J. et al. A clinician's perspective on boron neutron capture therapy: promising advances, ongoing trials, and future outlook. International Journal of Radiation Biology. 2024 100
24. Kashihara T, Nakamura S, Yamazaki N, Takahashi A, Namikawa K, Ogata D. et al. Boron neutron capture therapy for cutaneous angiosarcoma and malignant melanoma: First in-human phase I clinical trial. Radiotherapy and Oncology : Journal of the European Society for Therapeutic Radiology and Oncology. 2025 202
25. Kashihara T, Nakamura S, Yamazaki N, Takahashi A, Namikawa K, Ogata D. et al. Boron neutron capture therapy for cutaneous angiosarcoma and malignant melanoma: First in-human phase I clinical trial. Radiother Oncol. 2025;202:110607
26. Nakai K, Kumada H, Matsumoto Y, Baba K, Murakami M, Ishida T. et al. Boron neutron capture therapy (BNCT) phase I clinical trial for newly diagnosed glioblastoma by newly developed accelerator at University of Tsukuba. Appl Radiat Isot. 2025;226:112152
27. Soloway Ah, Hatanaka H, Davis Ma. Penetration of brain and brain tumor. VII. Tumor-binding sulfhydryl boron compounds. Journal of Medicinal Chemistry. 1967 10
28. Hatanaka H, Nakagawa Y. Clinical results of long-surviving brain tumor patients who underwent boron neutron capture therapy. International Journal of Radiation Oncology, Biology, Physics. 1994 28
29. Hideghéty K, Sauerwein W, Wittig A, Götz C, Paquis P, Grochulla F. et al. Tissue uptake of BSH in patients with glioblastoma in the EORTC 11961 phase I BNCT trial. Journal of Neuro-Oncology. 2003 62
30. Snyder HR, Reedy AJ, Lennarz WmJ. Synthesis of Aromatic Boronic Acids. Aldehydo Boronic Acids and a Boronic Acid Analog of Tyrosine1. Journal of the American Chemical Society. 1958 80
31. Mishima Y, Ichihashi M, Hatta S, Honda C, Yamamura K, Nakagawa T. New thermal neutron capture therapy for malignant melanoma: melanogenesis-seeking 10B molecule-melanoma cell interaction from in vitro to first clinical trial. Pigment Cell Research. 1989 2
32. Fukuda H, Honda C, Wadabayashi N, Kobayashi T, Yoshino K, Hiratsuka J. et al. Pharmacokinetics of 10B-p-boronophenylalanine in tumours, skin and blood of melanoma patients: a study of boron neutron capture therapy for malignant melanoma. Melanoma Research. 1999 9
33. Wongthai P, Hagiwara K, Miyoshi Y, Wiriyasermkul P, Wei L, Ohgaki R. et al. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Science. 2015;106:279-286
34. Hoppenz P, Els-Heindl S, Beck-Sickinger Ag. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Frontiers in Chemistry. 2020 8
35. Scholz M, Hey-Hawkins E. Carbaboranes as pharmacophores: properties, synthesis, and application strategies. Chemical Reviews. 2011 111
36. Chen Y, Du F, Tang L, Xu J, Zhao Y, Li M. et al. Carboranes as unique pharmacophores in antitumor medicinal chemistry. Molecular Therapy Oncolytics. 2022 24
37. Li R, Zhang J, Guo J, Xu Y, Duan K, Zheng J. et al. Application of Nitroimidazole-Carbobane-Modified Phenylalanine Derivatives as Dual-Target Boron Carriers in Boron Neutron Capture Therapy. Molecular Pharmaceutics. 2020 17
38. Gazvoda M, Dhanjee Hh, Rodriguez J, Brown Js, Farquhar Ce, Truex Nl. et al. Palladium-Mediated Incorporation of Carboranes into Small Molecules, Peptides, and Proteins. Journal of the American Chemical Society. 2022 144
39. Zhou Y-T, Cheng K, Liu B, Cao Y-C, Fan J-X, Liu Z-G. et al. Recent progress of nano-drugs in neutron capture therapy. Theranostics. 2024;14:3193-3212
40. Huang D, Sun L, Huang L, Chen Y. Nanodrug Delivery Systems Modulate Tumor Vessels to Increase the Enhanced Permeability and Retention Effect. Journal of Personalized Medicine. 2021 11
41. Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J. et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566-575
42. Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244:108-121
43. Xiong H, Wei X, Zhou D, Qi Y, Xie Z, Chen X. et al. Amphiphilic Polycarbonates from Carborane-Installed Cyclic Carbonates as Potential Agents for Boron Neutron Capture Therapy. Bioconjugate Chemistry. 2016 27
44. Mi P, Yanagie H, Dewi N, Yen H-C, Liu X, Suzuki M. et al. Block copolymer-boron cluster conjugate for effective boron neutron capture therapy of solid tumors. J Control Release. 2017;254:1-9
45. Takeuchi I, Nomura K, Makino K. Hydrophobic boron compound-loaded poly(l-lactide-co-glycolide) nanoparticles for boron neutron capture therapy. Colloids Surf B Biointerfaces. 2017;159:360-365
