13.3
Impact Factor
Theranostics 2018; 8(19):5336-5347. doi:10.7150/thno.27384 This issue Cite
Review
Recommendations for reporting on emerging optical imaging agents to promote clinical approval
1. Department of Radiology, Molecular Imaging Program, Stanford University, Stanford, CA.
2. Department of Surgery, Leiden University Medical Center, Leiden, Netherlands.
3. Department of Otolaryngology, University of Alabama at Birmingham, AL
4. Department of Otolaryngology, Stanford University, Stanford, CA.
Abstract
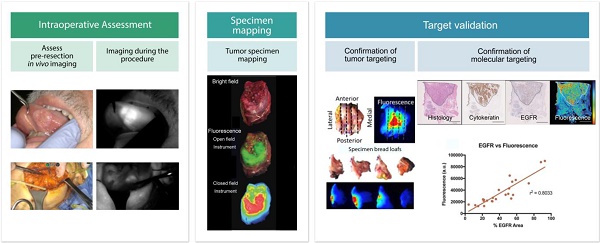
Intraoperative fluorescence imaging is particularly well-suited for surgical applications due to its inherently high sensitivity, resolution, and ability to provide images in real-time. To date, the intraoperative observation of fluorescence has largely been subjective. With the need to show objective evidence in order to demonstrate the benefit of this technique, quantitative data needs to be provided to overseeing regulatory bodies. Standardization of fluorescence imaging protocols would improve reproducibility and minimize inter- and intra-institution variance. This would allow studies to be conducted using the same injection techniques, imaging times, reconstruction methods, and analyses. Here, we provide recommendations for standardized methodologies with the goal of setting a minimum requirement for reporting fluorescence-guided surgery results based on both qualitative and (semi-) quantitative data collection. Clinical trials using fluorescence-guided surgery should present results of three critical elements; 1) intra-operative imaging, 2) specimen mapping and pathology correlation, and 3) target validation. Qualitative analyses should consist of a bright field image, black-and-white fluorescence image, pseudo-colored fluorescence overlay image, and/or heat-map whereby fluorescence signal intensity differences are displayed on a color spectrum. Quantitative analyses should include 1) intraoperative data (consisting of images or video, raw numeric values and ratios); 2) specimen mapping, for correlation of fluorescence with the presence of disease (performed using fresh tissue); and 3) target validation (designed to determine fluorescence intensity relative to receptor density of a specific area). Including the aforementioned methods of both qualitative and quantitative analyses will ensure that trial results are comparable and could be collated in future studies to expedite FDA approval.
Keywords: Optical imaging, fluorescence, clinical trial, standardization, result reporting
 Global reach, higher impact
Global reach, higher impact