13.3
Impact Factor
Theranostics 2024; 14(6):2442-2463. doi:10.7150/thno.93236 This issue Cite
Research Paper
The HSP90 inhibitor HVH-2930 exhibits potent efficacy against trastuzumab-resistant HER2-positive breast cancer
1. Division of Medical Oncology, Department of Internal Medicine, Korea University College of Medicine, Korea University, Seoul 02841, Republic of Korea.
2. Brain Korea 21 Program for Biomedical Science, Korea University College of Medicine, Korea University, Seoul 02841, Republic of Korea.
3. Department of Biomedical Research Center, Korea University Guro Hospital, Korea University, Seoul 08308, Republic of Korea.
4. Adelaide Medical School, Faculty of Health and Medical Sciences, The University of Adelaide, South Australia 5000, Australia.
5. Faculty of Pharmacy, PHENIKAA University, Hanoi 12116, Vietnam.
6. Department of Organic Chemistry, Hanoi University of Pharmacy, Hanoi 10000, Vietnam.
7. Laboratory of Medicinal Chemistry, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea.
8. School of Pharmacy, Sungkyunkwan University, Suwon, Gyeonggi-do 16419, Republic of Korea.
* M. Park and E. Jung contributed equally to this work.
Abstract
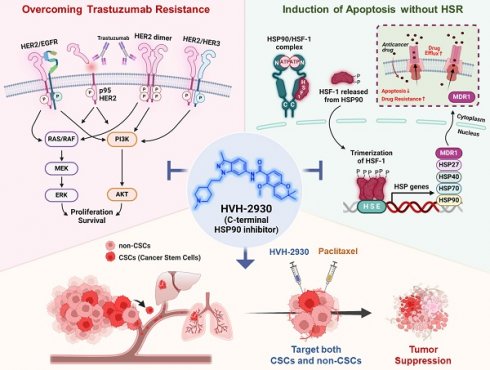
Rationale: Resistance to targeted therapies like trastuzumab remains a critical challenge for HER2-positive breast cancer patients. Despite the progress of several N-terminal HSP90 inhibitors in clinical trials, none have achieved approval for clinical use, primarily due to issues such as induction of the heat shock response (HSR), off-target effects, and unfavorable toxicity profiles. We sought to examine the effects of HVH-2930, a novel C-terminal HSP90 inhibitor, in overcoming trastuzumab resistance.
Methods: The effect of HVH-2930 on trastuzumab-sensitive and -resistant cell lines in vitro was evaluated in terms of cell viability, expression of HSP90 client proteins, and impact on cancer stem cells. An in vivo model with trastuzumab-resistant JIMT-1 cells was used to examine the efficacy and toxicity of HVH-2930.
Results: HVH-2930 was rationally designed to fit into the ATP-binding pocket interface cavity of the hHSP90 homodimer in the C-terminal domain of HSP90, stabilizing its open conformation and hindering ATP binding. HVH-2930 induces apoptosis without inducing the HSR but by specifically suppressing the HER2 signaling pathway. This occurs with the downregulation of HER2/p95HER2 and disruption of HER2 family member heterodimerization. Attenuation of cancer stem cell (CSC)-like properties was associated with the downregulation of stemness factors such as ALDH1, CD44, Nanog and Oct4. Furthermore, HVH-2930 administration inhibited angiogenesis and tumor growth in trastuzumab-resistant xenograft mice. A synergistic effect was observed when combining HVH-2930 and paclitaxel in JIMT-1 xenografts.
Conclusion: Our findings highlight the potent efficacy of HVH-2930 in overcoming trastuzumab resistance in HER2-positive breast cancer. Further investigation is warranted to fully establish its therapeutic potential.
Keywords: C-terminal HSP90 inhibitor, HVH-2930, HER2-positive breast cancer, trastuzumab resistance, cancer stem cells
 Global reach, higher impact
Global reach, higher impact