46. Wu W-C, Wang S-H, Ou S-T, Liu Y-WH, Liu B-H, Tseng F-G. Electrosprayed chitosan/alginate/polyvinyl alcohol nanoparticles as boric acid carriers for 10Boron neutron capture therapy. Nanomedicine (Lond). 2020;15:1067-1077
47. Liu J, Ai X, Zhang H, Zhuo W, Mi P. Polymeric Micelles with Endosome Escape and Redox-Responsive Functions for Enhanced Intracellular Drug Delivery. J Biomed Nanotechnol. 2019;15:373-381
48. Shi Y, Li J, Zhang Z, Duan D, Zhang Z, Liu H. et al. Tracing Boron with Fluorescence and Positron Emission Tomography Imaging of Boronated Porphyrin Nanocomplex for Imaging-Guided Boron Neutron Capture Therapy. ACS Appl Mater Interfaces. 2018;10:43387-43395
49. Yoneoka S, Park KC, Nakagawa Y, Ebara M, Tsukahara T. Synthesis and Evaluation of Thermoresponsive Boron-Containing Poly(N-isopropylacrylamide) Diblock Copolymers for Self-Assembling Nanomicellar Boron Carriers. Polymers (Basel). 2018;11:42
50. Quan H, Fan L, Huang Y, Xia X, He Y, Liu S. et al. Hyaluronic acid-decorated carborane-TAT conjugation nanomicelles: A potential boron agent with enhanced selectivity of tumor cellular uptake. Colloids Surf B Biointerfaces. 2021;204:111826
51. Fithroni AB, Kobayashi K, Uji H, Ishimoto M, Akehi M, Ohtsuki T. et al. Novel Self-Forming Nanosized DDS Particles for BNCT: Utilizing A Hydrophobic Boron Cluster and Its Molecular Glue Effect. Cells. 2022;11:3307
52. Tsygankova AR, Gruzdev DA, Kanygin VV, Ya Guselnikova T. Guselnikova T, Telegina AA, Kasatova AI, et al. Liposomes loaded with lipophilic derivative of closo-carborane as a potential boron delivery system for boron neutron capture therapy of tumors. Mendeleev Communications. 2021;31:659-661
53. Singh A, Kim BK, Mackeyev Y, Rohani P, Mahajan SD, Swihart MT. et al. Boron-Nanoparticle-Loaded Folic-Acid-Functionalized Liposomes to Achieve Optimum Boron Concentration for Boron Neutron Capture Therapy of Cancer. J Biomed Nanotechnol. 2019;15:1714-1723
54. Shtansky DV, Matveev AT, Permyakova ES, Leybo DV, Konopatsky AS, Sorokin PB. Recent Progress in Fabrication and Application of BN Nanostructures and BN-Based Nanohybrids. Nanomaterials (Basel). 2022;12:2810
55. Sun T, Li Y, Huang Y, Zhang Z, Yang W, Du Z. et al. Targeting glioma stem cells enhances anti-tumor effect of boron neutron capture therapy. Oncotarget. 2016;7:43095-43108
56. Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254-266
57. Wittig A, Sauerwein WA, Coderre JA. Mechanisms of transport of p-borono-phenylalanine through the cell membrane in vitro. Radiat Res. 2000;153:173-180
58. Tamanoi F, Chinnathambi S, Laird M, Komatsu A, Birault A, Takata T. et al. Construction of Boronophenylalanine-Loaded Biodegradable Periodic Mesoporous Organosilica Nanoparticles for BNCT Cancer Therapy. Int J Mol Sci. 2021;22:2251
59. Kim A, Suzuki M, Matsumoto Y, Fukumitsu N, Nagasaki Y. Non-isotope enriched phenylboronic acid-decorated dual-functional nano-assembles for an actively targeting BNCT drug. Biomaterials. 2021;268:120551
60. Lu Y, Low PS. Immunotherapy of folate receptor-expressing tumors: review of recent advances and future prospects. Journal of Controlled Release. 2003;91:17-29
61. Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Advanced Drug Delivery Reviews. 2002;54:675-693
62. Achilli C, Grandi S, Ciana A, Guidetti GF, Malara A, Abbonante V. et al. Biocompatibility of functionalized boron phosphate (BPO4) nanoparticles for boron neutron capture therapy (BNCT) application. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:589-597
63. Dai C, Cai F, Hwang KC, Zhou Y, Zhang Z, Liu X. et al. Folate receptor-mediated boron-10 containing carbon nanoparticles as potential delivery vehicles for boron neutron capture therapy of nonfunctional pituitary adenomas. Sci China Life Sci. 2013;56:163-173
64. Icten O, Kose DA, Matissek SJ, Misurelli JA, Elsawa SF, Hosmane NS. et al. Gadolinium borate and iron oxide bioconjugates: Nanocomposites of next generation with multifunctional applications. Materials Science and Engineering: C. 2018;92:317-328
65. Alberti D, Protti N, Franck M, Stefania R, Bortolussi S, Altieri S. et al. Theranostic Nanoparticles Loaded with Imaging Probes and Rubrocurcumin for Combined Cancer Therapy by Folate Receptor Targeting. ChemMedChem. 2017;12:502-509
66. Matović J, Järvinen J, Bland HC, Sokka IK, Imlimthan S, Ferrando RM. et al. Addressing the Biochemical Foundations of a Glucose-Based 'Trojan Horse'-Strategy to Boron Neutron Capture Therapy: From Chemical Synthesis to In Vitro Assessment. Mol Pharm. 2020;17:3885-3899
