13.3
Impact Factor
Theranostics 2020; 10(6):2659-2674. doi:10.7150/thno.42665 This issue Cite
Research Paper
Neuron labeling with rhodamine-conjugated Gd-based MRI contrast agents delivered to the brain via focused ultrasound
1. Department of Bioengineering, Imperial College London, South Kensington, London, SW7 2BP, UK.
2. Department of Chemistry, Imperial College London, Molecular Sciences Research Hub, White City, London, W12 0BZ, UK.
3. Biological Imaging Centre, Faculty of Medicine, Hammersmith Hospital, Du Cane Road, London, W12 0NN, UK.
Abstract
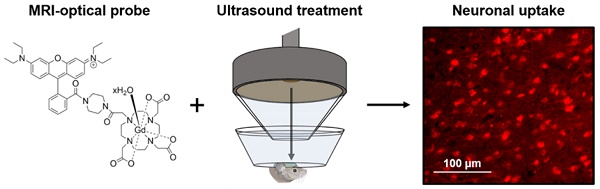
Gadolinium-based magnetic resonance imaging contrast agents can provide information regarding neuronal function, provided that these agents can cross the neuronal cell membrane. Such contrast agents are normally restricted to extracellular domains, however, by attaching cationic fluorescent dyes, they can be made cell-permeable and allow for both optical and magnetic resonance detection. To reach neurons, these agents also need to cross the blood-brain barrier. Focused ultrasound combined with microbubbles has been shown to enhance the permeability of this barrier, allowing molecules into the brain non-invasively, locally and transiently. The goal of this study was to investigate whether combining fluorescent rhodamine with a gadolinium complex would form a dual-modal contrast agent that could label neurons in vivo when delivered to the mouse brain with focused ultrasound and microbubbles.
Methods: Gadolinium complexes were combined with a fluorescent, cationic rhodamine unit to form probes with fluorescence and relaxivity properties suitable for in vivo applications. The left hemisphere of female C57bl/6 mice (8-10 weeks old; 19.07 ± 1.56 g; n = 16) was treated with ultrasound (centre frequency: 1 MHz, peak-negative pressure: 0.35 MPa, pulse length: 10 ms, repetition frequency: 0.5 Hz) while intravenously injecting SonoVue microbubbles and either the 1 kDa Gd(rhodamine-pip-DO3A) complex or a conventionally-used lysine-fixable Texas Red® 3 kDa dextran. The opposite right hemisphere was used as a non-treated control region. Brains were then extracted and either sectioned and imaged via fluorescence or confocal microscopy or imaged using a 9.4 T magnetic resonance imaging scanner. Brain slices were stained for neurons (NeuN), microglia (Iba1) and astrocytes (GFAP) to investigate the cellular localization of the probes.
Results: Rhodamine fluorescence was detected in the left hemisphere of all ultrasound treated mice, while none was detected in the right control hemisphere. Cellular uptake of Gd(rhodamine-pip-DO3A) was observed in all the treated regions with a uniform distribution (coefficient of variation = 0.4 ± 0.05). Uptake was confirmed within neurons, whereas the probe did not co-localize with microglia and astrocytes. Compared to the dextran molecule, Gd(rhodamine-pip-DO3A) distributed more homogeneously and was less concentrated around blood vessels. Furthermore, the dextran molecule was found to accumulate unselectively in microglia as well as neurons, whereas our probe was only taken up by neurons. Gd(rhodamine-pip-DO3A) was detected via magnetic resonance imaging ex vivo in similar regions to where fluorescence was detected.
Conclusion: We have introduced a method to image neurons with a dual-modal imaging agent delivered non-invasively and locally to the brain using focused ultrasound and microbubbles. When delivered to the mouse brain, the agent distributed homogeneously and was only uptaken by neurons; in contrast, conventionally used dextran distributed heterogeneously and was uptaken by microglia as well as neurons. This result indicates that our probe labels neurons without microglial involvement and in addition the probe was found to be detectable via both ex vivo MRI and fluorescence. Labeling neurons with such dual-modal agents could facilitate the study of neuronal morphology and physiology using the advantages of both imaging modalities.
Keywords: neurons, rhodamine, MRI contrast agents, focused ultrasound, blood-brain barrier
 Global reach, higher impact
Global reach, higher impact