13.3
Impact Factor
Theranostics 2025; 15(15):7346-7377. doi:10.7150/thno.115414 This issue Cite
Review
Artemisiae Annuae Herba: from anti-malarial legacy to emerging anti-cancer potential
1. Department of General Surgery, the Zhejiang Provincial People's Hospital, Hangzhou, Zhejiang, China.
2. Department of Medical Oncology, the Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou, Zhejiang 310015, China.
3. School of Pharmacy, Hangzhou Normal University, Hangzhou, Zhejiang 311121, China.
4. Henan Key Laboratory of Microbiome and Esophageal Cancer Prevention and Treatment, Henan Key Laboratory of Cancer Epigenetics, The First Affiliated Hospital, College of Clinical Medicine, Henan University of Science and Technology, Luoyang, Henan, China.
Received 2025-4-9; Accepted 2025-6-2; Published 2025-6-20
Abstract
Modern medical approaches to cancer treatment face significant obstacles, including limited therapeutic options, narrow drug applicability, and rapid development of drug resistance. Consequently, re-evaluating traditional medicinal plants and natural compounds has emerged as a promising strategy to address this public health issue, particularly amid challenges in developing novel pharmaceuticals. Artemisiae Annuae Herba, a versatile natural drug renowned for its established efficacy against malaria and for other diverse pharmacological activities, is gaining recognition for its anti-cancer potential due to the unique structures and biological effects of its constituents. This review comprehensively outlines the major components of Artemisiae Annuae Herba and their reported anti-cancer activities, beginning with an examination of the molecular structures of the foundational components and an exploration of derivatives of these compounds. Furthermore, through an analysis of observed pharmacological effects, we systematically elucidate the multifaceted influence of Artemisiae Annuae Herba on cancerous tissues, including cell cycle arrest, apoptosis induction, non-apoptotic cell death induction, angiogenesis inhibition, tumor microenvironment remodeling, and immune modulation. Finally, we discuss the feasibility of Artemisiae Annuae Herba in cancer therapy as well as the challenges and unresolved issues that require further investigation. We also consider ways that new drug formulations and routes of administration might overcome these translational hurdles. By synthesizing existing research on applications of Artemisiae Annuae Herba to cancer therapy, this review underscores potentially innovative clinical approaches, ultimately paving the way for the discovery of effective anti-cancer drugs with far-reaching benefits.
Keywords: Artemisiae Annuae Herba, traditional Chinese medicine, malaria, cancer, derivatives
Introduction
The biological complexity of tumors has presented significant challenges to the treatment of cancer. In addition, increasing levels of drug resistance have made some existing cancer therapies inadequate. Despite the development and clinical application of novel drugs, many patients still experience disease progression and unfavorable outcomes despite exhausting all relevant anti-cancer medications and participating in cutting-edge clinical trials. The processes of investigating the mechanisms of tumorigenesis and developing novel drugs require highly innovative thinking and are often hindered by the element of chance. Moreover, the protracted drug development timeline, from initial research to clinical application, stands in stark contrast to the urgent demand for effective cancer therapies. Consequently, re-evaluating and extending the anti-cancer potential of existing drugs, particularly those derived from traditional herbal medicines, has gained considerable scientific and medical significance. This approach offers a potentially more rapid and accessible avenue for addressing the pressing need for improved cancer treatments.
The integration of Traditional Chinese Medicine (TCM), including TCM-related natural products, with Western medicine [1,2], has recently become an important aspect of comprehensive cancer treatments [3-5]. Modern medical science researchers have noted that the anti-cancer effects of natural TCM products involve a complex array of mechanisms [6]. Previously, the absence of advanced micro-analysis techniques hindered the ability of researchers to analyze underlying mechanisms and effects. However, advances in technical methods have now enabled molecular isolation, purification, and structural analyses to uncover novel insights [7-9]. Given the existence of drugs or herbs with recognized anti-cancer properties, a comprehensive investigation is essential and promises to yield novel opportunities for cancer therapy.
Artemisiae Annuae Herba, a TCM herb derived from the plant Artemisia annua L. has a rich history [10] and has been demonstrated to hold a multifaceted therapeutic potential [11,12]. It is effective against multiple conditions, such as malaria and rheumatism, and it exhibits emerging promise in cancer therapy [13]. However, current research has predominantly focused on artemisinin and its derivatives [14-16], a component within the herb that gained attention for its anti-malarial properties, while neglecting the broader pharmacological actions of Artemisiae Annuae Herba's formulations and decoctions. It is worth noting that many researchers have recognized the potential of the herb's other constituents in cancer research and are actively investigating their effects. Despite challenges like purification complexity and interactions among the compounds, elucidating the mechanisms that underlie the anti-cancer activities of Artemisiae Annuae Herba and its components is a research avenue that promises to advance cancer treatment.
This review offers an in-depth analysis of Artemisiae Annuae Herba and its components or derivatives in cancer therapy, detailing their mechanisms, clinical applications, and usage considerations. In this review, we also identify critical research gaps and priorities to advance this field.
The Basics of Artemisiae Annuae Herba
Renaissance of ancient medicine: Artemisiae Annuae Herba
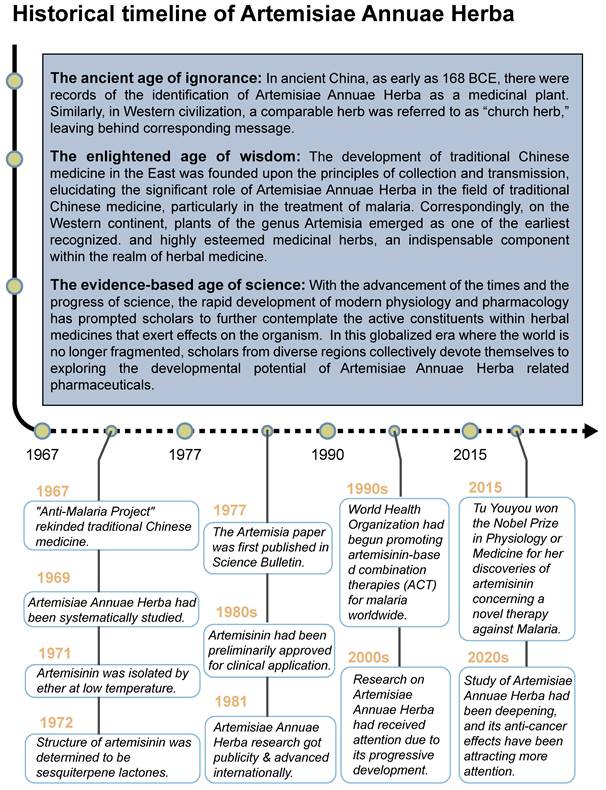
The medicinal application of Artemisiae Annuae Herba has a long and rich history (Figure 1), originating in ancient China. The Silk manuscript Prescription for Fifty-two Diseases, unearthed in the Mawangdui tomb (Changsha, Hunan Province, 168 BCE), documents one of its earliest medicinal uses, as part of a hemorrhoid treatment. Several other classical Chinese herbal medicine texts also provided detailed descriptions of the plant's therapeutic properties. For instance, Shennong's Herbal Classic (ca. 100 CE) characterizes Artemisiae Annuae Herba as “bitter and cold in taste,” with applications for treating scabies, itching, sores, lice infestations, and heat retention in the bones and for vision improvement. Similarly, New Repair of Materia Medica (ca. 659 CE) emphasizes its external use for “applying to sores, promoting hemostasis, regenerating tissue, and relieving pain.” These early records indicate that Artemisiae Annuae Herba was primarily employed for both internal and external treatments of skin infections, such as pruritus and scabies, with a secondary mention of its potential to kill lice and prevent insect-borne diseases.
In Western civilizations, a thorough definition of Artemisiae Annuae Herba emerged later, with the plant's original scientific name, Artemisia annua L., being established in 1753 by Carl Linnaeus. The name Artemisia refers to the genus within the Asteraceae (chrysanthemum) family, and annua denotes its annual growth cycle. Despite the relatively recent adoption of its scientific nomenclature, the plant's reputation had already spread widely across various cultures. In the Arab world, Artemisia annua L. was valued for its ability to treat ulcers, skin ailments, and hair loss, and it even served as a talisman against evil. Among Slavic communities, it was termed the “church herb,” while in Poland, it was affectionately referred to as “God's little tree.” These diverse cultural associations underscore the plant's longstanding significance in traditional medicine and its symbolic importance globally [17].
As scholars' knowledge has progressed and technology has advanced, our understanding of Artemisiae Annuae Herba has deepened significantly. In Asia, particularly within the context of TCM, the efficacy of this herb has been continuously verified and extended. Ge Hong (283-343 CE), a philosopher and pharmacist of China's Eastern Jin Dynasty, played a crucial role in identifying and validating the use of Artemisiae Annuae Herba for malaria treatment. In Handbook of Prescriptions for Emergency Treatment (ca. 326-341 CE), he detailed its anti-malarial applications, with instructions to extract its juice by soaking in water. This foundational understanding was further developed by subsequent TCM practitioners. Song Dynasty scholar Zhou Qufei (1134-1189 CE), documented the use of Artemisiae Annuae Herba as a key treatment for malaria and other ailments in his book Questions and Answers from Beyond Mountain (ca. 1178 CE); the use of Artemisiae Annuae Herba was often combined with acupuncture and bloodletting to improve effectiveness. Meanwhile, in Western Europe, research into herbal medicine also explored the potential of other Artemisia species. The herb A. abrotanum (southernwood), a congener species, held a significant place in European traditional medicine [18]. It was predominantly recommended for liver and biliary diseases and increasingly utilized as an efficient deworming and antipyretic agent for both adults and children. These parallel traditions in different regions underscore the global historical appreciation of Artemisia's therapeutic value.
The resurgence of interest in Artemisiae Annuae Herba in modern China aligns with the revitalization of TCM practices [19]. Since 1967, many researchers in China have systematically investigated TCM through modern scientific methodologies. Among these pioneers [20-23], Tu Youyou stands out as a particularly notable figure. She achieved a significant breakthrough by isolating and extracting sesquiterpene lactones artemisinin from Artemisiae Annuae Herba. Her innovative approach involved using cold brewing and ingredient analysis [24,25], rather than the traditional hot decoction methods described in ancient texts. This advancement marked a pivotal moment in the treatment of malaria [26,27] and redefined the therapeutic use of Artemisiae Annuae Herba [28,29]. Tu was awarded the 2015 Nobel Prize in Physiology or Medicine for this groundbreaking discovery [30].
Historical Timeline of Artemisiae Annuae Herba: Development of Artemisiae Annuae Herba as medicine can be summarized into three periods: ancient age of ignorance, enlightened age of wisdom, and evidence-based age of science. Current research on Artemisiae Annuae Herba is rooted in the enlightenment era of scientific exploration and is moving forward steadily. In the 1960s, the drug was investigated intensely due to the need for anti-malaria treatments, and results were achieved in the 1970s and early 1980s. Other effects of the drug were considered after further promotion of it in the 1990s. Research of Artemisiae Annuae Herba entered the 21st century, with the Nobel Prize serving as a sign of progress.
Beyond its established anti-malarial properties, Artemisiae Annuae Herba has garnered considerable scientific interest for its varied therapeutic effects [31] and biochemical properties [32,33], especially concerning artemisinin [34-37]. The anti-cancer potential of various components of Artemisiae Annuae Herba or their derivatives [38] has become a significant research focus. Since the 1990s, both Eastern and Western scientific communities have investigated the cytotoxicity of artemisinin and its derivatives [39,40], such as dihydroartemisinin (DHA) [41-43], artesunate (ART) [44-46], artemether (ARM) [47-50], and arteether (ARE) [51,52], across diverse cancer cell lines [53]. The early studies initially confirmed the anti-cancer potential of Artemisiae Annuae Herba components in-vitro [54-56]. In addition, an increasing number of in-vivo studies using mouse models of human xenograft tumors [57-59], along with clinical trials combining chemotherapy with Artemisiae Annuae Herba-derived drugs [60], are further substantiating the tumor-inhibitory effects of these compounds [61,62].
Accurate identification of Artemisiae Annuae Herba
A retrospective of the historical research on Artemisiae Annuae Herba reveals a diversity of appellations and naming conventions [63]. The plant from which authentic Artemisiae Annuae Herba is derived, most commonly known as A. annua L., is primarily identified by the morphologies of its rhizomes and leaves. The cylindrical rhizomes, branching in the upper section, exhibit yellowish green to brownish-yellow surfaces with longitudinal ridges. They are slightly hard, easily fractured, and possess a pith in the center of the fracture surface. The dark green to brownish-green leaves are fragile and often curled. Intact leaves display a tripinnately dissected morphology, with rectangular to oblong segments and lobules covered in short hairs. The herb possesses a distinct, aromatic odor and a slightly bitter taste.
Despite these distinctive features, other plants' products have been mistakenly identified or referred to as Artemisiae Annuae Herba, leading to some confusion. By synthesizing information from both ancient texts and modern scientific literature, it becomes evident that the medicinal herb commonly referred to as Qinghao in traditional and contemporary contexts is indeed Artemisiae Annuae Herba. Specifically, this formulation refers to the dried above-ground parts of A. annua L. [64,65], rather than other related species within the Asteraceae family, such as A. flavescens. The clarification is essential for the precise identification and applications of Artemisiae Annuae Herba in medicinal and scientific research.
Beyond identifying A. annua L. as the definitive source of Artemisiae Annuae Herba, its transformation from a simple plant to a medicinal herb warrants attention. Unlike other herbs requiring complex processing, traditional Artemisiae Annuae Herba preparation involved air-drying A. annua L. after removing aged stems in autumn. Modern methods, however, utilize continuous solvent extraction and product recovery. This purification reduces interactions between the various components, and it also allows for in-depth exploration of single compounds, enabling researchers to thoroughly analyze pharmacological effects and applications, offering unparalleled advantages. Decades of comprehensive chemical and biological analyses have isolated various bioactive compounds from Artemisiae Annuae Herba [66-68], including sesquiterpenoids [69,70], flavonoids [70,71], coumarins [72], steroids [24], phenolics [73], purines [74], and lipids [75]. To date, over 600 components have been characterized, with novel compounds still continuing to be discovered [69,76]. Based on the chemical structures of these components, their active structures, mechanisms of action, and anti-cancer potential deserve further analysis and investigation.
Biological characteristics of Artemisiae Annuae Herba
Artemisinin is the most famous of the many bioactive components in Artemisiae Annuae Herba. Extensive research on artemisinin continues to uncover novel applications and research directions. In addition, the growing diversity of research approaches has led to the production and application of a broad range of derivatives [77]. Derivative compounds such as artesunate, artemether, DHA, and others have already become first-line treatments for malaria [78-81]. The versatility of artemisinin derivatives in the treatment of cancer, viral infection, immunity, and parasitic infections, as compared to the parent component, underscores the importance of further research on Artemisiae Annuae Herba.
Advancing research on Artemisiae Annuae Herba depends on the comprehensive elucidation of its biochemical effects as a pharmaceutical agent (Figure 2). These effects can be categorized into several key activities: anti-parasitic, anti-viral, anti-bacterial and -fungal, anti-inflammatory, anti-obesity, anti-osteoporotic, and anti-cancer. The diverse therapeutic potential, especially the anti-cancer capability [13] of Artemisiae Annuae Herba has garnered increasing attention in the scientific community.
“World Tree” of Artemisiae Annuae Herba: Artemisiae Annuae Herba has a variety of components as its “roots,” and numerous biological activities and medicinal effects have been developed as its “branches and fruits.” Among the extracts of Artemisiae Annuae Herba, the main compounds are sesquiterpenoids, including artemisinin, artemisinic acid, and artemisitene, along with flavonoids, coumarins, steroids, phenolics, purines, and lipids. It has been developed for A) anti-parasitic, B) anti-viral, C) anti-bacterial and fungal, D) anti-inflammatory, E) anti-obesity, F) anti-osteoporotic, and G) anti-cancer applications.
