13.3
Impact Factor
Theranostics 2019; 9(4):1066-1084. doi:10.7150/thno.29754 This issue Cite
Research Paper
Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing
1. Leibniz-Institute for Plasma Science and Technology (INP Greifswald), Plasma Life Science and ZIK plasmatis, Felix-Hausdorff-Str. 2, 17489 Greifswald, Germany
2. Department of Hygiene and Environmental Medicine, University Medicine Greifswald, 17475 Greifswald, Germany
3. Institute for Experimental Surgery, University of Rostock, Schillingallee 69a, 18057 Rostock, Germany
Abstract
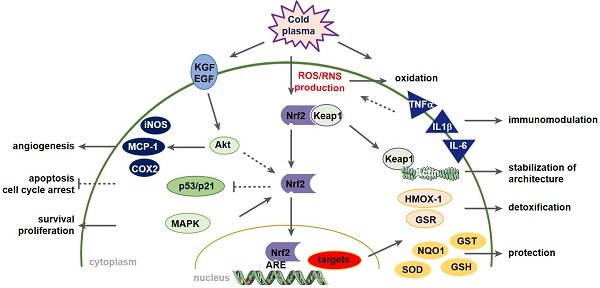
Wound healing is strongly associated with the presence of a balanced content of reactive species in which oxygen-dependent, redox-sensitive signaling represents an essential step in the healing cascade. Numerous studies have demonstrated that cold physical plasma supports wound healing due to its ability to deliver a beneficial mixture of reactive species directly to the cells.
Methods: We described a preclinical proof-of-principle-concept of cold plasma use in a dermal, full-thickness wound model in immunocompetent SKH1 mice. Quantitative PCR, Western blot analysis, immunohistochemistry and immunofluorescence were perfomed to evaluate the expression and cellular translocation of essential targets of Nrf2 and p53 signaling as well as immunomodulatory and angiogenetic factors. Apoptosis and proliferation were detected using TUNEL assay and Ki67 staining, respectively. Cytokine levels in serum were measured using bead-based multiplex cytokine analysis. Epidermal keratinocytes and dermal fibroblasts were isolated from mouse skin to perform functional knockdown experiments. Intravital fluorescence analysis was used to illustrate and quantified microvascular features.
Results: Plasma exerted significant effects on wound healing in mice, including the promotion of granulation and reepithelialization as a consequence of the migration of skin cells, the balance of antioxidant and inflammatory response, and the early induction of macrophage and neutrophil recruitment to the wound sites. Moreover, through an early and local plasma-induced p53 inhibition with a concomitant stimulation of proliferation, the upregulation of angiogenetic factors, and an increased outgrowth of new vessels, our findings explain why dermal skin repair is accelerated. The cellular redox homeostasis was maintained and cells were defended from damage by a strong modulation of the nuclear E2-related factor (Nrf2) pathway and redox-sensitive p53 signaling.
Conclusions: Although acute wound healing is non-problematic, the pathways highlighted that mainly the activation of Nrf2 signaling is a promising strategy for the clinical use of cold plasma in chronic wound healing.
Keywords: Nrf2, p53, plasma medicine, reactive species, redox regulation, wound healing
 Global reach, higher impact
Global reach, higher impact