13.3
Impact Factor
Theranostics 2018; 8(9):2329-2347. doi:10.7150/thno.21451 This issue Cite
Research Paper
Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy
1. Key Laboratory of Carcinogenesis and Invasion, Chinese Ministry of Education, Xiangya Hospital, Central South University, Changsha 410078, China
2. Cancer Research Institute, Xiangya School of Medicine, Central South University, Changsha 410078, China
3. Key Laboratory of Carcinogenesis, Chinese Ministry of Health, Changsha 410078, China
4. Research Center for Technologies of Nucleic Acid-Based Diagnostics and Therapeutics Hunan Province, Changsha 410078, China
5. Center for Theoretical Biological Physics, Rice University, Houston, Texas 77005, USA
6. Department of Neurosurgery, Xiangya Hospital, Central South University, Changsha 410078, China
7. Zhongshan Hospital and Shanghai Medical School, Fudan University, Key Laboratory for Carcinogenesis and Cancer Invasion, Chinese Ministry of Education, Shanghai 200000, China
8. The Hormel Institute, University of Minnesota, Austin, MN 55912, USA
*These authors contributed equally to this work.
Abstract
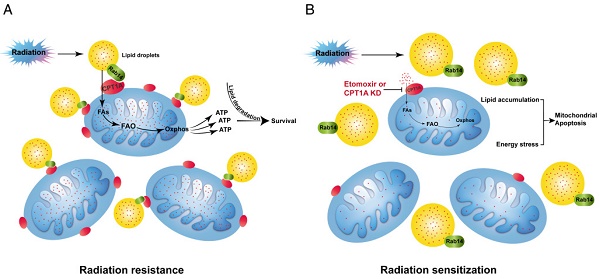
Nasopharyngeal carcinoma (NPC) has a particularly high prevalence in southern China, southeastern Asia and northern Africa. Radiation resistance remains a serious obstacle to successful treatment in NPC. This study aimed to explore the metabolic feature of radiation-resistant NPC cells and identify new molecular-targeted agents to improve the therapeutic effects of radiotherapy in NPC. Methods: Radiation-responsive and radiation-resistant NPC cells were used as the model system in vitro and in vivo. Metabolomics approach was used to illustrate the global metabolic changes. 13C isotopomer tracing experiment and Seahorse XF analysis were undertaken to determine the activity of fatty acid oxidation (FAO). qRT-PCR was performed to evaluate the expression of essential FAO genes including CPT1A. NPC tumor tissue microarray was used to investigate the prognostic role of CPT1A. Either RNA interference or pharmacological blockade by Etomoxir were used to inhibit CPT1A. Radiation resistance was evaluated by colony formation assay. Mitochondrial membrane potential, apoptosis and neutral lipid content were measured by flow cytometry analysis using JC-1, Annexin V and LipidTOX Red probe respectively. Molecular markers of mitochondrial apoptosis were detected by western blot. Xenografts were treated with Etomoxir, radiation, or a combination of Etomoxir and radiation. Mitochondrial apoptosis and lipid droplets content of tumor tissues were detected by cleaved caspase 9 and Oil Red O staining respectively. Liquid chromatography coupled with tandem mass spectrometry approach was used to identify CPT1A-binding proteins. The interaction of CPT1A and Rab14 were detected by immunoprecipitation, immunofluorescence and in situ proximity ligation analysis. Fragment docking and direct coupling combined computational protein-protein interaction prediction method were used to predict the binding interface. Fatty acid trafficking was measured by pulse-chase assay using BODIPY C16 and MitoTracker Red probe. Results: FAO was active in radiation-resistant NPC cells, and the rate-limiting enzyme of FAO, carnitine palmitoyl transferase 1 A (CPT1A), was consistently up-regulated in these cells. The protein level of CPT1A was significantly associated with poor overall survival of NPC patients following radiotherapy. Inhibition of CPT1A re-sensitized NPC cells to radiation therapy by activating mitochondrial apoptosis both in vitro and in vivo. In addition, we identified Rab14 as a novel CPT1A binding protein. The CPT1A-Rab14 interaction facilitated fatty acid trafficking from lipid droplets to mitochondria, which decreased radiation-induced lipid accumulation and maximized ATP production. Knockdown of Rab14 attenuated CPT1A-mediated fatty acid trafficking and radiation resistance. Conclusion: An active FAO is a vital signature of NPC radiation resistance. Targeting CPT1A could be a beneficial regimen to improve the therapeutic effects of radiotherapy in NPC patients. Importantly, the CPT1A-Rab14 interaction plays roles in CPT1A-mediated radiation resistance by facilitating fatty acid trafficking. This interaction could be an attractive interface for the discovery of novel CPT1A inhibitors.
Keywords: CPT1A, fatty acid oxidation, nasopharyngeal carcinoma, radiation therapy, Rab14, fatty acid trafficking
 Global reach, higher impact
Global reach, higher impact