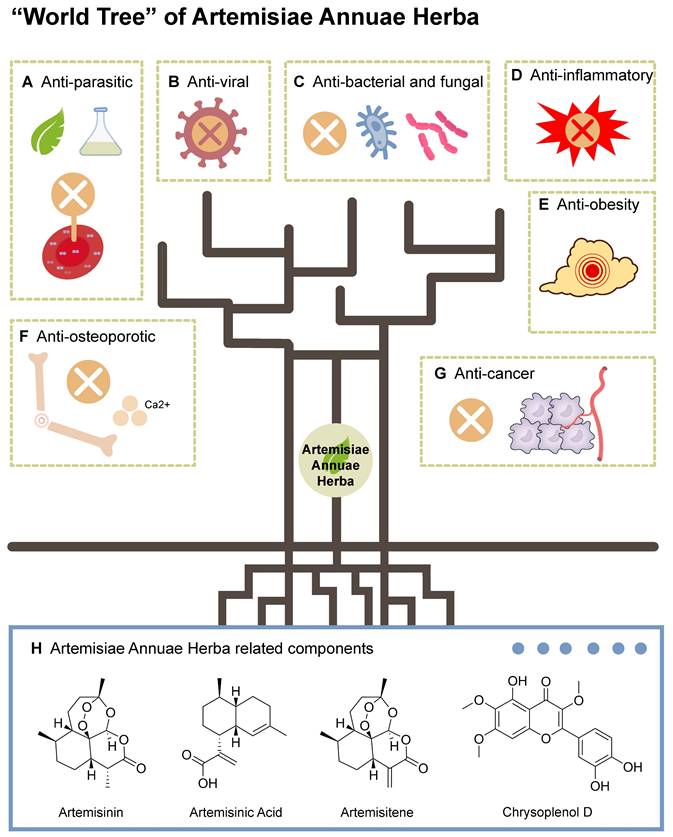
Anti-parasitic: Artemisiae Annuae Herba has been widely available and recommended for malaria prevention and treatment [82]. Artemisinin, in particular, is most renowned for its anti-parasitic properties [83-85], particularly its efficacy against the causative agents of malaria, parasites of the genus Plasmodium [86-88]. Malaria remains a leading cause of morbidity and mortality [89] in numerous countries [90,91], with infections varying from asymptomatic or mild to severe and potentially fatal. The World Health Organization has recommended Artemisinin-based Combination Therapies (ACTs) as the first-line treatment of uncomplicated Plasmodium falciparum malaria [92]. Apart from malaria, artemisinin derivatives have also been shown to be effective against a number of other parasites, including Toxoplasma gondii [85], Leishmania [93,94], Acanthamoeba [84,95], and Schistosoma [96]. Research has demonstrated the potent anti-parasitic effects of Artemisiae Annuae Herba components and derivatives, underscoring their therapeutic potential for various parasitic infections.
Anti-viral: Recent decades have brough substantial anti-viral research on artemisinin and derivatives, rather than the entirety of Artemisiae Annuae Herba. These compounds have exhibited efficacy against multiple viruses, including human herpesvirus 6, herpes simplex viruses 1 and 2, hepatitis B virus, and bovine viral diarrhea virus [10,97]. Less anti-viral research has explored the activities of other Artemisiae Annuae Herba components, such as artemisinic acid, scopoletin, and arteannuin B, but as the understanding of Artemisiae Annuae Herba has deepened, and new challenging viruses like coronaviruses have arisen, other extracts from Artemisiae Annuae Herba have been found to demonstrate notable virucidal and anti-viral properties. Baggieri et al. [98] demonstrated that natural components extracted from Artemisiae Annuae Herba interact with 3-chymotrypsin-like protease and the spike protein from SARS-CoV-2. These natural components exert anti-SARS-CoV-2 activity by disrupting viral pathways during insertion and replication [99,100]. The multiple anti-viral applications of Artemisiae Annuae Herba suggest that this herb is an important part of the clinical fight against viral infections.
Anti-bacterial and fungal: Recent studies have increasingly concentrated on the anti-bacterial and anti-fungal properties [101,102] of Artemisiae Annuae Herba, particularly its essential oils [103]. These oils have demonstrated activities against diverse bacterial species [104-106], including both Gram-positive and Gram-negative bacteria, as well as fungi [101,107]. The anti-microbial properties of Artemisiae Annuae Herba essential oils vary based on their geographical origin [102,108,109]; variations in the chemical composition of oils between studies may account for the observed discrepancies. The essential oils of Artemisiae Annuae Herba exhibit significant variability in chemical composition, with camphor, artemisia ketone, and 1,8-cineole being the main anti-fungal and anti-bacterial agents [105]. Elevated levels of these terpenoids correlate with enhanced anti-microbial activity [106,107]. Overall, Artemisiae Annuae Herba holds promising potential as a source of novel anti-microbial agents. Systematic studies are required to comprehensively characterize the anti-microbial properties of Artemisiae Annuae Herba and to evaluate its advantages and limitations.
Anti-inflammatory: As the core, most intensely studied component, Artemisinin has been widely applied in various inflammatory disease contexts [110], such as autoimmune diseases, allergic inflammation, and sepsis. The anti-inflammatory effects of Artemisiae Annuae Herba have been attributed primarily to inhibition of MAPK and PI3K/AKT signaling pathways, activation of NF-κB, and modulation of the expression of the toll-like receptors TLR4 and TLR9 [106]. Beyond artemisinin, several other components of Artemisiae Annuae Herba have also exhibited anti-inflammatory properties [112,113], underscoring the significant therapeutic potential of Artemisiae Annuae Herba component-based interventions in inflammation management.
Anti-obesity: It is not appropriate to discuss the relationship between fat metabolism and components derived from Artemisiae Annuae Herba without reference to artemisinic acid, which has been shown to have highly anti-adipogenic activity in-vitro [114]. Artemisinic acid has been shown to hinder adipogenic differentiation in adipose-derived mesenchymal stem cells by downregulating CCAAT/enhancer-binding protein δ expression through the inhibition of JNK [115]. Other extracts of Artemisiae Annuae Herba have been shown to inhibit the expression of a target of PPARγ, the gene encoding fatty acid-binding protein 4, in adipocytes [116]. In a study using a high-fat diet-induced rat model of obesity, Artemisiae Annuae Herba extracts notably reduced body weight, fat accumulation, adipocyte size, and serum levels of total cholesterol and triglycerides [114,117]. These findings suggest that Artemisiae Annuae Herba components could be effective in preventing and treating obesity and associated metabolic disorders.
Anti-osteoporotic: Several pieces of experimental evidence have suggested that additional exploration of roles for Artemisiae Annuae Herba in treatment of osteoporosis is warranted. For example, osteoporosis was prevented by an Artemisiae Annuae Herba extract in a study in ovariectomized mice, in which estrogen deficiency typically causes osteoporosis [118]. Additional research has indicated that Artemisiae Annuae Herba and its associated components [114], artemisinin and artemisinin B, exhibit anti-osteoporotic activity by downregulating the activity of the transcription factors c-Fos and nuclear factor of activated T cells 1 (NFATC1), which in turn inhibit osteoclast differentiation as induced by receptor activator of nuclear factor-κB ligand (RANKL) [120,121].
Beyond these diverse clinical activities, Artemisiae Annuae Herba components exhibit complex yet promising anti-cancer activities [122]. A deeper understanding how these agents benefit human health in multiple contexts promises to provide intriguing insights at the micro and macro levels into their potential use in cancer therapy.
From molecular structures to mechanisms: Possible anti-cancer mechanisms have been found upon structural analyses of various molecular components of Artemisiae Annuae Herba, and three possible exploration directions have emerged: specific anti-cancer structures, non-specific anti-proliferative structures, and complex regulatory structures. A) Specific anti-cancer structures rely on their unique peroxide bridge, which generates reactive oxygen species under certain conditions to kill tumor cells. B) Non-specific anti-proliferative structures are associated with the cyclic structures and certain functional groups of sesquiterpenoids, influencing cell proliferation through various mechanisms. C) Complex regulatory structures primarily involve diverse polysaccharides and polyphenols, which modulate immune responses and improve the body's anti-cancer capabilities.
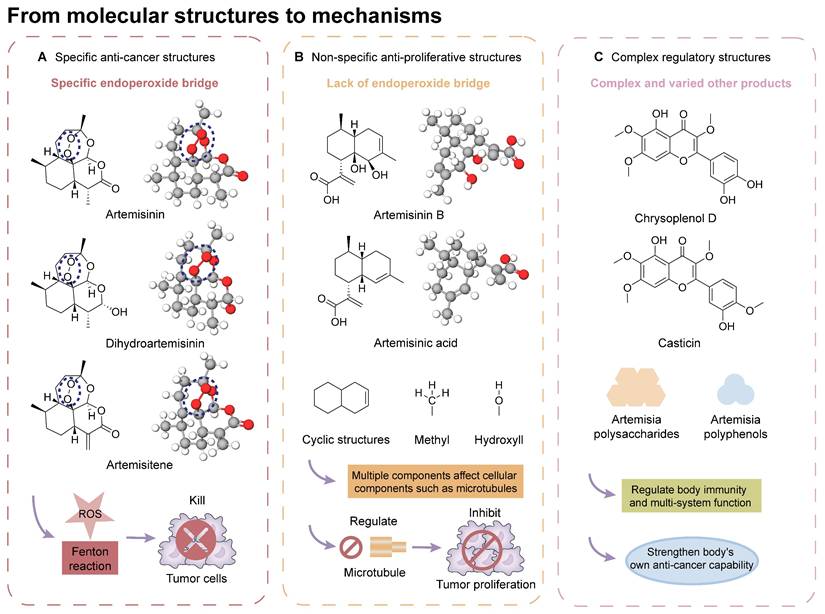
From Molecular Structure to Mechanisms: A Forward Reasoning Approach
Traditional Artemisiae Annuae Herba-based medications contain a multitude of components [123], including a series of sesquiterpenes, coumarins, lignans, phloroglucinol derivatives, and numerous polysaccharides and polypeptides, all of which can be isolated and purified [124]. Among these, certain unique constituents of Artemisiae Annuae Herba exhibit distinct biological properties and medicinal value. In addition, each component seems to work through distinct anti-cancer mechanisms. To further elucidate the possible associations and interactions between these components and tumor tissues, a micro-level analysis of Artemisiae Annuae Herba components and their foundational mechanisms serves as an insightful focus and exploratory direction (Figure 3). This approach could catalyze a substantial paradigm shift in Artemisiae Annuae Herba research.
Extensive structural analyses of Artemisiae Annuae Herba components have facilitated comparisons of the biological characteristics and bioactive mechanisms of its various components. Furthermore, inter-group comparisons and analyses have been conducted to identify similarities and differences between Artemisiae Annuae Herba components and anti-cancer drugs derived from other traditional herbal medicine and natural plants [120]. Overall, the potential anti-cancer capabilities of Artemisiae Annuae Herba, in terms of molecular structure and micro-foundational aspects, can be broadly categorized into the following three areas: specific anti-cancer structures, non-specific anti-proliferative structures, and structures that interact with complex regulatory components.
Chemical structures that specifically target cancer
Considering its potent anti-malarial properties and minimal side effects [126], a central question that has intrigued scholars is whether artemisinin and its derivatives can exhibit similar specificity against various proliferative tumors while minimizing harm to the body [127]. The exceptional anti-malarial effectiveness of artemisinin and its derivatives is primarily due to their unique “endoperoxide bridge” structure, a specific chemical bond between oxygen atoms in the ring, distinguishing them from other natural plant components and sesquiterpene compounds [128]. During the red blood cell stage of the Plasmodium lifecycle, the malaria parasite ingests and digests hemoglobin, releasing a substantial amount of reductive heme and ferrous ions. These reductive components serve as the basis for artemisinin's specific parasiticidal action. Catalyzed by divalent iron, the endoperoxide bridge opens, generating free radicals that target and disrupt proteins, lipids, and DNA [124,125]. This suggests that if artemisinin-related compounds possess a strong anti-cancer effect, the molecular mechanism is likely rooted in this distinctive endoperoxide bridge [38,126,127].
It is important to note, however, that the potential for this molecular structure to confer specific anti-cancer activity remains a topic of considerable debate [128,129]. The endoperoxide bridge and its related Fenton reaction, known for producing alkyl radicals and ROS, are well-documented [130], though their role in anti-cancer processes remains controversial [131-134]. Nan et al. demonstrated a direct link between the endoperoxide bridge of artemisinin and its derivatives and its anti-cancer action in the MCF-7 breast cancer cell line [135]. They observed that artemisinin, which contains the endoperoxide bridge, exhibited significantly stronger cytotoxicity compared to deoxyartemisinin, which lacks this structure. Nevertheless, many researchers and mainstream opinions remain skeptical of such specific anti-cancer effects [141,142]. Given the complex pathology and diverse microenvironments of various tumors, even though tumors are often rich in hemoglobin and transferrin due to extensive angiogenesis, they do not universally provide the ferrous ions and hemes necessary to support a Fenton reaction akin to malaria infection. Therefore, the potential anti-cancer mechanism established by the endoperoxide bridge structure may only be effective in patients with tumors rich in ferrous ions.
Chemical structures with non-specific anti-proliferative effects
In addition to artemisinin, structural analyses of other Artemisiae Annuae Herba components have also prompted new considerations. Artemisinic acid, a precursor to artemisinin, lacks the peroxide bridge, yet some studies have demonstrated its anti-cancer properties [138,139]. This finding suggests that the unique peroxide bridge of artemisinin is not the only aspect of Artemisiae Annuae Herba components that is endowed with anti-cancer potential. The most abundant components of Artemisiae Annuae Herba, terpenoids, are also noteworthy in existing pharmaceutical research [140]. In general, terpenoids are prevalent plant-derived natural products and include well-known chemotherapeutic agents such as paclitaxel, as well as several anti-cancer drugs have been refined from TCM components, such as elemene. The sesquiterpenoids in Artemisiae Annuae Herba share similarities with these recognized plant-based anti-cancer agents, presenting an intriguing avenue for exploration.
It is noteworthy that while the molecular foundations for the anti-cancer activities of paclitaxel, elemene, and other terpenoids differ, their mechanisms of action exhibit some similarities [141]. Specifically, multiple studies have suggested that the anti-cancer mechanisms of plant terpenoids are linked to varying degrees with interruption of the cell cycle and of tubulin microtubule polymerization [142-145]. For example, paclitaxel's anti-cancer efficacy primarily relies on its tricyclic structure, which facilitates binding to tubulin [146]; the two benzene rings in its tricyclic structure closely interact with the hydrophobic regions of microtubule proteins, leading to the formation of stable complexes [152,153]. Additionally, the acetyl group on carbon-10 of paclitaxel forms hydrogen bonds with tubulin, collectively inhibiting microtubule polymerization and cell cycle proliferation [149]. The mechanism underlying elemene's anti-cancer effect is more complex, with numerous studies indicating its association with cell cycle arrest [155,156]. The predominant theory suggests that elemene inhibits tubulin polymerization by inhibiting the MAPK pathway [152,153].
Research on several components of Artemisiae Annuae Herba has also suggested the existence of such mechanisms. For example, Wu et al. showed that DHA altered activity of the p38/MAPK signaling pathway [154], and in colitis-associated colorectal cancer, DHA has been shown to suppress phosphorylation associated with the p38/MAPK pathway, leading to cell cycle arrest [155,156]. While no direct evidence has shown that Artemisiae Annuae Herba components share structural similarities with plant-based anti-cancer agents that target tubulin directly, the influence of Artemisiae Annuae Herba components on intracellular signaling pathways [157-159], including MAPK/PI3K/Akt, highlights their role in cytoskeleton dynamics and cell cycle arrest [160-162]. These structures that exert nonspecific anti-proliferative actions may significantly contribute to the efficacy of Artemisiae Annuae Herba components against rapidly proliferating tumor cells.
Certainly, regulating signaling pathways, influencing intermolecular interactions of tubulin, inhibiting microtubule polymerization, and arresting the cell cycle would not have effects that are specific to tumor cells. More accurately, they mirror the nonspecific anti-proliferative effects of alkylating agents in traditional chemotherapy, targeting all rapidly proliferating tissues [168], with cancerous tissues being the most affected. Accordingly, if the biochemical basis of the anti-cancer activity of Artemisiae Annuae Herba components leans more towards this anti-proliferative capability, the dosage of these drugs becomes a critical concern, and associated side effects can no longer be overlooked [137,157,164,165]. Future research should aim to determine which Artemisiae Annuae Herba components exhibit optimal anti-proliferative effects with enhanced drug efficacy and minimized side effects.
Chemical structures with complex effects on regulatory mechanisms
In broadening the scope of components related to Artemisiae Annuae Herba, it is crucial to consider not only the structurally similar sesquiterpene compounds with relatively high molecular weights but also the diverse array of other components that play significant roles in the foundational conditions for anti-cancer activity [166]. Among these varied components, certain polysaccharides can enhance anti-cancer effects through their unique immune-related properties [11,167], which are rooted in their structural characteristics. For instance, Chen et al. demonstrated that Artemisia polysaccharides inhibit the growth of liver cancer tumors in mice [172]. This effect was primarily attributed to the distinctive molecular structure of these polysaccharides, which stimulates and enhances the antigen recognition capabilities of lymphocytes in the mouse model, thereby promoting the destruction of liver cancer cells through both cellular and humoral immune mechanisms [168].
The complex composition and lack of specific pharmacological properties in some secondary components, such as polysaccharides and polypeptides, prevent them from becoming the primary foundation of Artemisiae Annuae Herba's anti-cancer capabilities. Nevertheless, their presence underscores the significance of Artemisiae Annuae Herba as a TCM with indispensable auxiliary components. Research into the relationship between these components and anti-cancer activity holds promising potential for developing adjunctive anti-cancer regulatory mechanisms [169].
Overall, components related to Artemisiae Annuae Herba indeed possess micro-molecular structural foundations that strongly influence their anti-cancer mechanisms. These foundations include the specific endoperoxide bridge structures of artemisinin and its derivatives, non-specific cell cycle-related anti-proliferative structures, and various regulatory synergistic effects. The existence of these differential foundations further establishes the comprehensiveness of the anti-cancer activities of Artemisiae Annuae Herba components, highlighting the potential application value of this herb in the advancement of cancer treatment.