67. Matović J, Järvinen J, Sokka IK, Imlimthan S, Raitanen J-E, Montaser A. et al. Exploring the Biochemical Foundations of a Successful GLUT1-Targeting Strategy to BNCT: Chemical Synthesis and In Vitro Evaluation of the Entire Positional Isomer Library of ortho-Carboranylmethyl-Bearing Glucoconjugates. Mol Pharm. 2021;18:285-304
68. Lai C-H, Lin Y-C, Chou F-I, Liang C-F, Lin E-W, Chuang Y-J. et al. Design of multivalent galactosyl carborane as a targeting specific agent for potential application to boron neutron capture therapy. Chem Commun (Camb). 2012;48:612-614
69. Yamana K, Kawasaki R, Sanada Y, Tabata A, Bando K, Yoshikawa K. et al. Tumor-targeting hyaluronic acid/fluorescent carborane complex for boron neutron capture therapy. Biochem Biophys Res Commun. 2021;559:210-216
70. Wu G, Yang W, Barth RF, Kawabata S, Swindall M, Bandyopadhyaya AK. et al. Molecular targeting and treatment of an epidermal growth factor receptor-positive glioma using boronated cetuximab. Clin Cancer Res. 2007;13:1260-1268
71. Wu G, Barth RF, Yang W, Chatterjee M, Tjarks W, Ciesielski MJ. et al. Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjug Chem. 2004;15:185-194
72. Barth RF, Wu G, Yang W, Binns PJ, Riley KJ, Patel H. et al. Neutron capture therapy of epidermal growth factor (+) gliomas using boronated cetuximab (IMC-C225) as a delivery agent. Appl Radiat Isot. 2004;61:899-903
73. Wu C-Y, Lin J-J, Chang W-Y, Hsieh C-Y, Wu C-C, Chen H-S. et al. Development of theranostic active-targeting boron-containing gold nanoparticles for boron neutron capture therapy (BNCT). Colloids Surf B Biointerfaces. 2019;183:110387
74. Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM. et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510-520
75. Chen J, Yang Q, Liu M, Lin M, Wang T, Zhang Z. et al. Remarkable Boron Delivery Of iRGD-Modified Polymeric Nanoparticles For Boron Neutron Capture Therapy. Int J Nanomedicine. 2019;14:8161-8177
76. Zhang Y, Li J, Pu K. Recent advances in dual- and multi-responsive nanomedicines for precision cancer therapy. Biomaterials. 2022;291:121906
77. Soleimanbeigi M, Dousti F, Hassanzadeh F, Mirian M, Varshosaz J, Kasesaz Y. et al. Boron phenyl alanine targeted chitosan-PNIPAAm core-shell thermo-responsive nanoparticles: boosting drug delivery to glioblastoma in BNCT. Drug Dev Ind Pharm. 2021;47:1607-1623
78. Vares G, Jallet V, Matsumoto Y, Rentier C, Takayama K, Sasaki T. et al. Functionalized mesoporous silica nanoparticles for innovative boron-neutron capture therapy of resistant cancers. Nanomedicine. 2020;27:102195
79. Ciofani G, Raffa V, Menciassi A, Cuschieri A. Folate Functionalized Boron Nitride Nanotubes and their Selective Uptake by Glioblastoma Multiforme Cells: Implications for their Use as Boron Carriers in Clinical Boron Neutron Capture Therapy. Nanoscale Res Lett. 2008;4:113
80. Yamana K, Kawasaki R, Kondo K, Hirano H, Kawamura S, Sanada Y. et al. HER-2-targeted boron neutron capture therapy using an antibody-conjugated boron nitride nanotube/β-1,3-glucan complex. Nanoscale Adv. 2023;5:3857-3861
81. Kawasaki R, Oshige A, Yamana K, Hirano H, Nishimura K, Miura Y. et al. HER-2-Targeted Boron Neutron Capture Therapy with Carborane-integrated Immunoliposomes Prepared via an Exchanging Reaction. Chemistry. 2023;29:e202302486
82. Kuthala N, Shanmugam M, Yao C-L, Chiang C-S, Hwang KC. One step synthesis of 10B-enriched 10BPO4 nanoparticles for effective boron neutron capture therapeutic treatment of recurrent head-and-neck tumor. Biomaterials. 2022;290:121861
83. Shanmugam M, Kuthala N, Kong X, Chiang C-S, Hwang KC. Combined Gadolinium and Boron Neutron Capture Therapies for Eradication of Head-and-Neck Tumor Using Gd10B6 Nanoparticles under MRI/CT Image Guidance. JACS Au. 2023;3:2192-2205
84. Feng B, Tomizawa K, Michiue H, Miyatake S, Han X-J, Fujimura A. et al. Delivery of sodium borocaptate to glioma cells using immunoliposome conjugated with anti-EGFR antibodies by ZZ-His. Biomaterials. 2009;30:1746-1755
85. Pan X, Wu G, Yang W, Barth RF, Tjarks W, Lee RJ. Synthesis of Cetuximab-Immunoliposomes via a Cholesterol-Based Membrane Anchor for Targeting of EGFR. Bioconjugate Chem. 2007;18:101-108
86. Alamón C, Dávila B, García MF, Nievas S, Dagrosa MA, Thorp S. et al. A Potential Boron Neutron Capture Therapy Agent Selectively Suppresses High-Grade Glioma: In Vitro and in Vivo Exploration. Mol Pharmaceutics. 2023;20:2702-2713
