13.3
Impact Factor
Theranostics 2024; 14(1):17-32. doi:10.7150/thno.87345 This issue Cite
Review
Improving susceptibility of neuroendocrine tumors to radionuclide therapies: personalized approaches towards complementary treatments
1. Institute for Clinical Chemistry and Laboratory Medicine, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
2. Department of Internal Medicine III, University Clinic Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
3. Department of Endocrinology, Diabetology and Clinical Nutrition, University Hospital Zurich (USZ), and University of Zurich (UZH), Zurich, Switzerland.
4. Department of Radiopharmaceutical and Chemical Biology, Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden-Rossendorf (HZDR), Dresden, Germany.
5. University Hospital Würzburg, Division of Endocrinology and Diabetes, Würzburg, Germany.
6. Department of Medicine IV, University Hospital, Ludwig-Maximilians-University Munich, Munich, Germany.
7. Faculty of Chemistry and Food Chemistry, School of Science, Technische Universität Dresden, Dresden, Germany.
* Shared senior authorship.
Abstract
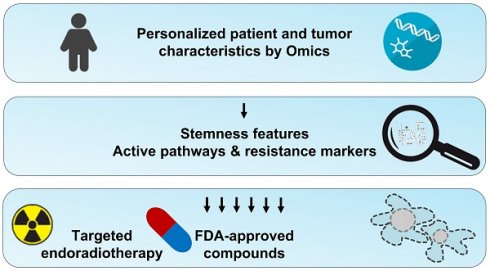
Radionuclide therapies are an important tool for the management of patients with neuroendocrine neoplasms (NENs). Especially [131I]MIBG and [177Lu]Lu-DOTA-TATE are routinely used for the treatment of a subset of NENs, including pheochromocytomas, paragangliomas and gastroenteropancreatic tumors. Some patients suffering from other forms of NENs, such as medullary thyroid carcinoma or neuroblastoma, were shown to respond to radionuclide therapy; however, no general recommendations exist. Although [131I]MIBG and [177Lu]Lu-DOTA-TATE can delay disease progression and improve quality of life, complete remissions are achieved rarely. Hence, better individually tailored combination regimes are required. This review summarizes currently applied radionuclide therapies in the context of NENs and informs about recent advances in the development of theranostic agents that might enable targeting subgroups of NENs that previously did not respond to [131I]MIBG or [177Lu]Lu-DOTA-TATE. Moreover, molecular pathways involved in NEN tumorigenesis and progression that mediate features of radioresistance and are particularly related to the stemness of cancer cells are discussed. Pharmacological inhibition of such pathways might result in radiosensitization or general complementary antitumor effects in patients with certain genetic, transcriptomic, or metabolic characteristics. Finally, we provide an overview of approved targeted agents that might be beneficial in combination with radionuclide therapies in the context of a personalized molecular profiling approach.
 Global reach, higher impact
Global reach, higher impact