From Efficacy to Mechanisms: A Reverse Reflection on Anti-Cancer Actions
A structural analysis of the molecules that make up Artemisiae Annuae Herba preparations suggests that the herb exhibits a comprehensive and multidirectional anti-cancer potential. This potential can be attributed to the complex composition of the formulations and to its varied interactions with biological components. Complementing this approach, a reverse efficacy analysis further supports the herb's potential as an anti-cancer agent. This potential has been rigorously validated through ongoing research and repeated experimental studies. Artemisinin and its derivatives, including artesunate and DHA, have been studied for their anti-cancer activities since the late 1990s [43,143,175,176]. These compounds have been shown to promote apoptosis of cancer cells [172-174], inhibit tumor angiogenesis [15,56,180], and block tumor invasion and metastasis [158,176].
Extracts of Artemisiae Annuae Herba, in addition to purified artemisinin, also exhibit significant anti-cancer activity and tumor-killing effects via multiple regulatory mechanisms, as supported by extensive in vitro, in vivo, and clinical research [127,182]. For instance, Michaelsen et al. reported a clinical study involving patients with advanced prostate cancer, where the long-term addition of Artemisiae Annuae Herba following short-term treatment with bicalutamide led to tumor regression and treatment remission, as confirmed by prostate-specific antigen (PSA), magnetic resonance imaging, and SPECT/CT indicators [122]. Furthermore, a wide range of natural components that have been isolated from Artemisiae Annuae Herba, including polysaccharides and polyphenols, have garnered significant attention for their potential as anti-cancer drugs [178-181].
After reviewing and synthesizing numerous studies on Artemisiae Annuae Herba components, we found that the primary anti-cancer mechanisms can be categorized into six key functions (Figure 4): (1) induction of cell cycle arrest; (2) induction of apoptosis; (3) induction of non-apoptotic cell death processes, including autophagy, ferroptosis, pyroptosis, and macrophage death; (4) inhibition of angiogenesis; (5) regulation of epithelial-mesenchymal transition (EMT); and 6) modulation of immune system functions. This analysis revealed unique primary effects and synergistic interactions of Artemisiae Annuae Herba components, collectively enhancing the plant's notable anti-cancer properties (Table 1).
From efficacy to mechanisms: Experiments investigating the anti-cancer activities of existing Artemisiae Annuae Herba components were analyzed and summarized, and possible anti-cancer mechanisms were deduced. Improvements to experimental strategies were proposed. At present, the mechanisms can be summarized into six aspects: cell cycle arrest, induction of apoptosis, induction of non-apoptotic cell death, inhibition of angiogenesis, regulation of EMT and inhibition of metastasis, and regulation of immune functions.
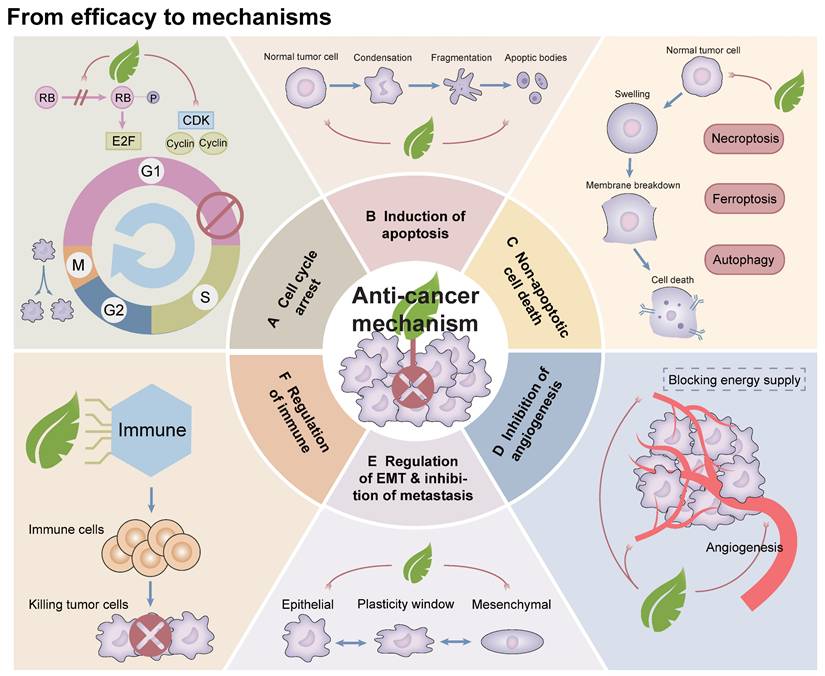
Induction of cell cycle arrest
Uncontrolled and exceptionally rapid cell division is a fundamental characteristic of tumor cells, driving their proliferation and contributing to disease progression [182]. The process of cell division, known as the cell cycle, consists of four major phases: G1, S, G2, and M phases. This cycle is tightly regulated by cyclins and cyclin-dependent kinases (CDKs), which play critical roles in ensuring orderly progression through each phase [188]. Recent studies have identified artemisinin and its derivatives as potential modulators of cyclins and CDKs, highlighting their promise in the development of cancer therapies [184,185]. For example, research on prostate cancer cells has demonstrated that artemisinin inhibits the action of the cell cycle-regulatory protein pRb by disrupting a complex formed among pRb, E2F transcription factors, and CDKs [122]. This activity halts the transition of cells from the G1 to the S phase, thereby inhibiting cell cycle progression and suppressing cancer cell proliferation [191]. Similarly, in a study on non-small cell lung cancer, Rassias et al. found that an extract of dried leaves from Artemisia annua induced G2/M phase and mitotic arrest in PC9 and H1299 cell lines, while also causing G1 phase arrest in A549 cells [53]. Collectively, these findings underscore the anti-cancer potential of Artemisiae Annuae Herba components, particularly through their ability to regulate the cell cycle and impede cancer progression [187,188].
Anti-cancer components in Artemisiae Annuae Herba and related mechanisms
| Component | Molecular structure | Tumor types | Mechanisms | Ref. | |
|---|---|---|---|---|---|
| Artemisinin | 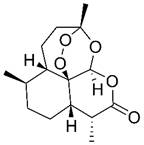 | Hematologic | Cell cycle arrest Induced autophagy | [336,337] | |
| Esophageal | Cell cycle arrest Regulate EMT Suppress metastasis | [338,339] | |||
| Gastric | Cell cycle arrest Induce ferroptosis | [340,341] | |||
| Colorectal | Cell cycle arrest Induce apoptosis | [54,205,342] | |||
| Lung | Cell cycle arrest Induce apoptosis Inhibit angiogenesis | [232,343-345] | |||
| Liver | Induce ferroptosis Modulate immune function | [346-348] | |||
| Pancreas | Induce apoptosis | [349] | |||
| Breast | Induce apoptosis Induce autophagy Inhibit angiogenesis Modulate immune function | [179,192,350-352] | |||
| Prostate | Cell cycle arrest Induce apoptosis | [127,191,353] | |||
| Dihydroartemisinin | 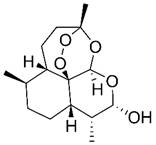 | Hematologic | Induce apoptosis Induce ferroptosis | [354,355] | |
| Lymphatic | Induce apoptosis Induce autophagy Induce ferroptosis | [159,356-358] | |||
| Gastric | Cell cycle arrest Induce apoptosis Induce ferroptosis Inhibit angiogenesis Regulate EMT Suppress metastasis | [167,359-363] | |||
| Colorectal | Induce apoptosis Inhibit angiogenesis Suppress metastasis Modulate immune function | [15,364-367] | |||
| Lung | Induce apoptosis Induce ferroptosis Inhibit angiogenesis Modulate immune function | [245,283,368-370] | |||
| Liver | Cell cycle arrest Induce ferroptosis Regulate EMT Modulate immune function | [371-374] | |||
| Pancreas | Induce apoptosis Induce ferroptosis Modulate immune function | [375-381] | |||
| Breast | Cell cycle arrest Inhibit angiogenesis Suppress metastasis | [243,382,383] | |||
| Ovarian | Induce apoptosis Suppress metastasis | [384-387] | |||
| Prostate | Induce apoptosis | [388-390] | |||
| Artesunate | 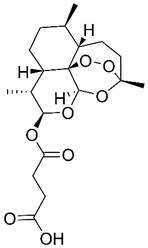 | Hematologic | Induce apoptosis Induce autophagy Induce ferroptosis | [286,391,392] | |
| Lymphatic | Induce apoptosis Induce autophagy Induce ferroptosis | [393-397] | |||
| Gastric | Cell cycle arrest Induce apoptosis Inhibit angiogenesis | [398-400] | |||
| Colorectal | Cell cycle arrest Induce apoptosis Induce ferroptosis Inhibit angiogenesis Suppress metastasis Modulate immune function | [165,249,401-403] | |||
| Lung | Cell cycle arrest Induce apoptosis Induce ferroptosis Regulate EMT Suppress metastasis | [245,318,404-406] | |||
| Liver | Induce apoptosis Induce autophagy Induce ferroptosis | [16,320,407-409] | |||
| Pancreas | Cell cycle arrest Induce ferroptosis | [410-413] | |||
| Breast | Cell cycle arrest Induce apoptosis Regulate EMT Suppress metastasis | [414-418] | |||
| Ovarian | Cell cycle arrest Induce apoptosis Induce ferroptosis Inhibit angiogenesis | [233,419-423] | |||
| Prostate | Cell cycle arrest Induce apoptosis | [127,424-426] | |||
| Artemether | 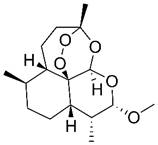 | Lymphatic | Cell cycle arrest Induce apoptosis Modulate immune function | [427,428] | |
| Lung | Cell cycle arrest Induce apoptosis | [429] | |||
| Breast | Inhibit angiogenesis | [48] | |||
| Artemisitene |  | Breast | Induce apoptosis Regulate EMT Suppress metastasis | [430,431] | |
| Artemisinic acid | 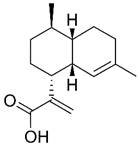 | Lung | Induce apoptosis Modulate immune function | [72,143] | |
| Artemisinin B | 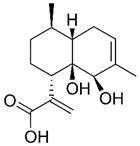 | Lung | Induce apoptosis | [432] | |
| Liver | Induce apoptosis | [143] | |||
| Casticin | 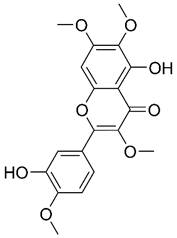 | Osteosarcoma | Induce ferroptosis | [433] | |
| Melanoma | Suppress metastasis | [434] | |||
| Glioblastoma | Induce apoptosis Induce autophagy | [435,436] | |||
| Chrysosplenol D | 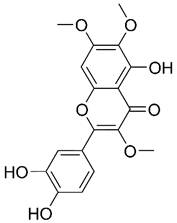 | Lung | Induce apoptosis | [185] | |
| Breast | Cell cycle arrest Induce apoptosis | [57,437] | |||
| Prostate | Induce autophagy | [438] | |||
| Polysaccharides | / | Liver | Induce apoptosis Suppress metastasis Modulate immune function | [171,173] | |
| Polyphenols | / | Colorectal | Cell cycle arrest Induce apoptosis | [282,439,440] | |
| Breast | Regulate EMT Suppress metastasis | [186] | |||
| Prostate | Cell cycle arrest | [440] | |||
Induction of apoptosis
Apoptosis, a form of programmed cell death, is regulated by a complex interplay of various apoptosis-related proteins [189]. This process involves two main pathways: the exogenous (death receptor) pathway and the endogenous (mitochondrial) pathway [190]. The Bcl-2 family proteins play a central role in both of these mechanisms, encompassing pro-apoptotic members such as Bcl-2-associated X protein (Bax), Bcl-2 homologous antagonist killer protein, and Bcl-2-associated death promoter, as well as anti-apoptotic proteins like Bcl-2 itself [191,192]. These proteins are tightly regulated by the tumor suppressor protein p53, which is critical in mediating programmed cell death, particularly in cancer cells under the influence of therapeutic agents [193,194]. Interactions with the apoptotic cell death machinery underlie one of the most important anti-cancer mechanisms of Artemisiae Annuae Herba components (Figure 5).
In colorectal cancer cell lines, artemisinin and its derivatives, the components of Artemisiae Annuae Herba, have been shown to induce apoptosis by activating Bax [195]. This activation leads to the release of cytochrome C, a key event in the intrinsic apoptotic pathway, ultimately resulting in cell death and an anti-cancer effect [196,197]. Additionally, the caspase-dependent pathway, another important mechanism of endogenous apoptosis, has garnered attention in anti-cancer research involving artemisinin and its derivatives [198-200]. For instance, in human gastric cancer cell lines, artesunate has been found to promote apoptosis through the activation of caspases-3 and -9, leading to tumor cell death [206]. Similarly, Lu et al. demonstrated that DHA activates caspase-3 in human lung adenocarcinoma cells (ASTC-a-1), thereby inducing apoptosis and exhibiting significant anti-cancer effects [207,208].
Beyond artemisinin and its derivatives, chrysosplenol D, a flavonol isolated from Artemisiae Annuae Herba, has also shown notable anti-cancer activity, particularly in oral squamous cell carcinoma [204]. The results of in vitro studies have indicated that PI3K/AKT, extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase are downregulated by chrysosplenol D. This inhibition synergizes with the induction of poly(ADP-ribose) polymerase by chrysosplenol D. Additionally, chrysosplenol D further upregulates heme oxygenase-1, leading to apoptosis. Both casticin and chrysosplenol D from Artemisiae Annuae Herba have been shown to induce apoptosis in another way by inhibiting topoisomerase IIα in human non-small-cell lung cancer cells [180]. Taken together, these findings underscore the idea that the induction and regulation of apoptosis constitute a pivotal mechanism underlying the anti-cancer effects of Artemisiae Annuae Herba components.
Seeking anti-cancer effects among interactions with cell death mechanisms: Artemisiae Annuae Herba components and derivatives can often exert their anti-cancer effects through multiple mechanisms that induce tumor cell death. These mechanisms include A) apoptosis, potentially through activation of caspase pathways (through caspase-3 and -9) or modulation of Bcl-2 family proteins, specifically Bcl-2, BAX and BAK; B) autophagy, influenced by IL-3 and the PI3K/AKT/mTOR pathway; and C) ferroptosis, which is associated with labile iron and the Fenton reaction.
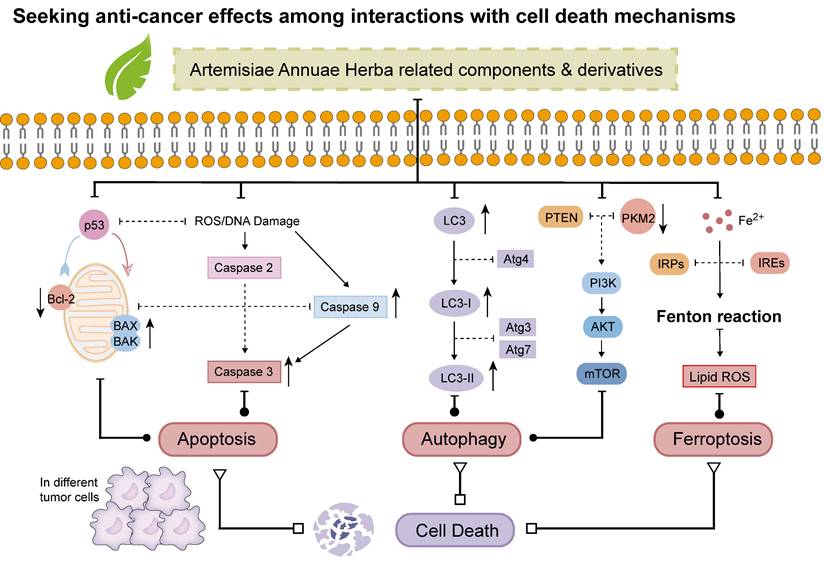
Induction of non-apoptotic cell death
In addition to the programmed cell death mechanism apoptosis, various forms of non-apoptotic cell death [205,206], such as autophagy [212] and ferroptosis [208], have emerged as critical targets in contemporary cancer therapies [209]. Consequently, whether Artemisiae Annuae Herba components induce tumor cell death through these non-apoptotic pathways has garnered significant attention (Figure 5).