87. Chiang C-W, Chien Y-C, Yu W-J, Ho C-Y, Wang C-Y, Wang T-W. et al. Polymer-Coated Nanoparticles for Therapeutic and Diagnostic Non-10B Enriched Polymer-Coated Boron Carbon Oxynitride (BCNO) Nanoparticles as Potent BNCT Drug. Nanomaterials (Basel). 2021;11:2936
88. Meher N, Seo K, Wang S, Bidkar AP, Fogarty M, Dhrona S. et al. Synthesis and Preliminary Biological Assessment of Carborane-Loaded Theranostic Nanoparticles to Target Prostate-Specific Membrane Antigen. ACS Appl Mater Interfaces. 2021;13:54739-54752
89. Zhang Z, Wang X, Dai Q, Qin Y, Sun X, Suzuki M. et al. Peptide-functionalized gold nanoparticles for boron neutron capture therapy with the potential to use in Glioblastoma treatment. Pharm Dev Technol. 2024;29:862-873
90. Nishikawa M, Yu J, Kang HG, Suzuki M, Komatsu N. Rational Design, Multistep Synthesis and in Vitro Evaluation of Poly(glycerol) Functionalized Nanodiamond Conjugated with Boron-10 Cluster and Active Targeting Moiety for Boron Neutron Capture Therapy. Chemistry - A European Journal. 2023;29:e202302073
91. Deng S, Hu L, Chen G, Ye J, Xiao Z, Guan T. et al. A PD-L1 siRNA-Loaded Boron Nanoparticle for Targeted Cancer Radiotherapy and Immunotherapy. Advanced Materials. 2025;37:2419418
92. Yang Q, Dai Q, Bao X, Zhou Y, Lu Y, Zhong H. et al. Evaluation of a Tumor-Targeting Oligosaccharide Nanosystem in BNCT on an Orthotopic Hepatocellular Carcinoma Model. Mol Pharm. 2023;20:1025-1038
93. Chen J, Dai Q, Yang Q, Bao X, Zhou Y, Zhong H. et al. Therapeutic nucleus-access BNCT drug combined CD47-targeting gene editing in glioblastoma. J Nanobiotechnol. 2022;20:102
94. Kuthala N, Vankayala R, Li Y-N, Chiang C-S, Hwang KC. Engineering Novel Targeted Boron-10-Enriched Theranostic Nanomedicine to Combat against Murine Brain Tumors via MR Imaging-Guided Boron Neutron Capture Therapy. Adv Mater. 2017 29
95. Chen X-P, Hsu F-C, Huang K-Y, Hsieh T-S, Farn S-S, Sheu R-J. et al. Fluorine-18 labeling PEGylated 6-boronotryptophan for PET scanning of mice for assessing the pharmacokinetics for boron neutron capture therapy of brain tumors. Bioorg Med Chem Lett. 2024;105:129744
96. Tang H, Wang Z, Hao H, Luo W, Yang J, Li M. et al. Boron-Containing Mesoporous Silica Nanoparticles with Effective Delivery and Targeting of Liver Cancer Cells for Boron Neutron Capture Therapy. ACS Appl Mater Interfaces. 2024;16:22934-22945
97. Coninx S, Kalot G, Godard A, Bodio E, Goze C, Sancey L. et al. Tailored hyaluronic acid-based nanogels as theranostic boron delivery systems for boron neutron cancer therapy. International Journal of Pharmaceutics: X. 2022;4:100134
98. Dai L, Liu J, Yang T, Yu X, Lu Y, Pan L. et al. Lipoic acid-boronophenylalanine-derived multifunctional vesicles for cancer chemoradiotherapy. Nat Commun. 2025;16:1329
99. Matsumoto Y, Arase H, Ishiki H, Takeuchi H, Sugawara Y, Taharabaru T. et al. Design and evaluation of a supramolecular boron compound using a cyclodextrin-based polyrotaxane for boron neutron capture therapy. Carbohydrate Polymers. 2025;354:123343
100. Li L, Zhao Q, Chen Z, Zhao Z, Du B, Wang M. et al. Size-Tunable Boron Nanoreactors for Boron Neutron Capture Synergistic Chemodynamic Therapy of Tumor. Adv Healthc Mater. 2025;14:e2402307
101. Sharma KS, Raju M S, Phapale S, Valvi SK, Dubey AK, Goswami D. et al. Multimodal Applications of Zinc Gallate-Based Persistent Luminescent Nanoparticles in Cancer Treatment: Tumor Margining, Diagnosis, and Boron Neutron Capture Therapy. ACS Appl Bio Mater. 2022;5:3134-3145
102. Tokura D, Konarita K, Suzuki M, Ogata K, Honda Y, Miura Y. et al. Active control of pharmacokinetics using light-responsive polymer-drug conjugates for boron neutron capture therapy. J Control Release. 2024;371:445-454
103. Li J, Sun Q, Lu C, Xiao H, Guo Z, Duan D. et al. Boron encapsulated in a liposome can be used for combinational neutron capture therapy. Nat Commun. 2022;13:2143
104. Qin M, Du G, Sun X. Biomimetic cell-derived nanocarriers for modulating immune responses. Biomater Sci. 2020;8:530-543
105. Wang M, Zhao J, Jiang H, Wang X. Tumor-targeted nano-delivery system of therapeutic RNA. Mater Horiz. 2022;9:1111-1140
106. Heide F, McDougall M, Harder-Viddal C, Roshko R, Davidson D, Wu J. et al. Boron rich nanotube drug carrier system is suited for boron neutron capture therapy. Sci Rep. 2021;11:15520
107. Michiue H, Kitamatsu M, Fukunaga A, Tsuboi N, Fujimura A, Matsushita H. et al. Self-assembling A6K peptide nanotubes as a mercaptoundecahydrododecaborate (BSH) delivery system for boron neutron capture therapy (BNCT). Journal of Controlled Release. 2021;330:788-796
