13.3
Impact Factor
Theranostics 2019; 9(6):1523-1537. doi:10.7150/thno.32461 This issue Cite
Research Paper
Molecular Imaging of Immune Cell Dynamics During De- and Remyelination in the Cuprizone Model of Multiple Sclerosis by [18F]DPA-714 PET and MRI
1. European Institute for Molecular Imaging (EIMI), University of Münster, Münster, Germany
2. Bio-Imaging Laboratory, Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium
3. Imaging Neuroinflammation in Neurodegenerative Diseases (INMIND) EU FP7 consortium
4. PET Imaging in Drug Design and Development (PET3D)
5. Department of Nuclear Medicine, Universitätsklinikum Münster, Münster, Germany
6. Department of Geriatrics, Johanniter Hospital, Evangelische Kliniken, Bonn, Germany
7. Current affiliation: TECHNA Institute for the Advancement of Technology for Health, University Health Network; Institute of Biomaterials and Biomedical Engineering, University of Toronto; both Toronto, Ontario, Canada.
Abstract
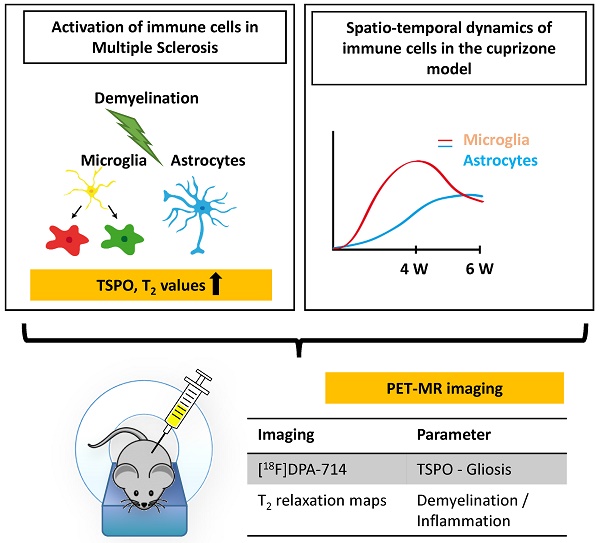
Background: Activation and dysregulation of innate, adaptive and resident immune cells in response to damage determine the pathophysiology of demyelinating disorders. Among the plethora of involved cells, microglia/macrophages and astrocytes play an important role in the pathogenesis of demyelinating disorders. The in-depth investigation of the spatio-temporal profile of these cell types in vivo may inform about the exact disease state and localization as well as may allow to monitor therapeutic modulation of the components of the neuroinflammatory response during the course of multiple sclerosis (MS).
In this study, we aimed to non-invasively decipher the degree and temporal profile of neuroinflammation (TSPO - [18F]DPA-714 PET) in relation to selected magnetic resonance imaging (MRI) parameters (T2 maps) in the cuprizone (CPZ)-induced model of demyelination.
Methods: C57Bl6 (n=30) mice were fed with a standard chow mixed with 0.2% (w/w) CPZ for 4 (n=10; demyelination) and 6 weeks (n=10; spontaneous remyelination). The degree of neuroinflammation at de- and remyelination was assessed by [18F]DPA-714 PET, multi-echo T2 MRI, autoradiography and immunohistochemistry.
Results: CPZ-induced brain alterations were confirmed by increase of T2 relaxation times in both white and grey matter after 3 and 5 weeks of CPZ. Peak [18F]DPA-714 was found in the corpus callosum (CC, white matter), the hippocampus (HC, grey matter) and thalamus (grey matter) after 4 weeks of CPZ treatment and declined after 6 weeks of CPZ. Ex vivo autoradiography and dedicated immunofluorescence showed demyelination/remyelination with corresponding increased/decreased TSPO levels in the CC and hippocampus, confirming the spatial distribution of [18F]DPA-714 in vivo. The expression of TSPO microglia and astrocytes is time-dependent in this model. Microglia predominantly express TSPO at demyelination, while the majority of astrocytes express TSPO during remyelination.
The combination of PET- and MRI-based imaging biomarkers demonstrated the regional and temporal development of the CPZ model-associated neuroinflammatory response in grey and white matter regions.
Conclusions: The combination of [18F]DPA-714 PET and T2 mapping may allow to further elucidate the regional and temporal profile of inflammatory signals depending on the myelination status, although the underlying inflammatory microenvironment changes. A combination of the described imaging biomarkers may facilitate the development of patient-tailored strategies for immunomodulatory and neuro-restorative therapies in MS.
Keywords: multiple sclerosis, cuprizone, microglia, TSPO, imaging biomarker, neuroinflammation, DPA-714, MRI
 Global reach, higher impact
Global reach, higher impact