Evidence suggests a connection between Artemisiae Annuae Herba components and the induction of autophagy in tumor cells [165]. For example, Hsieh et al. demonstrated that chrysosplenol D enhances autophagy in oral squamous cell carcinoma cells [204]. Specifically, cells treated with chrysosplenol D exhibited an increased accumulation of microtubule-associated protein 1A/1B-light chain 3 within autophagosomes and autophagolysosomes, alongside enhanced autophagosome formation. Similarly, Son et al. reported the effects of MC-4, a partially purified artemisinin-based material, in a clinical trial involving patients with metastatic renal cell carcinoma [210]. MC-4 was shown to enhance the expression of phosphatase and tensin homolog (PTEN), subsequently downregulating the downstream effector Akt/pyruvate kinase muscle isozyme M2 (PKM2). This reduction in PKM2 activity decreased glucose transporter 1 expression, effectively disrupting cancer cell metabolism. Ultimately, these effects promoted autophagy-related cell death through the regulation of the PI3K/Akt/PKM2 and mTORC1 pathways. Meanwhile, in breast cancer research, ART has been found to upregulate the expression of beclin 1, an autophagy initiator, ultimately exerting anti-cancer effects through downstream cascade reactions [211].
Ferroptosis, an iron-dependent form of cell death driven by the accumulation of lipid peroxides on cell membranes, has emerged as a promising therapeutic strategy for various diseases, particularly cancer [217-219]. Artemisiae Annuae Herba components, including artemisinin and its derivatives such as DHA, artesunate, and artemether, have demonstrated the ability to upregulate free iron levels in cancer cells and promote the accumulation of intracellular lipid peroxides [174,220]. This dual action induces ferroptosis in cancer cells, thereby inhibiting tumor progression. Notably, these compounds hold potential not only as direct inducers of ferroptosis but also as adjuncts that enhance the efficacy of other cancer therapies [221]. For example, Chen et al. showed that DHA induces lysosomal degradation of ferritin, a process distinct from traditional autophagy, thereby increasing intracellular free iron and sensitizing cells to ferroptosis [142]. Additionally, DHA interacts with intracellular free iron to stimulate iron regulatory proteins (IRPs), which bind to mRNA molecules containing iron-responsive elements (IREs). This interaction modulates the IRP/IRE system, further disrupting iron homeostasis and elevating free iron levels. Furthermore, DHA has been shown to amplify ferroptosis in cancer cells with high tolerance to this form of cell death, particularly by enhancing the effects of glutathione peroxidase 4 inhibition, as demonstrated in vitro and in mouse models [14,215]. Collectively, these findings suggest that Artemisiae Annuae Herba components sensitize tumor cells to ferroptosis by intricately regulating cellular iron homeostasis.
Inhibition of tumor angiogenesis
During tumor growth and proliferation, the tumor requires a substantial supply of nutrients and energy to sustain its development [217,218]. Solid tumors disrupt the balance between pro-angiogenic and anti-angiogenic factors [219,220], leading to the upregulation of angiogenic stimulators such as matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF), while simultaneously suppressing angiogenesis inhibitors like thrombospondin and tissue inhibitors of metalloproteinases (TIMP) [226]. This imbalance facilitates the enhanced blood supply necessary to support the unrestricted growth of tumor cells [227]. Furthermore, under hypoxic and nutrient-deficient conditions, tumor cells activate transcription factors such as hypoxia-inducible factor-1α (HIF-1α) and NF-κB, which further drive the expression of VEGF to promote angiogenesis [223,224].
Building on this understanding of tumor vascular regulation, studies have shown that Artemisiae Annuae Herba components exhibit anti-angiogenic properties [72,230]. For instance, in mouse embryonic stem cells, artemisinin was found to reduce the levels of HIF-1α and VEGF [226]. Wang et al. showed that oral administration of artemisinin significantly suppressed tumor angiogenesis in a C57BL/6 mouse Lewis lung cancer model by downregulating VEGF-C expression [227]. Similarly, Chen et al. demonstrated that artesunate inhibited tumor angiogenesis in BALB/c nude mice implanted with human ovarian cancer cells [228]. This inhibition was achieved by reducing the expression of VEGF and its receptor KDR/flk-1, ultimately leading to the suppression of tumor growth.
Regulation of EMT and suppression of metastasis
The invasion and metastasis of malignant tumors typically begin with the detachment of tumor cells and the reorganization and degradation of the extracellular matrix (ECM) [234-236]. This process enables tumor cells to spread and colonize other parts of the body through direct extension, blood circulation, lymphatic pathways, and other mechanisms [237,238]. Tumor cell detachment is strongly associated with the downregulation of E-cadherin [239,240] and the degradation of ECM components by proteases such as MMPs [241], both of which play pivotal roles in tumor migration, invasion, and metastasis. Thus, interventions targeting these molecular mechanisms have the potential to impede tumor progression and enhance the efficacy of cancer therapies.
The impact of Artemisiae Annuae Herba components on these targets has garnered significant attention [161]. Artemisinin has been shown to markedly reduce MMP2 levels, thereby preventing tumor cell migration in human melanoma cells [242]. DHA inhibits cell migration and metastasis by suppressing NF-κB activity and reducing MMP2 and/or MMP9 expression in human breast cancer cells [243] and pancreatic cancer cells [239]. Similarly, in non-small cell lung cancer cells, artemisinin was found to inhibit metastasis by downregulating MMP activity and NF-κB signaling [240,241].
Further evidence has highlighted artemisinin's role in modulating E-cadherin expression and ECM stability [242,243]. In hepatocellular carcinoma cells, artemisinin significantly upregulated E-cadherin expression while downregulating MMP2 and TIMP-2 levels, contributing to ECM stabilization [158]. Similarly, in human colorectal cancer cell lines, artemisinin enhanced E-cadherin expression, altered β-catenin subcellular localization, and suppressed the activity of the Wnt signaling pathway [244]. These effects collectively induced apoptosis and inhibited tumor migration and metastasis.
Modulation of the immune system
Both specific and non-specific immunotherapies play important roles in current cancer treatment strategies [245,246]. Specific immunotherapies, such as immune checkpoint inhibitors, CAR T-cell therapy, and tumor vaccines, are advancing rapidly and are often hailed as potential solutions to many cancer-related challenges [252]. However, non-specific immunotherapy also remains an indispensable component of cancer treatment [248,249]. This approach leverages the innate immune system to target and eliminate tumor cells by activating non-specific immune cells through immunomodulators, thereby promoting the proliferation of immune-active substances and lymphocytes to exert anti-cancer effects [255].
Regarding Artemisiae Annuae Herba, previous studies have predominantly highlighted its role as an immunosuppressant [251,252]. This is largely attributed to the anti-inflammatory properties of artemisinin, which enhance its efficacy in treating autoimmune and allergic diseases [253-255]. Nevertheless, the broad-spectrum anti-parasitic, anti-bacterial, and anti-fungal activities of Artemisiae Annuae Herba have led researchers to recognize its complex and multifaceted immunoregulatory effects [261]. Notably, polysaccharides AAP-1, AAP-2, and AAP-3 extracted from Artemisiae Annuae Herba have demonstrated significant immunomodulatory activities in mouse macrophage experiments. These studies revealed a positive correlation between the levels of interleukin-6 and tumor necrosis factor-α and the supplementation of AAP in-vitro, indicating the potent immunostimulatory activity of these polysaccharides [11,171]. Further investigation into the underlying mechanisms revealed that AAPs from Artemisiae Annuae Herba extracts can be recognized by TLRs, leading to macrophage activation and the subsequent release of immune-related cytokines.
Drawing from extensive scientific literature and clinical guidelines, immunoregulatory therapies have consistently maintained a significant role in cancer treatment [262]. The polysaccharide components isolated and purified from Artemisiae Annuae Herba have shown potential as immune function regulators, paving the way for the development of anti-cancer agents.
Duality and unity of Artemisiae Annuae Herba in cancer therapy
Clearly, the anti-cancer effects of Artemisiae Annuae Herba extend beyond merely targeting a specific gene or signaling pathway. Instead, its action represents a multidirectional and comprehensive macro-control approach against cancer. The efficacy of Artemisiae Annuae Herba in treating tumors is influenced by the organismal status of various cancer types and the composition of different drug groups, which determine the dominant components and effective mechanisms at play [13]. Notably, the anti-cancer potential of Artemisiae Annuae Herba is primarily driven by cell death induced through the tumor cytotoxicity of Artemisiae Annuae Herba components [119,258]. Additionally, the modest immunosuppressive effects of artemisinin, along with the complementary role of polysaccharides in non-specific immune activation, further enhance its anti-cancer capabilities [168]. The anti-cancer efficacy of Artemisiae Annuae Herba is thus elevated through the favorable interaction and synergistic unity among its various components.
Feasibility of Using Artemisiae Annuae Herba in Cancer Treatment
Artemisiae Annuae Herba exhibits diverse and rational anti-cancer mechanisms, with significant anti-cancer effects observed in numerous experiments both in vitro and in vivo. However, in recent years, there has been a noticeable lack of breakthroughs in clinical research related to the anti-cancer effects of this preparation [13], representing a critical gap that poses challenges for the use of Artemisiae Annuae Herba to address cancer by the medical community. This issue is largely attributable to the entrenched perception of artemisinin as primarily an anti-malarial agent. Both clinicians and cancer patients often fail to associate Artemisiae Annuae Herba with its potential anti-cancer applications and may even dismiss such claims. This cognitive bias has hindered the exploration and development of Artemisiae Annuae Herba as an anti-cancer agent. In this regard, it is essential to recognize that Artemisiae Annuae Herba components encompass more than a few isolated derivatives [264,265]; the plant contains numerous bioactive components with potential therapeutic benefits. Therefore, a comprehensive feasibility assessment of artemisinin and other complex components of Artemisiae Annuae Herba as candidates for cancer therapies remains a critical area for further investigation (Figure 6).
Anti-cancer applications of Artemisiae Annuae Herba: The traditional Chinese medicine Artemisiae Annuae Herba may be utilized in cancer therapies as direct killer drugs and as indirect adjunctive drugs in connection with the application of other Chinese medicines.
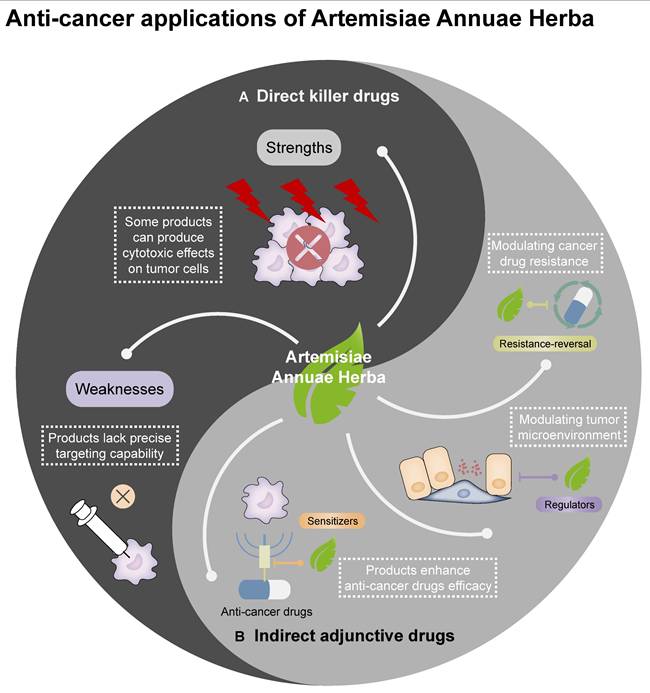
Comparisons of the anti-cancer activities of Artemisiae Annuae Herba components and existing drugs
Feasibility analyses and evaluations of Artemisiae Annuae Herba components as anti-cancer agents can be initiated by comparing them with conventional anti-cancer drugs approved by the FDA. Current anti-cancer combination therapies primarily include chemotherapy drugs, molecular targeted drugs, immunotherapy drugs, and endocrine therapy drugs, among other less conventional drugs. Due to the complex composition of Artemisiae Annuae Herba components, it is challenging to categorize all of the components under any single existing drug class; instead, artemisinin and other components often below within multiple categories.
The primary components of Artemisiae Annuae Herba are sesquiterpenes, which exhibit structural similarities to anti-cancer agents derived from other medicinal plants [261,262]. As discussed above, structural analyses have revealed that Artemisiae Annuae Herba mediates cytotoxic effects and proliferation inhibition via the distinctive peroxide bridge structure of artemisinin and via other non-specific structural interactions [169,225,263]. However, compared to current alkylating agents and chemotherapeutics (e.g., paclitaxel and cisplatin), Artemisiae Annuae Herba exhibits weaker cytotoxicity against tumor cells. Nevertheless, the peroxide bridge structure and iron-dependent Fenton reaction mechanisms merit rigorous investigation to validate their therapeutic feasibility and efficacy.
In advancing the herb's anti-cancer applications, the combination of Artemisiae Annuae Herba components with transferrin-mediated drug delivery systems has emerged as a particularly promising strategy [269-271]. This approach leverages both the intrinsic anti-cancer properties of Artemisiae Annuae Herba and the tumor-targeting capabilities of transferrin receptors (TfR1), which are overexpressed in many malignancies. Transferrin, a plasma glycoprotein responsible for iron transport, binds to TfR1 with high affinity. Since TfR1 is overexpressed in many cancers, including breast [267,268], lung [269,270], and pancreatic [271,272], transferrin has been widely explored as a “Trojan horse” for targeted drug delivery. By conjugating artemisinin derivatives to transferrin or encapsulating them within transferrin-coated nanoparticles, researchers aim to enhance tumor-specific accumulation while minimizing off-target effects. For instance, a 2022 study demonstrated that transferrin-modified liposomes loaded with DHA achieved higher uptake in TfR1-positive triple-negative breast cancer cells compared to normal cells, significantly improving therapeutic efficacy in models [278]. In glioblastoma models, transferrin-conjugated artemisinin nanoparticles penetrated the blood-brain barrier and reduced tumor volume compared to free artemisinin [274].
Leveraging its targeted recognition mechanisms and combination-mediated precision therapy, Artemisiae Annuae Herba differentiates itself from traditional chemotherapy by selectively inducing tumor cell death with minimal systemic toxicity and a favorable safety profile, key advantages for anti-cancer drug development. These safety advantages are supported by clinical studies. For instance, Rassias et al. demonstrated that Artemisiae Annuae Herba components effectively suppress tumor growth while exhibiting minimal cytotoxicity toward normal cells, underscoring its translational potential for relatively safe cancer therapies [53]. These findings validate Artemisiae Annuae Herba's feasibility as a targeted anti-cancer agent with distinct clinical advantages [122,275].
In contrast to the rapid advancements in immunotherapy, particularly specific therapies like immune checkpoint inhibition and CAR-T therapy, polysaccharides from Artemisiae Annuae Herba primarily exert a non-specific immune regulatory effect akin to thymosin drugs [11,173,281]. They influence immune factor-related components within the tumor microenvironment [282]. When considering only the immunomodulatory capabilities of Artemisiae Annuae Herba, the isolated and purified components may not exhibit sufficient anti-cancer efficacy. However, its non-specific immune modulation enhances the therapeutic activity of Artemisiae Annuae Herba-derived anti-cancer agents. Moreover, the multi-mechanistic profile can enable Artemisiae Annuae Herba to be developed into a single drug with synergistic component interactions that amplifying its anti-cancer effects. Just as with the specific delivery systems based on transferrin, Artemisiae Annuae Herba-related immunomodulatory preparations are also being further developed. In addition to the synergistic effects on drug resistance and tumor suppression exhibited by combinations of artemisinin with transferrin-based delivery systems [283,284], transferrin-coupled artemisinin formulations have also shown immunomodulatory effects. In a hepatocellular carcinoma study, these nanoparticles promoted M1 macrophage polarization and increased CD8+ T-cell infiltration, suggesting potential for multi-mechanism cancer treatment [280].
Beyond comparisons with common drugs, Artemisiae Annuae Herba components share characteristics with certain specialized anti-cancer agents. For instance, some preparations can serve as auxiliary anti-cancer agents with apoptosis inducers like bortezomib [281,282] and can also inhibit tumor neovascularization, akin to angiogenesis inhibitors such as thalidomide [51,283]. Additionally, differentiation inducers play a crucial role in treating hematological malignancies [289,290]. The potential of artemisinin to function as a differentiation inducer, comparable to all-trans-retinoic acid or arsenical agents, remains underexplored and warrants further research and evaluation [291].
Despite their promising potential, Artemisiae Annuae Herba components exhibit certain weaknesses in their application as anti-cancer agents [127]. Unlike the widely used and highly regarded targeted therapies, these preparations lack the capability to specifically target mutated genes or abnormal signaling pathways, which are essential for precise cancer treatment. Consequently, compared to molecularly targeted drugs, Artemisiae Annuae Herba components are less effective in achieving precision-guided tumor cell eradication [127]. Their therapeutic specificity for particular tumor mutations is lower, and they cannot minimize individual drug-related side effects to the same extent as molecularly targeted treatments. However, this limitation may also present a unique advantage. While lacking precision, the broad and multifaceted anti-cancer mechanisms of Artemisiae Annuae Herba significantly expand their potential therapeutic applications. Notably, experimental evidence supports the anti-cancer effects of Artemisiae Annuae Herba components in both hematologic malignancies [196,287] and a variety of solid tumors [185,186,293]. This versatility underscores their potential utility across a wide range of cancer types. Taking these factors into account, Artemisiae Annuae Herba components hold considerable promise as candidates for future anti-cancer strategies.