108. Yoneoka S, Nakagawa Y, Uto K, Sakura K, Tsukahara T, Ebara M. Boron-incorporating hemagglutinating virus of Japan envelope (HVJ-E) nanomaterial in boron neutron capture therapy. Sci Technol Adv Mater. 2019;20:291-304
109. Hirase S, Aoki A, Hattori Y, Morimoto K, Noguchi K, Fujii I. et al. Dodecaborate-Encapsulated Extracellular Vesicles with Modification of Cell-Penetrating Peptides for Enhancing Macropinocytotic Cellular Uptake and Biological Activity in Boron Neutron Capture Therapy. Mol Pharm. 2022;19:1135-1145
110. Kawasaki R, Oshige A, Kono N, Yamana K, Hirano H, Miura Y. et al. Extracellular Vesicles Comprising Carborane Prepared by a Host Exchanging Reaction as a Boron Carrier for Boron Neutron Capture Therapy. ACS Appl Mater Interfaces. 2024;16:47137-47149
111. He J, Zhang X, Liu L, Wang Y, Liu R, Li M. et al. Acute and Subacute Toxicity Evaluation of Erythrocyte Membrane-Coated Boron Nitride Nanoparticles. J Funct Biomater. 2023;14:181
112. Li H, Qiao W, Shen Y, Xu H, Fan Y, Liu Y. et al. Biomimetic Boron Nitride Nanoparticles for Targeted Drug Delivery and Enhanced Antitumor Activity. Pharmaceutics. 2023;15:1269
113. Zhong T, Yang Y, Pang M, Pan Y, Jing S, Qi Y. et al. Human Serum Albumin-Coated 10B Enriched Carbon Dots as Targeted 'Pilot Light' for Boron Neutron Capture Therapy. Adv Sci (Weinh). 2024;11:e2406577
114. Raskolupova VI, Wang M, Dymova MA, Petrov GO, Shchudlo IM, Taskaev SYu. et al. Design of the New Closo-Dodecarborate-Containing Gemcitabine Analogue for the Albumin-Based Theranostics Composition. Molecules. 2023;28:2672
115. Ye M, Li B, Shi W, Liu H, Wang Y, Chen W. et al. Preparation and tumor-targeting evaluation of BS-CyP albumin nanoparticles modified with hyaluronic acid based on boron neutron capture therapy. J Biomed Mater Res A. 2023;111:1176-1184
116. Monti Hughes A, Goldfinger JA, Palmieri MA, Ramos P, Santa Cruz IS, De Leo L. et al. Boron Neutron Capture Therapy (BNCT) Mediated by Maleimide-Functionalized Closo-Dodecaborate Albumin Conjugates (MID:BSA) for Oral Cancer: Biodistribution Studies and In Vivo BNCT in the Hamster Cheek Pouch Oral Cancer Model. Life (Basel). 2022;12:1082
117. Nicolini A, Ferrari P, Biava PM. Exosomes and Cell Communication: From Tumour-Derived Exosomes and Their Role in Tumour Progression to the Use of Exosomal Cargo for Cancer Treatment. Cancers (Basel). 2021;13:822
118. Raskolupova VI, Wang M, Dymova MA, Petrov GO, Shchudlo IM, Taskaev SY. et al. Design of the New Closo-Dodecarborate-Containing Gemcitabine Analogue for the Albumin-Based Theranostics Composition. Molecules. 2023;28:2672
119. Fan C-H, Wang T-W, Hsieh Y-K, Wang C-F, Gao Z, Kim A. et al. Enhancing Boron Uptake in Brain Glioma by a Boron-Polymer/Microbubble Complex with Focused Ultrasound. ACS Appl Mater Interfaces. 2019;11:11144-11156
120. Feiner IVJ, Pulagam KR, Uribe KB, Passannante R, Simó C, Zamacola K. et al. Pre-targeting with ultra-small nanoparticles: boron carbon dots as drug candidates for boron neutron capture therapy. J Mater Chem B. 2021;9:410-420
121. Li L, Wang M, Zhao Q, Bai P, Hao H, Zhang Z. et al. Intratumoral Transforming Boron Nanosensitizers for Amplified Boron Neutron Capture Therapy. Angew Chem Int Ed Engl. 2025;64:e202413232
122. Li L, Li J, Shi Y, Du P, Zhang Z, Liu T. et al. On-Demand Biodegradable Boron Nitride Nanoparticles for Treating Triple Negative Breast Cancer with Boron Neutron Capture Therapy. ACS Nano. 2019;13:13843-13852
123. Nomoto T, Inoue Y, Yao Y, Suzuki M, Kanamori K, Takemoto H. et al. Poly(vinyl alcohol) boosting therapeutic potential of p-boronophenylalanine in neutron capture therapy by modulating metabolism. Sci Adv. 2020;6:eaaz1722
124. Nomoto T, Yao Y, Inoue Y, Suzuki M, Kanamori K, Takemoto H. et al. Fructose-functionalized polymers to enhance therapeutic potential of p-boronophenylalanine for neutron capture therapy. J Control Release. 2021;332:184-193
125. Konarita K, Kanamori K, Suzuki M, Tokura D, Tanaka S, Honda Y. et al. Poly(vinyl alcohol) potentiating an inert d-amino acid-based drug for boron neutron capture therapy. J Control Release. 2025;377:385-396
126. Gabel D, Foster S, Fairchild RG. The Monte Carlo simulation of the biological effect of the 10B(n, alpha)7Li reaction in cells and tissue and its implication for boron neutron capture therapy. Radiat Res. 1987;111:14-25