Feasibility of combination therapies that include Artemisiae Annuae Herba components
Cancer therapies often include numerous drugs that do not directly kill tumors but instead work synergistically with other anti-cancer agents [294,295]. These drugs, known as sensitizers, are crucial in enhancing the efficacy and reducing the toxicity of treatment regimens [291-293]. A significant category of these sensitizers is derived from TCM [294-296].
Among TCM-based sensitizers, Artemisiae Annuae Herba components possess distinctive similarities to certain established compounds and enhance the therapeutic effects of primary drugs by modulating signaling pathways or protein expression [302-304]. For instance, in research involving HCT116 colorectal cancer cells, polyphenols isolated from Artemisiae Annuae Herba were found to enhance the anti-cancer effects of β-lapachone against oxaliplatin-resistant strains by inducing DNA damage and regulating apoptosis through multiple mechanisms [179]. Similarly, in hematological acute lymphoblastic leukemia, the methanolic extract of Artemisiae Annuae Herba has been shown to potentiate the anti-cancer effects of vincristine by mediating cytotoxicity [263].
Another mechanism by which these drugs operate is by modifying the tumor cell microenvironment, thereby optimizing conditions for the primary anti-cancer drugs [305,306]. For example, quercetin, which can be isolated from Artemisiae Annuae Herba, has been shown by Guo et al. to regulate the tumor microenvironment in endometrial cancer, inhibiting tumor cell growth and migration [177]. However, this study did not further assess the efficacy of Artemisiae Annuae Herba in combination with conventional anti-cancer drugs for endometrial cancer.
Certain drugs can interact with chemotherapy agents or specific structural components of tumor cells through their molecular configurations, enhancing the efficacy of cancer therapies or weakening tumor cell activity to achieve improved therapeutic outcomes [307-309]. For instance, Wang et al. demonstrated that artemisinin derivatives can bind to rhein to exert dual inhibition on heat shock protein 72 and heat shock cognate 70, thereby enhancing rhein's therapeutic efficacy against liver cancer [305]. Similarly, Fu et al. revealed that Artemisiae Annuae Herba-derived compounds such as casticin and chrysosplenol D can bind to apical Iα-DNA. This interaction disrupts DNA replication, induces DNA damage, and consequently boosts the effectiveness of anti-cancer drugs in treating non-small-cell lung cancer [180].
Beyond sensitizing agents that enhance the efficacy and mitigate the toxicity of anti-cancer adjuvant drugs, there is growing interest in resistance-reversal agents, which counteract tumor cell drug resistance—a major challenge in current cancer therapies [306-309]. To address the frequent emergence of drug resistance among existing anti-cancer agents, research has increasingly turned to TCM for potential solutions [310,311]. Notably, Artemisiae Annuae Herba components not only exhibit direct anti-cancer activity across various cancer types but also hold significant potential for reversing drug resistance through multilayered regulatory mechanisms [312-314]. For example, Ma et al. showed that the artemisinin derivative artesunate can reverse the resistance of hepatocellular carcinoma to sorafenib [315]. This effect is achieved by downregulation of expression of actin filament associated protein 1 like 2 protein expression, inhibiting the phosphorylation of inhibiting SRC and FUN14 domain-containing 1, and inducing mitochondrial autophagy and apoptosis in sorafenib-resistant cancer cells. Additionally, flavonoids extracted from Artemisiae Annuae Herba have been shown to regulate P-glycoprotein, a protein closely associated with multidrug resistance in tumor cells [321,322]. These findings underscore the potential of Artemisiae Annuae Herba components in overcoming tumor resistance and advancing cancer treatment strategies.
Challenges in Artemisiae Annuae Herba-Based Cancer Therapy
There are many aspects of Artemisiae Annuae Herba worth discussing in relation to its anti-cancer properties, and its actual efficacy and potential mechanisms have gained significant recognition. However, focusing solely on its positive aspects can lead to undue optimism. It is important to acknowledge that numerous challenges remain before Artemisiae Annuae Herba components can be widely recognized and formally used in cancer treatments (Figure 7).
Challenges of guiding the components to the target area
While Artemisiae Annuae Herba shows promise as an anti-cancer agent, reducing it to a conventional plant-derived chemotherapeutic would undermine its unique advantages. Similar to its anti-malarial action, the herb's anti-cancer advantages rely on interactions with specific molecular targets, which guide the compounds to the necessary site of action. However, the targets of Artemisiae Annuae Herba components are abundant in the context of malaria, but tumor heterogeneity makes such targets more scarce in the realm of cancer. For example, key components of Artemisiae Annuae Herba, such as artemisinin, artemisitene, and DHA, are known to target heme and ferrous ions [88,323,324]. In malaria, heme is abundant due to the parasite's lifecycle, but in tumors, heme/ferrous ion targets are rare owing to genetic diversity and mutations.
To address this challenge, two strategic approaches are proposed. The first approach involves the development of synthetic targeting carriers. These carriers, potentially constructed from nanomaterials, would bind to tumor-specific antigens and locally concentrate heme/ferrous ions, enabling precise tumor targeting by Artemisiae Annuae Herba. Inspired by malaria's heme-targeting mechanism, nanocarriers could be engineered with dual-functional surfaces: one end binds tumor-specific antigens, while the other captures ferrous ions or promotes tumor cells to produce heme. This process would mimic the red blood cell invasion of Plasmodium, hijacking tumor cells to amplify drug targeting. The second strategy involves the utilization of heme metabolic precursors. Here, tumor metabolism would be leveraged by administering heme precursors, like aminolevulinic acid, which accumulates in tumors and converts to heme, creating localized targets for Artemisiae Annuae Herba agents [325,326].
Both strategies face significant hurdles: carrier design demands interdisciplinary innovation, while precursor optimization requires extensive trial-and-error validation. Overcoming these challenges is critical to advancing Artemisiae Annuae Herba-based therapies into clinical practice.
Challenges facing the application of Artemisiae Annuae Herba: Although Artemisiae Annuae Herba has bright prospects as cancer treatment, the challenges to its implementation cannot be ignored. The main dilemmas include: A) Challenges of guiding compounds to the cancer site: two strategic approaches are proposed, including synthetic targeting carriers and metabolic precursor utilization; B) Challenges of solubility and delivery: liposomal formulations are a possible solution; C) Challenges of potential risks related to drug metabolism: albumin-bound formulations are proposed as a key strategy; D) Challenges of side effects and safety related to long-term management: multicenter large-scale research must be prioritized and expanded.
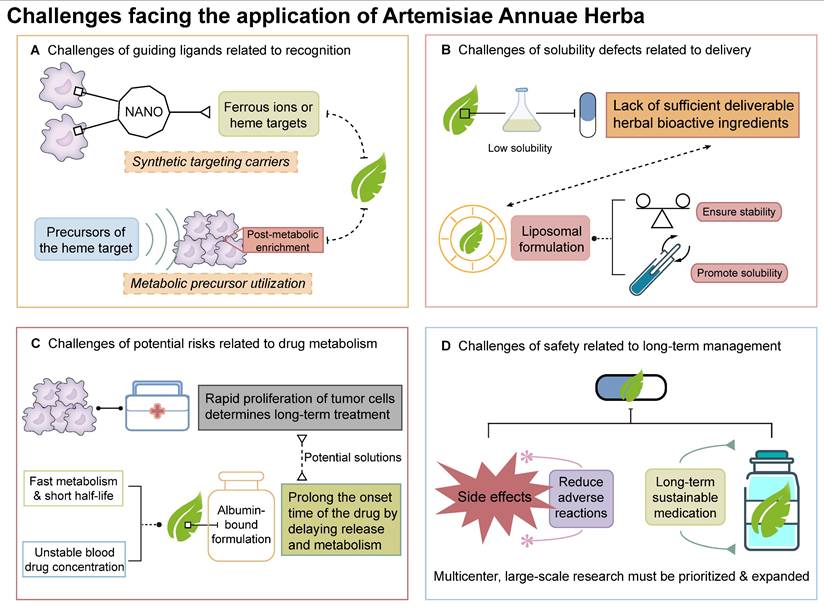
Delivery challenges due to low water solubility
Artemisiae Annuae Herba faces hurdles in anti-cancer applications that are akin to its historical limitations in the context of malaria treatment. Initially, the herb's anti-malarial efficacy was limited by suboptimal extraction methods and the poor aqueous solubility of its active sesquiterpene compounds. The lipophilic nature of sesquiterpenes, the key bioactive components, results in low aqueous solubility, hindering clinical translation [322]. Even solubility-enhanced derivatives face stability issues, creating a dilemma between precise dosing requirements for cancer therapy and physicochemical limitations.
Similar solubility challenges plague other plant-derived chemotherapeutics (e.g., paclitaxel and vincristine), yet advanced delivery systems have enabled their clinical success. Innovative drug delivery strategies, particularly liposomal formulations, offer transformative solutions for low-solubility agents. Liposomes enhance stability and enable precise drug loading, mitigating solubility-related bioavailability fluctuations. While Artemisiae Annuae Herba-derived liposomes (e.g., artesunate liposomes) remain experimental, optimizing drug-carrier compatibility, encapsulation efficiency, and payload capacity represent critical research frontiers [323-325].
Challenges of rapid drug metabolism
In addition to the challenges that are related to those faced for malaria treatment, Artemisiae Annuae Herba agents also face hurdles that are unique to clinical translation for oncology. Unlike malaria, an acute infection, tumor management requires chronic suppression of proliferating cells to maintain a low tumor burden, a distinction rooted in disease pathophysiology. Anti-malarial therapy aims to eradicate parasites in a single course, whereas tumors demand prolonged, cyclical treatment due to their self-renewing, proliferative nature. These prolonged treatments thus require the optimization of dosing regimens for Artemisiae Annuae Herba in oncology.
One potential solution is referred to as short-cycle therapy. Considering the precision anti-cancer action of Artemisiae Annuae Herba components and their association with fewer side effects compared with traditional chemotherapy, it is worth considering shortening dosing intervals during cancer therapy to achieve a shorter total cycle and to allow patients to return to normal life earlier while ensuring the same survival benefit. A second potential solution is to use these agents in maintenance therapy. As long as the risk of significant side effects remains low, it is worth considering long-term administration of drugs derived from Artemisiae Annuae Herba and to no longer routinely discontinue the drug when the tumor burden has decreased. The goal would be to maintain a low-level balance of tumor burden through tumor killing and inhibition of tumor proliferation in order to reduce tumor recurrence and metastasis. Finally, a third possible option is to implement a pulse-dosing strategy. This option is essentially a combination of the other two options in which the agents are delivered at a low level for long-term administration, but the patient receives regular high-dose pulsative shock treatments with appropriate Artemisiae Annuae Herba agents. This strategy could further reduce the occurrence of drug resistance, and we posit that pre-clinical and clinical studies are warranted.
Dosing regimens for Artemisiae Annuae Herba also face pharmacokinetic challenges, particularly in maintaining therapeutic drug concentrations. In malaria therapy, rapid drug clearance of Artemisiae Annuae Herba derivatives can lead to subtherapeutic drug levels, leading to pseudo-resistance. Pharmacokinetic studies have revealed that Artemisiae Annuae Herba components achieve rapid peak plasma concentrations but exhibit short half-lives due to fast metabolism and excretion [326,327]. This transient subtherapeutic exposure may be misinterpreted as drug resistance in malaria, complicating treatment outcomes. For anti-cancer applications, these pharmacokinetic limitations pose significant challenges for sustaining therapeutic drug levels. To address this, albumin-bound formulations could prolong the onset time of the drug by delaying drug release and metabolism. Alternatively, developing derivatives with extended half-lives represents a promising research direction.
Challenges of side effects and safety related to long-term management
Despite the potential of Artemisiae Annuae Herba components to reverse drug resistance in certain tumors, they also exhibit notable resistance issues themselves when employed in cancer therapy. For instance, in a clinical study on Artemisiae Annuae Herba-assisted prostate cancer treatment, researchers observed that although some patients initially experienced tumor control, prolonged oral administration of Artemisiae Annuae Herba capsules was associated with a progressive rise in PSA levels [122]. This underscores the necessity for further investigation into the mechanisms of drug resistance and the development of strategies to mitigate this issue.
Anti-cancer efficacy of Artemisiae Annuae Herba components is further complicated by insufficient pharmacotoxicological data on chronic exposure. While acute exposure to artemisinin in anti-malarial therapy has rarely resulted in neurotoxicity [333,334] or anaphylaxis [335], the use of Artemisiae Annuae Herba as a direct anti-cancer chemotherapeutic agent necessitates comprehensive pharmacokinetic and toxicological data, particularly concerning the short-term effects of high doses. When Artemisiae Annuae Herba components are considered for long-term adjuvant cancer therapy, it is imperative to conduct studies on chronic toxicology and drug interactions, especially for patients with chronic diseases requiring prolonged medication. Given these challenges, multicenter, large-scale research on Artemisiae Annuae Herba must be prioritized and expanded.
Despite these challenges, the potential of Artemisiae Annuae Herba components to deliver breakthroughs in cancer treatment is undeniable. The scientific community has amassed a wealth of basic and clinical research data on the anti-cancer effects of Artemisiae Annuae Herba over the past two decades. It is now important to further investigate their clinical application value to benefit cancer patients globally.
Conclusion and Prospects
The development of new cancer therapies has faced significant bottlenecks, prompting exploration into repurposing natural drugs and TCM agents for cancer treatment. This review examines the potential of Artemisiae Annuae Herba components as anti-cancer agents. It begins by reviewing the historical context of Artemisiae Annuae Herba in both Eastern and Western medicine, highlighting the increasingly sophisticated isolation and purification of its medicinal components, as well as the ongoing redefinition and expansion of its pharmacological effects. The article then elaborates on the diverse and complex, yet interacting, pharmacological effects of Artemisiae Annuae Herba components, including sesquiterpenes, polysaccharides, and peptides, encompassing anti-parasitic, anti-viral, anti-bacterial, anti-fungal, anti-inflammatory, anti-obesity, anti-osteoporosis, and anti-cancer activities. To elucidate the mechanisms underlying its anti-cancer effects, two analytical approaches have been employed. First, a forward reasoning approach from molecular structures to anti-cancer effects posits three independent but synergistic herb structures: the specific peroxy-bridge structures, the non-specific anti-proliferation structures, and the immunomodulatory structures. Second, a reverse reflection from herb response to potential anti-cancer mechanisms details the various modes of action and applications of Artemisiae Annuae Herba components in cancer treatment. These mechanisms include cell cycle arrest, induction of tumor cell apoptosis, non-apoptotic cell death, inhibition of tumor angiogenesis, regulation of EMT, and modulation of immune function. This analysis of the multiple synergistic mechanisms underlying Artemisiae Annuae Herba's anti-cancer activity supports its potential as an urgently needed anti-cancer drug and underscores the feasibility of its application in cancer therapies. This potential is further strengthened by early anti-cancer experiments suggesting its wide applicability.
It is important to note that the anti-cancer capabilities of various components of Artemisiae Annuae Herba differ due to their distinct pharmacological activities and mechanisms of action. Sesquiterpenoids, the core components, exert direct anti-cancer effects, demonstrating demonstrable efficacy in inhibiting proliferation and inducing tumor cell death, while coumarins and lignans offer unique advantages in modulating the tumor microenvironment. Furthermore, the auxiliary anti-cancer role of polysaccharide-related components in regulating immune function should not be underestimated.
However, despite the increasing recognition of the anti-cancer effects of Artemisiae Annuae Herba, current research faces several gaps. Existing studies have relied heavily on in vitro cell experiments, which, while useful for demonstrating anti-cancer potential in a simplified setting, fail to replicate the complexity of the tumor microenvironment and the host's immune system. This limitation hinders the accurate assessment of the translational potential of Artemisiae Annuae Herba. Furthermore, research has often focused on single-agent artemisinin and its derivatives, neglecting the synergistic regulatory anti-cancer effects of other components, such as polysaccharides and peptides. This isolation of components fragments the complex interactions within Artemisiae Annuae Herba and obscures the potential contributions of regulatory molecules. In addition, many studies do not explicitly state whether the tested component is a pure component or a hybrid product containing small amounts of other regulatory components, leading to inconsistencies in reported anti-cancer efficacy. Finally, the field lacks precision in defining the specific roles of different Artemisiae Annuae Herba components in cancer therapy. Grouping components with diverse mechanisms, such as those primarily targeting tumor cells, inhibiting angiogenesis, or modulating immune function, under the umbrella term “Artemisinin derivatives” impedes clinical translation. Addressing these research gaps requires more comprehensive experimental studies, utilizing animal models, organoid models, and, critically, clinical trials. Increased clinical investigation will elucidate the true efficacy of Artemisiae Annuae Herba in treating cancers, assess the influence of the tumor microenvironment and host immunity on its anti-cancer effects, and identify potential synergistic interactions between its various components through comparative trials. Concurrently, the implementation of additional clinical studies will facilitate the standardized classification and characterization of Artemisiae Annuae Herba components, streamlining research efforts and promoting a more efficient cycle of discovery and clinical translation.
Meanwhile, further development of Artemisiae Annuae Herba in cancer treatment faces several challenges, including guiding ligands related to their desired targets, issues with solubility and drug delivery, potential risks related to drug metabolism, side effects, and safety concerns related to long-term management. Addressing the challenge of ligand targeting through artificially constructed ligands or target precursors could enable Artemisiae Annuae Herba to achieve a breakthrough in precision treatment. This would allow it to specifically identify and target tumor lesions, similar to its anti-malarial activity, potentially facilitating the clinical translation of its cancer therapeutic effects. Overcoming challenges related to drug solubility, delivery, and distribution could also break through the blockade of the strict dosage requirements that currently limit many anti-cancer drugs, enabling standardized regulation of Artemisiae Annuae Herba-based anti-cancer agents. Validating and evaluating sufficient research data in the future could lead to a departure from the current rigid chemotherapy treatment cycles, providing cancer patients with more treatment options and improved time management, ultimately enhancing their quality of life. Furthermore, a deeper understanding of Artemisiae Annuae Herba drug metabolism, coupled with the use of albumin and other related carriers to slow down drug half-life and maintain blood drug concentrations, could provide a solution for long-term and stable anti-cancer needs, positioning it advantageously for clinical translation. Finally, collecting comprehensive experimental data on long-term and high-dose pulse use, and analyzing the corresponding effectiveness, side effects, and potential problems, can effectively prevent subsequent adverse events before it becomes a widely accepted anti-cancer drug.
Artemisiae Annuae Herba has evolved beyond its initial recognition as a specialized anti-malarial drug. Research into its anti-cancer properties is now well established and increasingly recognized. Continued basic research and sustained efforts are actively advancing its practical application in cancer therapies, marking a promising direction for future exploration.
Abbreviations
3CLpro: 3C-like proteinase
ACTs: artemisinin combination therapies
ADP-ribose: adenosine diphosphate ribose
AFAP1L2: actin filament associated protein 1-like 2
AKT: protein kinase B
ALL: acute lymphoblastic leukemia
ARE: arteether
ARM: artemether
ART: artesunate
BAD: Bcl-2-associated death promoter
BAK: Bcl-2 homologous antagonist killer protein
BAX: Bcl-2-associated X protein
CDKs: cyclin-dependent kinases
DAT: dihydroartemisinin
DHA: dihydroartemisinin
E2F: E2 promoter binding factor
ECM: extracellular matrix
EMT: epithelial-mesenchymal transition
GPX4: glutathione peroxidase 4
HIF-1α: hypoxia-inducible factor-1α
HO-1: heme oxygenase-1
HSC70: heat shock protein70
HSP72: human heat shock protein 72
IREs: iron-responsive elements
IRPs: iron regulatory proteins
KDR: kinase insert domain receptor
LC3: microtubule-associated protein 1A/1B-light chain 3
mTOR: mammalian target of rapamycin
MAPK: mitogen-activated protein kinase
MMPs: matrix metalloproteinases
NF-κB: nuclear factor kappa-B
NFATc1: nuclear factor of activated T-cells 1
NSCLC: non-small cell lung cancer
OSCC: oral squamous cell carcinoma
pRb: phosphorylated retinoblastoma protein
PI3K: phosphatidylinositol 3-kinase
PKM2: pyruvate kinase muscle isozyme M2
PPARγ: peroxisome proliferator-activated receptor γ
PSA: prostate-specific antigen
PTEN: phosphatase and tensin homolog
RANKL: receptor activator of nuclear factor-κ B ligand
ROS: reactive oxygen species
TfR: transferrin receptor
TCM: traditional Chinese medicine
TIMP: tissue inhibitors of metalloproteinase
TLR: toll-like receptors
TSP: thrombospondin
VEGF: vascular endothelial growth factor
Acknowledgements
We are grateful to all the researchers who have contributed to the field of Artemisiae Annuae Herba. Thanks for the help with adobe illustrator in drawing the components of the figures.
Funding
This study is supported by the Joint Fund of Henan Science and Technology Research (232103810048), the Joint Fund of the Major Science and Technology Projects in Henan Province (235200810002), the Leading Academic Discipline Project of Henan Provincial Health Commission, the High-level Innovative Talents Program, the National Clinical Key Specialty Construction Foundation of China (ZLKFJJ20230401).
Author contributions
X.S. and S.G. guided and designed this review. J.S., J.H., J.X., Z.Q., Q.G., and Z.Z. wrote the original manuscript and drew the figures and tables. J.H. revised the manuscript. S.G. obtained financial support. All authors contributed to the article and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yao CL, Zhang JQ, Li JY, Wei WL, Wu SF, Guo DA. Traditional Chinese medicine (TCM) as a source of new anticancer drugs. Nat Prod Rep. 2021;38:1618-33
2. Sui XB, Xie T. Combination of Chinese and Western medicine to prevent and reverse resistance of cancer cells to anticancer drugs. Chin J Integr Med. 2020;26:251-5
3. Li Z, Feiyue Z, Gaofeng L. Traditional Chinese medicine and lung cancer-From theory to practice. Biomed Pharmacother. 2021;137:111381
4. Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15:283-98
5. Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front Immunol. 2020;11:609705
6. Lai JQ, Zhao LL, Hong C, Zou QM, Su JX, Li SJ. et al. Baicalein triggers ferroptosis in colorectal cancer cells via blocking the JAK2/STAT3/GPX4 axis. Acta Pharmacol Sin. 2024;45:1715-26
7. Abbasi Holasou H, Valizadeh N, Mohammadi SA. Molecular insights into the genetic diversity and population structure of Artemisia annua L. as revealed by insertional polymorphisms. Rev Bras Bot. 2023;46:51-60
8. Ding X, Pan H, Shi P, Zhao S, Bao S, Zhong S. et al. A comparative analysis of chloroplast genomes revealed the chloroplast heteroplasmy of Artemisia annua. Front Pharmacol. 2024;15:1466578
9. Li Y, Yang Y, Li L, Tang K, Hao X, Kai G. Advanced metabolic engineering strategies for increasing artemisinin yield in Artemisia annua L. Hortic Res. 2024;11:uhad292
10. Feng X, Cao S, Qiu F, Zhang B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol Ther. 2020;216:107650
11. Zhang L, Reddy N, Khoo CS, Koyyalamudi SR. Structural characterization and in-vitro antioxidant and immunomodulatory activities of polysaccharide fractions isolated from Artemisia annua L. Molecules. 2022;27:3643
12. Mirbehbahani FS, Hejazi F, Najmoddin N, Asefnejad A. Artemisia annua L. as a promising medicinal plant for powerful wound healing applications. Prog Biomater. 2020;9:139-51
13. Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol. 2017;46:65-83
14. Chen Y, Mi Y, Zhang X, Ma Q, Song Y, Zhang L. et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J Exp Clin Cancer Res. 2019;38:402
15. Dai X, Chen W, Qiao Y, Chen X, Chen Y, Zhang K. et al. Dihydroartemisinin inhibits the development of colorectal cancer by GSK-3β/TCF7/MMP9 pathway and synergies with capecitabine. Cancer Lett. 2024;582:216596
16. Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH, Wang ZX. et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol Sin. 2021;42:301-10
17. Liu H, Tian X, Zhang Y, Wang C, Jiang H. The discovery of Artemisia annua L. in the Shengjindian cemetery, Xinjiang, China and its implications for early uses of traditional Chinese herbal medicine qinghao. J Ethnopharmacol. 2013;146:278-86
18. Ekiert H, Knut E, Świątkowska J, Klin P, Rzepiela A, Tomczyk M. et al. Artemisia abrotanum L. (Southern Wormwood)-history, current knowledge on the chemistry, biological activity, traditional use and possible new pharmaceutical and cosmetological applications. Molecules. 2021;26:2503
19. Acton N, Klayman DL, Rollman IJ. Reductive electrochemical HPLC assay for artemisinin (qinghaosu). Planta Med. 1985;51:445-6
20. Liu HM, Liu GL, Wu HZ. Studies on the constituents of Qing-hao (Artemisia annua L.). Yao Xue Xue Bao. 1981;16:65-7
21. Zhang XQ, Xu LX. Determination of qinghaosu (arteannuin) in Artemisia annua L. by pulse polarography. Yao Xue Xue Bao. 1985;20:383-6
22. Huang L, Liu JF, Liu LX, Li DF, Zhang Y, Nui HZ. et al. Antipyretic and anti-inflammatory effects of Artemisia annua L. Zhongguo Zhong Yao Za Zhi. 1993;18:44-8 63-4
23. Tan Y, Zhao Y, Lin Q, Xie G, Yang P, Yin X. Experimental study on antiendotoxin effect of extracts from Artemisia annua L. Zhongguo Zhong Yao Za Zhi. 1999;24:166-71 192
24. Tu YY, Ni MY, Zhong YR, Li LN, Cui SL, Zhang MQ. et al. Studies on the constituents of Artemisia annua L. Yao Xue Xue Bao. 1981;16:366-70
25. Tu YY. The constituents of young Artemisia annua. Zhong Yao Tong Bao. 1985;10:35-6 18
26. Tu YY. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17:1217-20
27. Miller LH, Su X. Artemisinin: discovery from the Chinese herbal garden. Cell. 2011;146:855-8
28. Tu YY. The development of the antimalarial drugs with new type of chemical structure-qinghaosu and dihydroqinghaosu. Southeast Asian J Trop Med Public Health. 2004;35:250-1
29. Tu YY. Artemisinin-a gift from traditional Chinese medicine to the world (Nobel Lecture). Angew Chem Int Ed Engl. 2016;55:10210-26
30. Efferth T, Zacchino S, Georgiev MI, Liu L, Wagner H, Panossian A. Nobel Prize for artemisinin brings phytotherapy into the spotlight. Phytomedicine. 2015;22:A1-3
31. Kim DY, Son SR, Kim JY, Min JW, Kong CH, Park K. et al. Effects of Artemisia annua L. on postmenopausal syndrome in ovariectomized mice. J Ethnopharmacol. 2023;317:116800
32. Kondža M, Mandić M, Ivančić I, Vladimir-Knežević S, Brizić I. Artemisia annua L. extracts irreversibly inhibit the activity of CYP2B6 and CYP3A4 enzymes. Biomedicines. 2023;11:232
33. Nair MS, Huang Y, Fidock DA, Towler MJ, Weathers PJ. Artemisia annua L. hot-water extracts show potent activity in vitro against Covid-19 variants including delta. J Ethnopharmacol. 2022;284:114
34. Jiang B, Liu J, Qu Z, Wang Y, Wang Y, Li Z. et al. Mechanism of dihydroartemisinin in the treatment of ischaemia/reperfusion-induced acute kidney injury via network pharmacology, molecular dynamics simulation and experiments. Int Immunopharmacol. 2025;144:113705
35. Long Z, Xiang W, Xiao W, Min Y, Qu F, Zhang B. et al. Advances in the study of artemisinin and its derivatives for the treatment of rheumatic skeletal disorders, autoimmune inflammatory diseases, and autoimmune disorders: a comprehensive review. Front Immunol. 2024;15:1432625
36. Feng XT, Ma JX, Wang Y, Shen JH, Zhou LY, Li YY. et al. Research progress on artemisinin and its derivatives in treatment of orthopedics-related diseases. Zhongguo Zhong Yao Za Zhi. 2024;49:4829-40
37. Liu MK, Tang JJ, Li H, Chen XY, Cai JL, Lin GY. et al. Artemisitene ameliorates carbon tetrachloride-induced liver fibrosis by inhibiting NLRP3 inflammasome activation and modulating immune responses. Int Immunopharmacol. 2024;146:113818
38. Gao F, Sun Z, Kong F, Xiao J. Artemisinin-derived hybrids and their anticancer activity. Eur J Med Chem. 2020;188:112044
39. Woerdenbag HJ, Moskal TA, Pras N, Malingré TM, el-Feraly FS, Kampinga HH. et al. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J Nat Prod. 1993;56:849-56
40. Beekman AC, Barentsen AR, Woerdenbag HJ, Van Uden W, Pras N, Konings AW. et al. Stereochemistry-dependent cytotoxicity of some artemisinin derivatives. J Nat Prod. 1997;60:325-30
41. Xu Q, Deng H, Huang X, Chen GQ, Quan YS, Wang YL. et al. Design, synthesis, and in vitro and in vivo biological evaluation of dihydroartemisinin derivatives as potent anti-cancer agents with ferroptosis-inducing and apoptosis-activating properties. Eur J Med Chem. 2025;281:117018
42. Zhao S, Liu P, Li Y. Biomineralized apoferritin nanoparticles delivering dihydroartemisinin and calcium for synergistic breast cancer therapy. Sci Rep. 2024;14:29402
43. Lai H, Singh NP. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995;91:41-6
44. Deng D, Xu N, Wang M, Zhang G, Su Y, Fang H. et al. An artesunate-modified half-sandwich iridium(iii) complex inhibits colon cancer cell proliferation and metastasis through the STAT3 pathway. RSC Chem Biol. 2024;6:218-226
45. Wang DD, He L, Qi MH, Zhao HY, Yu AX, Huang SW. Mitochondria-targeting artesunate-rhein conjugates: linker-modulated cell-permeability, heme-affinity and anticancer activity. Eur J Med Chem. 2025;282:117100
46. Efferth T, Rücker G, Falkenberg M, Manns D, Olbrich A, Fabry U. et al. Detection of apoptosis in KG-1a leukemic cells treated with investigational drugs. Arzneimittelforschung. 1996;46:196-200
47. Lanna EG, Siqueira RP, Machado MGC, de Souza A, Trindade IC, Branquinho RT. et al. Lipid-based nanocarriers co-loaded with artemether and triglycerides of docosahexaenoic acid: effects on human breast cancer cells. Biomed Pharmacother. 2021;134:111114
48. Mirzaei-Parsa MJ, Najafabadi MRH, Haeri A, Zahmatkeshan M, Ebrahimi SA, Pazoki-Toroudi H. et al. Preparation, characterization, and evaluation of the anticancer activity of artemether-loaded nano-niosomes against breast cancer. Breast Cancer. 2020;27:243-51
49. Pirali-Hamedani Z, Abbasi A, Hassan ZM. Synthesis of artemether-loaded albumin nanoparticles and measurement of their anti-cancer effects. Biomedicines. 2022;10:2713
50. Efferth T, Olbrich A, Bauer R. mRNA expression profiles for the response of human tumor cell lines to the antimalarial drugs artesunate, arteether, and artemether. Biochem Pharmacol. 2002;64:617-23
51. Azimi Mohamadabadi M, Hassan ZM, Zavaran Hosseini A, Gholamzad M, Noori S, Mahdavi M. et al. Arteether exerts antitumor activity and reduces CD4+CD25+FOXP3+ T-reg cells in vivo. Iran J Immunol. 2013;10:139-49
52. Nam W, Tak J, Ryu JK, Jung M, Yook JI, Kim HJ. et al. Effects of artemisinin and its derivatives on growth inhibition and apoptosis of oral cancer cells. Head Neck. 2007;29:335-40
53. Efferth T, Oesch F. Oxidative stress response of tumor cells: microarray-based comparison between artemisinins and anthracyclines. Biochem Pharmacol. 2004;68:3-10
54. Firouzi Amandi A, Jokar E, Eslami M, Dadashpour M, Rezaie M, Yazdani Y. et al. Enhanced anti-cancer effect of artemisinin- and curcumin-loaded niosomal nanoparticles against human colon cancer cells. Med Oncol. 2023;40:170
55. Lu X, Blatt S, Dawood M, Klauck SM, Fleischer E, Kämmerer PW. et al. Novel artemisinin derivative FO8643 with anti-angiogenic activity inhibits growth and migration of cancer cells via VEGFR2 signaling. Eur J Pharmacol. 2022;930:175158
56. Lu X, Elbadawi M, Blatt S, Saeed MEM, Xiao X, Ma X. et al. Artemisinin derivative FO-ARS-123 as a novel VEGFR2 inhibitor suppresses angiogenesis, cell migration, and invasion. Chem Biol Interact. 2022;365:110062
57. Lang SJ, Schmiech M, Hafner S, Paetz C, Steinborn C, Huber R. et al. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomedicine. 2019;62:152962
58. Rassias DJ, Weathers PJ. Dried leaf Artemisia annua efficacy against non-small cell lung cancer. Phytomedicine. 2019;52:247-53
59. Zhang CZ, Zhang H, Yun J, Chen GG, Lai PBS. Dihydroartemisinin exhibits antitumor activity toward hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol. 2012;83:1278-89
60. Wang R, Zhang Q, Chen M. Artemisinin-isatin hybrids tethered via ethylene linker and their anti-lung cancer activity. Arch Pharm (Weinheim). 2023;356:e2200563