127. Chen S, Cao R, Xiang L, Li Z, Chen H, Zhang J. et al. Research progress in nucleus-targeted tumor therapy. Biomater Sci. 2023;11:6436-6456
128. Dozzo P, Koo M-S, Berger S, Forte TM, Kahl SB. Synthesis, Characterization, and Plasma Lipoprotein Association of a Nucleus-Targeted Boronated Porphyrin. J Med Chem. 2005;48:357-359
129. Suzuki M, Sakurai Y, Nagata K, Kinashi Y, Masunaga S, Ono K. et al. Impact of intra-arterial administration of boron compounds on dose-volume histograms in boron neutron capture therapy for recurrent head-and-neck tumors. International Journal of Radiation Oncology, Biology, Physics. 2006 66
130. Fuwa N, Suzuki M, Sakurai Y, Nagata K, Kinashi Y, Masunaga S. et al. Treatment results of boron neutron capture therapy using intra-arterial administration of boron compounds for recurrent head and neck cancer. The British Journal of Radiology. 2008 81
131. Barth Rf, Yang W, Rotaru Jh, Moeschberger Ml, Boesel Cp, Soloway Ah. et al. Boron neutron capture therapy of brain tumors: enhanced survival and cure following blood-brain barrier disruption and intracarotid injection of sodium borocaptate and boronophenylalanine. International Journal of Radiation Oncology, Biology, Physics. 2000 47
132. Yanagie H, Kumada H, Nakamura T, Higashi S, Ikushima I, Morishita Y. et al. Feasibility evaluation of neutron capture therapy for hepatocellular carcinoma using selective enhancement of boron accumulation in tumour with intra-arterial administration of boron-entrapped water-in-oil-in-water emulsion. Applied Radiation and Isotopes : Including Data, Instrumentation and Methods for Use in Agriculture, Industry and Medicine. 2011 69
133. Yanagie H, Dewi N, Higashi S, Ikushima I, Seguchi K, Mizumachi R. et al. Selective boron delivery by intra-arterial injection of BSH-WOW emulsion in hepatic cancer model for neutron capture therapy. The British Journal of Radiology. 2017 90
134. Bitar Gg, Persad M, Dragan A, Alade A, Jiménez-Labaig P, Johnston E. et al. Ultrasound-guided intra-tumoral administration of directly-injected therapies: a review of the technical and logistical considerations. Cancer Imaging : The Official Publication of the International Cancer Imaging Society. 2024 24
135. Melero I, Castanon E, Alvarez M, Champiat S, Marabelle A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat Rev Clin Oncol. 2021;18:558-576
136. Li L, Dai K, Li J, Shi Y, Zhang Z, Liu T. et al. A Boron-10 nitride nanosheet for combinational boron neutron capture therapy and chemotherapy of tumor. Biomaterials. 2021;268:120587
137. Ebenryter-Olbińska K, Kaniowski D, Sobczak M, Wojtczak BA, Janczak S, Wielgus E. et al. Versatile Method for the Site-Specific Modification of DNA with Boron Clusters: Anti-Epidermal Growth Factor Receptor (EGFR) Antisense Oligonucleotide Case. Chemistry. 2017;23:16535-16546
138. Kwiatkowska A, Sobczak M, Mikolajczyk B, Janczak S, Olejniczak AB, Sochacki M. et al. siRNAs modified with boron cluster and their physicochemical and biological characterization. Bioconjug Chem. 2013;24:1017-1026
139. Kaniowski D, Suwara J, Ebenryter-Olbińska K, Jakóbik-Kolon A, Nawrot B. EGFR-Targeted Cellular Delivery of Therapeutic Nucleic Acids Mediated by Boron Clusters. Int J Mol Sci. 2022;23:14793
140. Kaniowski D, Ebenryter-Olbińska K, Sobczak M, Wojtczak B, Janczak S, Leśnikowski ZJ. et al. High Boron-loaded DNA-Oligomers as Potential Boron Neutron Capture Therapy and Antisense Oligonucleotide Dual-Action Anticancer Agents. Molecules. 2017;22:1393
141. Kaniowski D, Kulik K, Suwara J, Ebenryter-Olbińska K, Nawrot B. Boron Clusters as Enhancers of RNase H Activity in the Smart Strategy of Gene Silencing by Antisense Oligonucleotides. Int J Mol Sci. 2022;23:12190
142. Pulagam KR, Henriksen-Lacey M, B Uribe K, Renero-Lecuna C, Kumar J, Charalampopoulou A. et al. In Vivo Evaluation of Multifunctional Gold Nanorods for Boron Neutron Capture and Photothermal Therapies. ACS Appl Mater Interfaces. 2021;13:49589-49601
143. Icten O, Erdem Tuncdemir B, Mergen H. Design and Development of Gold-Loaded and Boron-Attached Multicore Manganese Ferrite Nanoparticles as a Potential Agent in Biomedical Applications. ACS Omega. 2022;7:20195-20203
144. Wang Y, Reina G, Kang HG, Chen X, Zou Y, Ishikawa Y. et al. Polyglycerol Functionalized 10 B Enriched Boron Carbide Nanoparticle as an Effective Bimodal Anticancer Nanosensitizer for Boron Neutron Capture and Photothermal Therapies. Small. 2022;18:e2204044