61. Lu Z, Jiang J, Yao X, Hou G. Network pharmacological mechanism and molecular experimental validation of artemisinin in the treatment of lung adenocarcinoma. Toxicol Appl Pharmacol. 2025;495:117226
62. Zhou Z, Farhan M, Xing X, Zhou W, Lin R, Zeng S. et al. Artemisinin suppressed melanoma recurrence and metastasis after radical surgery through the KIT/PI3K/AKT pathway. Int J Biol Sci. 2025;21:75-94
63. Malan D, Van Niekerk S, Häberli C, Keiser J, Van der Kooy F. In vitro antischistosomal activity of Artemisia species. Acta Trop. 2025;262:107535
64. Desrosiers MR, Weathers PJ. Effect of leaf digestion and artemisinin solubility for use in oral consumption of dried Artemisia annua leaves to treat malaria. J Ethnopharmacol. 2016;190:313-8
65. Weathers PJ, Towler M, Hassanali A, Lutgen P, Engeu PO. Dried-leaf Artemisia annua: A practical malaria therapeutic for developing countries. World J Pharmacol. 2014;3:39-55
66. Gu HM, Lu BF, Qu ZX. Antimalarial activities of 25 derivatives of artemisinine against chloroquine-resistant plasmodium berghei. Zhongguo Yao Li Xue Bao. 1980;1:48-50
67. Zhang RB, Xu SL, Li Y. Separation of artemisinine and its derivatives by reversed phase high performance liquid chromatography. Yao Xue Xue Bao. 1981;16:460-5
68. Li Y, Yu PL, Chen YX, Li LQ, Gai YZ, Wang DS. et al. Studies on analogs of artemisinine. I. The synthesis of ethers, carboxylic esters and carbonates of dihydroartemisinine. Yao Xue Xue Bao. 1981;16:429-39
69. He XF, Li TZ, Ma YB, Wang MF, Chen JJ. Unusual cadinane-involved sesquiterpenoid dimers from Artemisia annua and their antihepatoma effect. Phytochemistry. 2024;226:114216
70. Bilia AR, Melillo de Malgalhaes P, Bergonzi MC, Vincieri FF. Simultaneous analysis of artemisinin and flavonoids of several extracts of Artemisia annua L. obtained from a commercial sample and a selected cultivar. Phytomedicine. 2006;13:487-93
71. Stermitz FR, Scriven LN, Tegos G, Lewis K. Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 2002;68:1140-1
72. Zhu XX, Yang L, Li YJ, Zhang D, Chen Y, Kostecká P. et al. Effects of sesquiterpene, flavonoid and coumarin types of compounds from Artemisia annua L. on production of mediators of angiogenesis. Pharmacol Rep. 2013;65:410-20
73. Carbonara T, Pascale R, Argentieri MP, Papadia P, Fanizzi FP, Villanova L. et al. Phytochemical analysis of a herbal tea from Artemisia annua L. J Pharm Biomed Anal. 2012;62:79-86
74. Nguyen KT, Towler MJ, Weathers PJ. The effect of roots and media constituents on trichomes and artemisinin production in Artemisia annua L. Plant Cell Rep. 2013;32:207-18
75. Perazzo FF, Carvalho JC, Carvalho JE, Rehder VL. Central properties of the essential oil and the crude ethanol extract from aerial parts of Artemisia annua L. Pharmacol Res. 2003;48:497-502
76. He XF, Wang MF, Li TZ, Ma YB, Chen JJ. Artemannuols A-C, novel sesquiterpenoid-flavonol hybrids with antihepatoma activity from Artemisia annua. Org Biomol Chem. 2023;21:5451-5456
77. Tan RX, Zheng WF, Tang HQ. Biologically active substances from the genus Artemisia. Planta Med. 1998;64:295-302
78. Marwa K, Kapesa A, Baraka V, Konje E, Kidenya B, Mukonzo J. et al. Therapeutic efficacy of artemether-lumefantrine, artesunate-amodiaquine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Sub-Saharan Africa: a systematic review and meta-analysis. PloS One. 2022;17:e0264339
79. Talman AM, Clain J, Duval R, Ménard R, Ariey F. Artemisinin bioactivity and resistance in malaria parasites. Trends Parasitol. 2019;35:953-963
80. Daily JP, Minuti A, Khan N. Diagnosis, treatment, and prevention of malaria in the US: a review. JAMA. 2022;328:460-471
81. Rosenthal PJ, Asua V, Conrad MD. Emergence, transmission dynamics and mechanisms of artemisinin partial resistance in malaria parasites in Africa. Nat Rev Microbiol. 2024;22:373-384
82. Mueller MS, Karhagomba IB, Hirt HM, Wemakor E. The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: agricultural, chemical and clinical aspects. J Ethnopharmacol. 2000;73:487-93
83. Islamuddin M, Chouhan G, Farooque A, Dwarakanath BS, Sahal D, Afrin F. Th1-biased immunomodulation and therapeutic potential of Artemisia annua in murine visceral leishmaniasis. PLoS Negl Trop Dis. 2015;9:e3321
84. Derda M, Hadaś E, Cholewiński M, Skrzypczak Ł, Grzondziel A, Wojtkowiak-Giera A. Artemisia annua L. as a plant with potential use in the treatment of acanthamoebiasis. Parasitol Res. 2016;115:1635-9
85. de Oliveira TC, Silva DA, Rostkowska C, Béla SR, Ferro EA, Magalhães PM. et al. Toxoplasma gondii: effects of Artemisia annua L. on susceptibility to infection in experimental models in vitro and in vivo. Exp Parasitol. 2009;122:233-41
86. Li J, Zhang C, Gong M, Wang M. Combination of artemisinin-based natural compounds from Artemisia annua L. for the treatment of malaria: pharmacodynamic and pharmacokinetic studies. Phytother Res. 2018;32:1415-1420
87. Gruessner BM, Weathers PJ. In vitro analyses of Artemisia extracts on Plasmodium falciparum suggest a complex antimalarial effect. PloS One. 2021;16:e0240874
88. Gao P, Wang J, Qiu C, Zhang H, Wang C, Zhang Y. et al. Photoaffinity probe-based antimalarial target identification of artemisinin in the intraerythrocytic developmental cycle of Plasmodium falciparum. Imeta. 2024;3:e176
89. Venkatesan P. The 2023 WHO world malaria report. Lancet Microbe. 2024;5:e214
90. Ghosh SK, Rahi M. Malaria elimination in India-The way forward. J Vector Borne Dis. 2019;56:32-40
91. Kołodziej D, Wilczyńska W, Marchelek-Myśliwiec M, Świetlik D, Ammi HZ, Athumani MO. et al. Asymptomatic malaria cases and plasmodium species in Mainland Tanzania and Zanzibar Archipelago (Pemba). Pathogens. 2024;13:1140
92. Newby G, Bennett A, Larson E, Cotter C, Shretta R, Phillips AA. et al. The path to eradication: a progress report on the malaria-eliminating countries. Lancet. 2016;387:1775-84
93. Islamuddin M, Chouhan G, Tyagi M, Abdin MZ, Sahal D, Afrin F. Leishmanicidal activities of Artemisia annua leaf essential oil against Visceral Leishmaniasis. Front Microbiol. 2014;5:626
94. Mesa LE, Vasquez D, Lutgen P, Vélez ID, Restrepo AM, Ortiz I. et al. In vitro and in vivo antileishmanial activity of Artemisia annua L. leaf powder and its potential usefulness in the treatment of uncomplicated cutaneous leishmaniasis in humans. Rev Soc Bras Med Trop. 2017;50:52-60
95. Wojtkowiak-Giera A, Derda M, Kosik-Bogacka D, Kolasa-Wołosiuk A, Wandurska-Nowak E, Jagodziński PP. et al. The modulatory effect of Artemisia annua L. on toll-like receptor expression in Acanthamoeba infected mouse lungs. Exp Parasitol. 2019;199:24-29
96. Liu YX, Wu W, Liang YJ, Jie ZL, Wang H, Wang W. et al. New uses for old drugs: the tale of artemisinin derivatives in the elimination of schistosomiasis japonica in China. Molecules. 2014;19:15058-74
97. Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis. 2008;47:804-11
98. Baggieri M, Gioacchini S, Borgonovo G, Catinella G, Marchi A, Picone P. et al. Antiviral, virucidal and antioxidant properties of Artemisia annua against SARS-CoV-2. Biomed Pharmacother. 2023;168:115682
99. Tang Y, Li X, Yuan Y, Zhang H, Zou Y, Xu Z. et al. Network pharmacology-based predictions of active components and pharmacological mechanisms of Artemisia annua L. for the treatment of the novel Corona virus disease 2019 (COVID-19). BMC Complement Med Ther. 2022;22:56
100. Nair MS, Huang Y, Fidock DA, Polyak SJ, Wagoner J, Towler MJ. et al. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J Ethnopharmacol. 2021;274:114016
101. Huang X, Liu T, Zhou C, Huang Y, Liu X, Yuan H. Antifungal activity of essential oils from three Artemisia species against colletotrichum gloeosporioides of Mango. Antibiotics (Basel). 2021;10:1331
102. Chebbac K, Benziane Ouaritini Z, El Moussaoui A, Chalkha M, Lafraxo S, Bin Jardan YA. et al. Antimicrobial and antioxidant properties of chemically analyzed essential oil of Artemisia annua L. (Asteraceae) native to mediterranean area. Life (Basel). 2023;13:807
103. Zhigzhitzhapova SV, Dylenova EP, Gulyaev SM, Randalova TE, Taraskin VV, Tykheev ZA. et al. Composition and antioxidant activity of the essential oil of Artemisia annua L. Nat Prod Res. 2020;34:2668-2671
104. Rolta R, Sharma A, Sourirajan A, Mallikarjunan PK, Dev K. Combination between antibacterial and antifungal antibiotics with phytocompounds of Artemisia annua L: a strategy to control drug resistance pathogens. J Ethnopharmacol. 2021;266:113420
105. Bilia AR, Santomauro F, Sacco C, Bergonzi MC, Donato R. Essential oil of Artemisia annua L.: an extraordinary component with numerous antimicrobial properties. Evid Based Complement Altern Med. 2014;2014:159819
106. Santomauro F, Donato R, Pini G, Sacco C, Ascrizzi R, Bilia AR. Liquid and vapor-phase activity of Artemisia annua essential oil against pathogenic Malassezia spp. Planta Med. 2018;84:160-167
107. Santomauro F, Donato R, Sacco C, Pini G, Flamini G, Bilia AR. Vapour and liquid-phase Artemisia annua essential oil activities against several clinical strains of Candida. Planta Med. 2016;82:1016-20
108. Aćimović M, Jeremić JS, Todosijević M, Kiprovski B, Vidović S, Vladić J. et al. Comparative study of the essential oil and hydrosol composition of Sweet Wormwood (Artemisia annua L.) from Serbia. Chem Biodivers. 2022;19:e202100954
109. Habibi Z, Ghanian S, Ghasemi S, Yousefi M. Chemical composition and antibacterial activity of the volatile oil from seeds of Artemisia annua L. from Iran. Nat Prod Res. 2013;27:198-200
110. Ho WE, Peh HY, Chan TK, Wong WSF. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142:126-39
111. Wang KS, Li J, Wang Z, Mi C, Ma J, Piao LX. et al. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol Immunotoxicol. 2017;39:28-36
112. Shinyuy LM, Loe GE, Jansen O, Mamede L, Ledoux A, Noukimi SF. et al. Secondary metabolites isolated from Artemisia afra and Artemisia annua and their anti-malarial, anti-inflammatory and immunomodulating properties-pharmacokinetics and pharmacodynamics: a review. Metabolites. 2023;13:613
113. Wu QG, Huang LY, Fan MH, Chou GX, Wang YL. Anti-inflammatory activities of monoterpene and sesquiterpene glycosides from the aqueous extract of Artemisia annua L. Chem Biodivers. 2023;20:e202201237
114. Baek HK, Shim H, Lim H, Shim M, Kim CK, Park SK. et al. Anti-adipogenic effect of Artemisia annua in diet-induced-obesity mice model. J Vet Sci. 2015;16:389-96
115. Lee J, Kim MH, Lee JH, Jung E, Yoo ES, Park D. Artemisinic acid is a regulator of adipocyte differentiation and C/EBP δ expression. J Cell Biochem. 2012;113:2488-99
116. Song Y, Lee SJ, Jang SH, Kim TH, Kim HD, Kim SW. et al. Annual wormwood leaf inhibits the adipogenesis of 3T3-L1 and bbesity in high-fat diet-induced obese rats. Nutrients. 2017;9:554
117. Hwang DI, Won KJ, Kim DY, Yoon SW, Park JH, Kim B. et al. Anti-adipocyte differentiation activity and chemical composition of essential oil from Artemisia annua. Nat Prod Commun. 2016;11:539-42
118. Lee SK, Kim H, Park J, Kim HJ, Kim KR, Son SH. et al. Artemisia annua extract prevents ovariectomy-induced bone loss by blocking receptor activator of nuclear factor kappa-B ligand-induced differentiation of osteoclasts. Sci Rep. 2017;7:17332
119. Gao T, Yu C, Shi X, Hu Y, Chang Y, Zhang J. et al. Artemisinic acid attenuates osteoclast formation and titanium particle-induced osteolysis via inhibition of RANKL-induced ROS accumulation and MAPK and NF-κB signaling pathways. Front Pharmacol. 2024;15:1345380
120. Sun W, Yang G, Zhang F, Feng C, Liang M, Jia P. et al. Effects of Artemisia annua L. essential oil on osteoclast differentiation and function induced by RANKL. Evid Based Complement Altern Med. 2022;2022:1322957
121. Wu H, Hu B, Zhou X, Zhou C, Meng J, Yang Y. et al. Artemether attenuates LPS-induced inflammatory bone loss by inhibiting osteoclastogenesis and bone resorption via suppression of MAPK signaling pathway. Cell Death Dis. 2018;9:498
122. Kolesar JM, Seeberger PH. Editorial: anticancer potential of Artemisia annua. Front Oncol. 2022;12:853406
123. Septembre-Malaterre A, Lalarizo Rakoto M, Marodon C, Bedoui Y, Nakab J, Simon E. et al. Artemisia annua, a traditional plant brought to light. Int J Mol Sci. 2020;21:4986
124. Efferth T, Herrmann F, Tahrani A, Wink M. Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine. 2011;18:959-69
125. Graziose R, Lila MA, Raskin I. Merging traditional Chinese medicine with modern drug discovery technologies to find novel drugs and functional foods. Curr Drug Discov Technol. 2010;7:2-12
126. Huang Y, Yang Y, Liu G, Xu M. New clinical application prospects of artemisinin and its derivatives: a scoping review. Infect Dis Poverty. 2023;12:115
127. Michaelsen FW, Saeed MEM, Schwarzkopf J, Efferth T. Activity of Artemisia annua and artemisinin derivatives, in prostate carcinoma. Phytomedicine. 2015;22:1223-31
128. Meunier B, Robert A. Heme as trigger and target for trioxane-containing antimalarial drugs. Acc Chem Res. 2010;43:1444-51
129. Meshnick SR. Artemisinin antimalarials: mechanisms of action and resistance. Med Trop (Mars). 1998;58:13-7
130. Meshnick SR, Yang YZ, Lima V, Kuypers F, Kamchonwongpaisan S, Yuthavong Y. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu). Antimicrob Agents Chemother. 1993;37:1108-14
131. Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28-33
132. Efferth T, Benakis A, Romero MR, Tomicic M, Rauh R, Steinbach D. et al. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med. 2004;37:998-1009
133. Zhong W, Wong KH, Xu F, Zhao N, Chen M. NIR-responsive polydopamine-based calcium carbonate hybrid nanoparticles delivering artesunate for cancer chemo-photothermal therapy. Acta Biomater. 2022;145:135-145
134. Hu Y, Guo N, Yang T, Yan J, Wang W, Li X. The potential mechanisms by which artemisinin and its derivatives induce ferroptosis in the treatment of cancer. Oxid Med Cell Longev. 2022;2022:1458143
135. Shao Y, Wang Z, Hao Y, Zhang X, Wang N, Chen K. et al. Cascade catalytic nanoplatform based on 'butterfly effect' for enhanced immunotherapy. Adv Healthc Mater. 2021;10:e2002171
136. Fan Z, Xia G, Wang Q, Chen S, Li J, Hou Z. et al. Endogenous Fe2+-triggered self-targeting nanomicelles for self-amplifying intracellular oxidative stress. Anim Models Exp Med. 2025;8:307-321
137. Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18:767-73
138. Gruber L, Abdelfatah S, Fröhlich T, Reiter C, Klein V, Tsogoeva SB. et al. Treatment of multidrug-resistant leukemia cells by novel artemisinin-, egonol-, and thymoquinone-derived hybrid compounds. Molecules. 2018;23:841
139. Firestone GL, Sundar SN. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med. 2009;11:e32
140. Qiu N, Abegg D, Guidi M, Gilmore K, Seeberger PH, Adibekian A. Artemisinin inhibits NRas palmitoylation by targeting the protein acyltransferase ZDHHC6. Cell Chem Biol. 2022;29:530-537.e7
141. Slezakova S, Ruda-Kucerova J. Anticancer activity of artemisinin and its derivatives. Anticancer Res. 2017;37:5995-6003
142. Chen GQ, Benthani FA, Wu J, Liang D, Bian ZX, Jiang X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020;27:242-254
143. Sun WC, Han JX, Yang WY, Deng DA, Yue XF. Antitumor activities of 4 derivatives of artemisic acid and artemisinin B in vitro. Zhongguo Yao Li Xue Bao. 1992;13:541-3
144. Kotammagari TK, Paul S, Barik GK, Santra MK, Bhattacharya AK. Synthesis of artemisinic acid derived glycoconjugates and their anticancer studies. Org Biomol Chem. 2020;18:2252-63