145. Ignatova AA, Korostey YS, Fedotova MK, Sivaev IB, Bregadze VI, Mironov AF. et al. Conjugate of chlorin е6 with iron bis(dicarbollide) nanocluster: synthesis and biological properties. Future Med Chem. 2020;12:1015-1023
146. Shi Y, Fu Q, Li J, Liu H, Zhang Z, Liu T. et al. Covalent Organic Polymer as a Carborane Carrier for Imaging-Facilitated Boron Neutron Capture Therapy. ACS Appl Mater Interfaces. 2020;12:55564-55573
147. Conway-Kenny R, Ferrer-Ugalde A, Careta O, Cui X, Zhao J, Nogués C. et al. Ru(ii) and Ir(iii) phenanthroline-based photosensitisers bearing o-carborane: PDT agents with boron carriers for potential BNCT. Biomater Sci. 2021;9:5691-5702
148. Hiramatsu R, Kawabata S, Miyatake S-I, Kuroiwa T, Easson MW, Vicente MGH. Application of a novel boronated porphyrin (H₂OCP) as a dual sensitizer for both PDT and BNCT. Lasers Surg Med. 2011;43:52-58
149. Wang J, Chen L, Ye J, Li Z, Jiang H, Yan H. et al. Carborane Derivative Conjugated with Gold Nanoclusters for Targeted Cancer Cell Imaging. Biomacromolecules. 2017;18:1466-1472
150. Popova TV, Pyshnaya IA, Zakharova OD, Akulov AE, Shevelev OB, Poletaeva J. et al. Rational Design of Albumin Theranostic Conjugates for Gold Nanoparticles Anticancer Drugs: Where the Seed Meets the Soil? Biomedicines. 2021;9:74
151. Lai C-H, Lai N-C, Chuang Y-J, Chou F-I, Yang C-M, Lin C-C. Trivalent galactosyl-functionalized mesoporous silica nanoparticles as a target-specific delivery system for boron neutron capture therapy. Nanoscale. 2013;5:9412-9418
152. Lou X, Yu F, Cao Z, Xu Y, Yang L, Liu H. Surface motif sensitivity of dual emissive gold nanoclusters for robust ratiometric intracellular imaging. Chem Commun (Camb). 2020;56:7112-7115
153. Lin X-H, Song L, Chen S, Chen X-F, Wei J-J, Li J. et al. Kiwifruit-like Persistent Luminescent Nanoparticles with High-Performance and in Situ Activable Near-Infrared Persistent Luminescence for Long-Term in Vivo Bioimaging. ACS Appl Mater Interfaces. 2017;9:41181-41187
154. Oleshkevich E, Morancho A, Saha A, Galenkamp KMO, Grayston A, Crich SG. et al. Combining magnetic nanoparticles and icosahedral boron clusters in biocompatible inorganic nanohybrids for cancer therapy. Nanomedicine. 2019;20:101986
155. Korolkov IV, Zibert AV, Lissovskaya LI, Ludzik K, Anisovich M, Kozlovskiy AL. et al. Boron and Gadolinium Loaded Fe3O4 Nanocarriers for Potential Application in Neutron Capture Therapy. Int J Mol Sci. 2021;22:8687
156. Torresan V, Guadagnini A, Badocco D, Pastore P, Muñoz Medina GA, Fernàndez van Raap MB. et al. Biocompatible Iron-Boron Nanoparticles Designed for Neutron Capture Therapy Guided by Magnetic Resonance Imaging. Adv Healthc Mater. 2021;10:e2001632
157. Wu C-Y, Hsieh H-H, Chang T-Y, Lin J-J, Wu C-C, Hsu M-H. et al. Development of MRI-Detectable Boron-Containing Gold Nanoparticle-Encapsulated Biodegradable Polymeric Matrix for Boron Neutron Capture Therapy (BNCT). Int J Mol Sci. 2021;22:8050
158. Qin Y, Sun X, Zhang Z, Yang Y, Dai Q, Tan X. et al. Dual-Functional Nanoliposome with High BPA Loading for Targeted MRI-Guided BNCT of Glioblastoma. ACS Appl Mater Interfaces. 2025;17:52474-52487
159. Geninatti-Crich S, Deagostino A, Toppino A, Alberti D, Venturello P, Aime S. Boronated compounds for imaging guided BNCT applications. Anticancer Agents Med Chem. 2012;12:543-553
160. Silva WM, Ribeiro H, Taha-Tijerina JJ. Potential Production of Theranostic Boron Nitride Nanotubes (64Cu-BNNTs) Radiolabeled by Neutron Capture. Nanomaterials (Basel). 2021;11:2907
161. Li J, Shi Y, Zhang Z, Liu H, Lang L, Liu T. et al. A Metabolically Stable Boron-Derived Tyrosine Serves as a Theranostic Agent for Positron Emission Tomography Guided Boron Neutron Capture Therapy. Bioconjug Chem. 2019;30:2870-2878
162. Ahn JH, Im C, Kim S, Bibi I, Ko IO, Lee YJ. et al. 64Cu-Labeled Boronic-Acid-Conjugated Porphyrin: A Novel Agent for PET Imaging and Boron Neutron Capture Therapy. ACS Med Chem Lett. 2025;16:1533-1537
163. Chen J, Xu M, Li Z, Kong Z, Cai J, Wang C. et al. A Bis-Boron Amino Acid for Positron Emission Tomography and Boron Neutron Capture Therapy. Angewandte Chemie International Edition. 2025;64:e202413249
164. Chiu Y-L, Fu WY, Huang W-Y, Hsu F-T, Chen H-W, Wang T-W. et al. Enhancing Cancer Therapy: Boron-Rich Polyboronate Ester Micelles for Synergistic Boron Neutron Capture Therapy and PD-1/PD-L1 Checkpoint Blockade. Biomater Res. 2024;28:0040
165. Lan K-W, Huang W-Y, Chiu Y-L, Hsu F-T, Chien Y-C, Hsiau Y-Y. et al. In vivo investigation of boron-rich nanodrugs for treating triple-negative breast cancers via boron neutron capture therapy. Biomaterials Advances. 2023;155:213699
166. Ciani L, Bortolussi S, Postuma I, Cansolino L, Ferrari C, Panza L. et al. Rational design of gold nanoparticles functionalized with carboranes for application in Boron Neutron Capture Therapy. International Journal of Pharmaceutics. 2013;458:340-346
167. Sinclair WK, Morton RA. X-ray sensitivity during the cell generation cycle of cultured Chinese hamster cells. Radiat Res. 1966;29:450-474
168. Sun T, Zhang Z, Li B, Chen G, Xie X, Wei Y. et al. Boron neutron capture therapy induces cell cycle arrest and cell apoptosis of glioma stem/progenitor cells in vitro. Radiation Oncology. 2013;8:195
169. Yoshida F, Matsumura A, Shibata Y, Yamamoto T, Nakauchi H, Okumura M. et al. Cell cycle dependence of boron uptake from two boron compounds used for clinical neutron capture therapy. Cancer Lett. 2002;187:135-141
170. Dillon MT, Good JS, Harrington KJ. Selective targeting of the G2/M cell cycle checkpoint to improve the therapeutic index of radiotherapy. Clin Oncol (R Coll Radiol). 2014;26:257-265
171. Matsuya Y, Sato T, Kusumoto T, Yachi Y, Seino R, Miwa M. et al. Cell-cycle dependence on the biological effects of boron neutron capture therapy and its modification by polyvinyl alcohol. Sci Rep. 2024;14:16696
172. Maier P, Hartmann L, Wenz F, Herskind C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. International Journal of Molecular Sciences. 2016;17:102
173. Qi J, Geng C, Tang X, Tian F, Han Y, Liu H. et al. Effect of spatial distribution of boron and oxygen concentration on DNA damage induced from boron neutron capture therapy using Monte Carlo simulations. Int J Radiat Biol. 2021;97:986-996
174. Chen K-H, Lai Z-Y, Li D-Y, Lin Y-C, Chou F-I, Chuang Y-J. Analysis of DNA Damage Responses After Boric Acid-mediated Boron Neutron Capture Therapy in Hepatocellular Carcinoma. Anticancer Res. 2019;39:6661-6671
175. Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409-425
176. Sanada Y, Takata T, Tanaka H, Sakurai Y, Watanabe T, Suzuki M. et al. HIF-1α affects sensitivity of murine squamous cell carcinoma to boron neutron capture therapy with BPA. Int J Radiat Biol. 2021;97:1441-1449
177. Wada Y, Hirose K, Harada T, Sato M, Watanabe T, Anbai A. et al. Impact of oxygen status on 10B-BPA uptake into human glioblastoma cells, referring to significance in boron neutron capture therapy. J Radiat Res. 2018;59:122-128
178. Harada T, Hirose K, Wada Y, Sato M, Ichise K, Aoki M. et al. YC-1 sensitizes the antitumor effects of boron neutron capture therapy in hypoxic tumor cells. J Radiat Res. 2020;61:524-534
179. Miyatake S-I, Kawabata S, Hiramatsu R, Furuse M, Kuroiwa T, Suzuki M. Boron neutron capture therapy with bevacizumab may prolong the survival of recurrent malignant glioma patients: four cases. Radiat Oncol. 2014;9:6
180. Yoneyama T, Hatakeyama S, Sutoh Yoneyama M, Yoshiya T, Uemura T, Ishizu T. et al. Tumor vasculature-targeted 10B delivery by an Annexin A1-binding peptide boosts effects of boron neutron capture therapy. BMC Cancer. 2021;21:72
181. Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Sig Transduct Target Ther. 2022;7:258
182. Wu Y, Yi M, Niu M, Zhou B, Mei Q, Wu K. Beyond success: unveiling the hidden potential of radiotherapy and immunotherapy in solid tumors. Cancer Communications. 2024;44:739-760
183. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313-322
184. Trivillin VA, Langle YV, Palmieri MA, Pozzi ECC, Thorp SI, Benitez Frydryk DN. et al. Evaluation of local, regional and abscopal effects of Boron Neutron Capture Therapy (BNCT) combined with immunotherapy in an ectopic colon cancer model. Br J Radiol. 2021;94:20210593
185. Shi Y, Guo Z, Fu Q, Shen X, Zhang Z, Sun W. et al. Localized nuclear reaction breaks boron drug capsules loaded with immune adjuvants for cancer immunotherapy. Nat Commun. 2023;14:1884
186. Lv L, Zhang J, Wang Y, Liang H, Liu Q, Hu F. et al. Boron Neutron Capture Therapy-Derived Extracellular Vesicles via DNA Accumulation Boost Antitumor Dendritic Cell Vaccine Efficacy. Adv Sci (Weinh). 2024;11:e2405158
Author contact
![]() Corresponding authors: Tel.: +86 571 88206017.E-mail addresses: qichun_weiedu.cn (Qichun Wei), bianqiongedu.cn (Qiong Bian).
Corresponding authors: Tel.: +86 571 88206017.E-mail addresses: qichun_weiedu.cn (Qichun Wei), bianqiongedu.cn (Qiong Bian).
 Global reach, higher impact
Global reach, higher impact