145. El Omari N, Bakrim S, Bakha M, Lorenzo JM, Rebezov M, Shariati MA. et al. Natural bioactive compounds targeting epigenetic pathways in cancer: a review on alkaloids, terpenoids, quinones, and isothiocyanates. Nutrients. 2021;13:3714
146. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665-7
147. Naaz F, Haider MR, Shafi S, Yar MS. Anti-tubulin agents of natural origin: targeting taxol, vinca, and colchicine binding domains. Eur J Med Chem. 2019;171:310-31
148. Li C, Wang E, Cheng Y, Bao J. Oridonin: an active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol. 2011;43:701-4
149. Li E, Gao Y, Mou L, Zhang Z. Anticancer activity of Germacrone terpenoid in human osteosarcoma cells is mediated via autophagy induction, cell cycle disruption, downregulating the cell cycle regulatory protein expressions and cell migration inhibition. Acta Biochim Pol. 2022;69:305-8
150. Hitora Y, Sejiyama A, Honda K, Ise Y, Losung F, Mangindaan REP. et al. Fluorescent image-based high-content screening of extracts of natural resources for cell cycle inhibitors and identification of a new sesquiterpene quinone from the sponge, Dactylospongia metachromia. Bioorg Med Chem. 2021;31:115968
151. Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980;77:1561-5
152. Prota AE, Lucena-Agell D, Ma Y, Estevez-Gallego J, Li S, Bargsten K. et al. Structural insight into the stabilization of microtubules by taxanes. Elife. 2023;12:e84791
153. Ganesh T, Yang C, Norris A, Glass T, Bane S, Ravindra R. et al. Evaluation of the tubulin-bound paclitaxel conformation: synthesis, biology, and SAR studies of C-4 to C-3' bridged paclitaxel analogues. J Med Chem. 2007;50:713-25
154. Snyder JP, Nettles JH, Cornett B, Downing KH, Nogales E. The binding conformation of Taxol in beta-tubulin: a model based on electron crystallographic density. Proc Natl Acad Sci U S A. 2001;98:5312-6
155. Song GQ, Wu P, Dong XM, Cheng LH, Lu HQ, Lin YY. et al. Elemene induces cell apoptosis via inhibiting glutathione synthesis in lung adenocarcinoma. J Ethnopharmacol. 2023;311:116409
156. Li QQ, Wang G, Huang F, Li JM, Cuff CF, Reed E. Sensitization of lung cancer cells to cisplatin by β-elemene is mediated through blockade of cell cycle progression: antitumor efficacies of β-elemene and its synthetic analogs. Med Oncol. 2013;30:488
157. Wang J, Xu C, Chen Y, Shao L, Li T, Fan X. et al. β-elemene enhances the antitumor activity of erlotinib by inducing apoptosis through AMPK and MAPK pathways in TKI-resistant H1975 lung cancer cells. J Cancer. 2021;12:2285-94
158. Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ, Xu YH. Anti-tumor effect of beta-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008;264:127-34
159. Wu X, Liu Y, Zhang E, Chen J, Huang X, Yan H. et al. Dihydroartemisinin modulates apoptosis and autophagy in multiple myeloma through the P38/MAPK and Wnt/β-Catenin signaling pathways. Oxid Med Cell Longev. 2020;2020:6096391
160. Bai B, Wu F, Ying K, Xu Y, Shan L, Lv Y. et al. Therapeutic effects of dihydroartemisinin in multiple stages of colitis-associated colorectal cancer. Theranostics. 2021;11:6225-6239
161. Liang R, Chen W, Fan H, Chen X, Zhang J, Zhu JS. Dihydroartemisinin prevents dextran sodium sulphate-induced colitisthrough inhibition of the activation of NLRP3 inflammasome and p38 MAPK signaling. Int Immunopharmacol. 2020;88:106949
162. Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO. et al. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol. 2003;64:382-94
163. Weifeng T, Feng S, Xiangji L, Changqing S, Zhiquan Q, Huazhong Z. et al. Artemisinin inhibits in vitro and in vivo invasion and metastasis of human hepatocellular carcinoma cells. Phytomedicine. 2011;18:158-62
164. Xu Z, Liu X, Zhuang D. Artesunate inhibits proliferation, migration, and invasion of thyroid cancer cells by regulating the PI3K/AKT/FKHR pathway. Biochem Cell Biol. 2022;100:85-92
165. Huang Z, Gan S, Zhuang X, Chen Y, Lu L, Wang Y. et al. Artesunate inhibits the cell growth in colorectal cancer by promoting ROS-dependent cell senescence and autophagy. Cells. 2022;11:2472
166. Farhan M, Silva M, Xingan X, Zhou Z, Zheng W. Artemisinin inhibits the migration and invasion in uveal melanoma via inhibition of the PI3K/AKT/mTOR signaling pathway. Oxid Med Cell Longev. 2021;2021:9911537
167. Zhang S, Shi L, Ma H, Li H, Li Y, Lu Y. et al. Dihydroartemisinin induces apoptosis in human gastric cancer cell line BGC-823 through activation of JNK1/2 and p38 MAPK signaling pathways. J Recept Signal Transduct Res. 2017;37:174-80
168. Tohyama N, Tanaka S, Onda K, Sugiyama K, Hirano T. Influence of anticancer agents on cell survival, proliferation, and CD4+CD25+Foxp3+ regulatory T cell-frequency in human peripheral-blood mononuclear cells activated by T cell-mitogen. Int Immunopharmacol. 2013;15:160-6
169. Xu C, Zhang H, Mu L, Yang X. Artemisinins as anticancer drugs: novel therapeutic approaches, molecular mechanisms, and clinical trials. Front Pharmacol. 2020;11:529881
170. Wang Z, Hu W, Zhang JL, Wu XH, Zhou HJ. Dihydroartemisinin induces autophagy and inhibits the growth of iron-loaded human myeloid leukemia K562 cells via ROS toxicity. FEBS Open Bio. 2012;2:103-12
171. Huo J, Lu Y, Xia L, Chen D. Structural characterization and anticomplement activities of three acidic homogeneous polysaccharides from Artemisia annua. J Ethnopharmacol. 2020;247:112281
172. Chen J, Chen J, Wang X, Liu C. Anti-tumour effects of polysaccharides isolated from Artemisia annua L by inducing cell apoptosis and immunomodulatory anti-hepatoma effects of polysaccharides. Afr J Tradit Complement Altern Med. 2013;11:15-22
173. Yan L, Xiong C, Xu P, Zhu J, Yang Z, Ren H. et al. Structural characterization and in vitro antitumor activity of A polysaccharide from Artemisia annua L. (Huang Huahao). Carbohydr Polym. 2019;213:361-9
174. Wang Y, Yuan X, Ren M, Wang Z. Ferroptosis: a new research direction of artemisinin and its derivatives in anti-cancer treatment. Am J Chin Med. 2024;52:161-81
175. Deng DA, Xu CH, Cai JC. Derivatives of arteannuin B with antileukemia activity. Yao Xue Xue Bao. 1992;27:317-20
176. Zheng GQ. Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Med. 1994;60:54-7
177. Chen K, Hua H, Zhu Z, Wu T, Jia Z, Liu Q. Artemisinin and dihydroartemisinin promote β-cell apoptosis induced by palmitate via enhancing ER stress. Apoptosis. 2020;25:192-204
178. Zhong Y, Li ZN, Jiang XY, Tian X, Deng MH, Cheng MS. et al. Identification of novel artemisinin hybrids induce apoptosis and ferroptosis in MCF-7 cells. Int J Mol Sci. 2022;23:15768
179. Cao Y, Feng YH, Gao LW, Li XY, Jin QX, Wang YY. et al. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int Immunopharmacol. 2019;70:110-6
180. Chen HH, Zhou HJ, Fang X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res. 2003;48:231-6
181. Zhang X, Li N, Zhang G, Li J, Liu Y, Wang M. et al. Nano strategies for artemisinin derivatives to enhance reverse efficiency of multidrug resistance in breast cancer. Curr Pharm Des. 2023;29:3458-66
182. Guo W, Wang W, Lei F, Zheng R, Zhao X, Gu Y. et al. Identifying the main components and mechanisms of action of Artemisia annua L. in the treatment of endometrial cancer using network pharmacology. ACS Omega. 2024;9:8055-66
183. Ferreira JFS, Luthria DL, Sasaki T, Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15:3135-70
184. Jung EJ, Kim HJ, Shin SC, Kim GS, Jung JM, Hong SC. et al. Artemisia annua L. polyphenols enhance the anticancer effect of β-lapachone in xxaliplatin-resistant HCT116 colorectal cancer cells. Int J Mol Sci. 2023;24:17505
185. Fu C, Zhang K, Wang M, Qiu F. Casticin and chrysosplenol D from Artemisia annua L. induce apoptosis by inhibiting topoisomerase IIα in human non-small-cell lung cancer cells. Phytomedicine. 2022;100:154095
186. Ko YS, Lee WS, Panchanathan R, Joo YN, Choi YH, Kim GS. et al. Polyphenols from Artemisia annua L inhibit adhesion and EMT of highly metastatic breast cancer cells MDA-MB-231. Phytother Res. 2016;30:1180-8
187. Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472-82
188. Icard P, Fournel L, Wu Z, Alifano M, Lincet H. Interconnection between metabolism and cell cycle in cancer. Trends Biochem Sci. 2019;44:490-501
189. Zhu JJ, Ai Y, Wu JH, Zeng CG, Cui Z, Zhang ZP. et al. Ring-contracted artemisinin derivatives as novel CDK 4/6 inhibitors: synthesis and anti-breast cancer evaluation. Chem Biodivers. 2024;21:e202400086
190. Chen H, Sun B, Wang S, Pan S, Gao Y, Bai X. et al. Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-kappaB. J Cancer Res Clin Oncol. 2010;136:897-903
191. Willoughby JA, Sundar SN, Cheung M, Tin AS, Modiano J, Firestone GL. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J Biol Chem. 2009;284:2203-13
192. Guan X, Guan Y. Artemisinin induces selective and potent anticancer effects in drug resistant breast cancer cells by inducing cellular apoptosis and autophagy and G2/M cell cycle arrest. J BUON. 2020;25:1330-6
193. Jia J, Qin Y, Zhang L, Guo C, Wang Y, Yue X. et al. Artemisinin inhibits gallbladder cancer cell lines through triggering cell cycle arrest and apoptosis. Mol Med Rep. 2016;13:4461-8
194. Obeng E. Apoptosis (programmed cell death) and its signals - A review. Braz J Biol. 2021;81:1133-43
195. Yuan J, Ofengeim D. A guide to cell death pathways. Nat Rev Mol Cell Biol. 2024;25:379-95
196. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205-19
197. Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49-63
198. Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749-58
199. Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030-40
200. Lin L, Lu W, Dai T, Chen H, Wang T, Yang L. et al. Novel artemisinin derivatives with potent anticancer activities and the anti-colorectal cancer effect by the mitochondria-mediated pathway. Bioorg Chem. 2021;106:104496
201. Hill KS, Schuler EE, Ellingson SR, Kolesar JM. Artesunate acts through cytochrome c to inhibit growth of pediatric AML cells. Sci Rep. 2023;13:22383
202. Poupel F, Aghaei M, Movahedian A, Jafari SM, Shahrestanaki MK. Dihydroartemisinin induces apoptosis in human bladder cancer cell lines through reactive oxygen species, mitochondrial membrane potential, and cytochrome C pathway. Int J Prev Med. 2017;8:78
203. Xiao F, Gao W, Wang X, Chen T. Amplification activation loop between caspase-8 and -9 dominates artemisinin-induced apoptosis of ASTC-a-1 cells. Apoptosis. 2012;17:600-11
204. Gao W, Xiao F, Wang X, Chen T. Artemisinin induces A549 cell apoptosis dominantly via a reactive oxygen species-mediated amplification activation loop among caspase-9, -8 and -3. Apoptosis. 2013;18:1201-13
205. Gong RH, Yang DJ, Kwan HY, Lyu AP, Chen GQ, Bian ZX. Cell death mechanisms induced by synergistic effects of halofuginone and artemisinin in colorectal cancer cells. Int J Med Sci. 2022;19:175-85
206. Zhang P, Luo HS, Li M, Tan SY. Artesunate inhibits the growth and induces apoptosis of human gastric cancer cells by downregulating COX-2. OncoTargets Ther. 2015;8:845-54
207. Lu YY, Chen TS, Wang XP, Li L. Single-cell analysis of dihydroartemisinin-induced apoptosis through reactive oxygen species-mediated caspase-8 activation and mitochondrial pathway in ASTC-a-1 cells using fluorescence imaging techniques. J Biomed Opt. 2010;15:046028
208. Lu YY, Chen TS, Qu JL, Pan WL, Sun L, Wei XB. Dihydroartemisinin (DHA) induces caspase-3-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J Biomed Sci. 2009;16:16
209. Hsieh MJ, Lin CC, Lo YS, Chuang YC, Ho HY, Chen MK. Chrysosplenol D triggers apoptosis through heme oxygenase-1 and mitogen-activated protein kinase signaling in oral squamous cell carcinoma. Cancers. 2021;13:4327
210. Hadian K, Stockwell BR. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat Rev Drug Discov. 2023;22:723-42
211. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-64
212. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3-12
213. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107-25
214. Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7:196
215. Son JY, Yoon S, Tae IH, Park YJ, De U, Jeon Y. et al. Novel therapeutic roles of MC-4 in combination with everolimus against advanced renal cell carcinoma by dual targeting of Akt/pyruvate kinase muscle isozyme M2 and mechanistic target of rapamycin complex 1 pathways. Cancer Med. 2018;7:5083-95
216. Chen K, Shou LM, Lin F, Duan WM, Wu MY, Xie X. et al. Artesunate induces G2/M cell cycle arrest through autophagy induction in breast cancer cells. Anticancer Drugs. 2014;25:652-62
217. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-82
218. Mou Y, Wang J, Wu J, He D, Zhang C, Duan C. et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12:34
219. Zhao L, Zhou X, Xie F, Zhang L, Yan H, Huang J. et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun (Lond). 2022;42:88-116
220. Zhu S, Yu Q, Huo C, Li Y, He L, Ran B. et al. Ferroptosis: a novel mechanism of artemisinin and its derivatives in cancer therapy. Curr Med Chem. 2021;28:329-45
221. Gao Z, Li Y, You C, Sun K, An P, Sun C. et al. Iron oxide nanocarrier-mediated combination therapy of cisplatin and artemisinin for combating drug resistance through highly increased toxic reactive oxygen species generation. ACS Appl Bio Mater. 2018;1:270-80
222. Altea-Manzano P, Cuadros AM, Broadfield LA, Fendt S-M. Nutrient metabolism and cancer in the in vivo context: a metabolic game of give and take. EMBO Rep. 2020;21:e50635
223. Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: still emerging. Cell Metab. 2022;34:355-77
224. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401-10
225. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745-70
226. Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023;8:198
227. Lopes-Coelho F, Martins F, Pereira SA, Serpa J. Anti-angiogenic therapy: current challenges and future perspectives. Int J Mol Sci. 2021;22:3765
228. Korbecki J, Simińska D, Gąssowska-Dobrowolska M, Listos J, Gutowska I, Chlubek D. et al. Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int J Mol Sci. 2021;22:10701
229. Lee SH, Golinska M, Griffiths JR. HIF-1-independent mechanisms regulating metabolic adaptation in hypoxic cancer cells. Cells. 2021;10:2371
230. Bernatoniene J, Nemickaite E, Majiene D, Marksa M, Kopustinskiene DM. In vitro and in silico anti-glioblastoma activity of hydroalcoholic extracts of Artemisia annua L. and Artemisia vulgaris L. Molecules. 2024;29:2460
231. Wartenberg M, Wolf S, Budde P, Grünheck F, Acker H, Hescheler J. et al. The antimalaria agent artemisinin exerts antiangiogenic effects in mouse embryonic stem cell-derived embryoid bodies. Lab Invest. 2003;83:1647-55
232. Wang J, Zhang B, Guo Y, Li G, Xie Q, Zhu B. et al. Artemisinin inhibits tumor lymphangiogenesis by suppression of vascular endothelial growth factor C. Pharmacology. 2008;82:148-55
233. Chen HH, Zhou HJ, Wu GD, Lou XE. Inhibitory effects of artesunate on angiogenesis and on expressions of vascular endothelial growth factor and VEGF receptor KDR/flk-1. Pharmacology. 2004;71:1-9
234. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786-801
235. Walker C, Mojares E, Del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19:3028
236. Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu X. et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. 2023;22:48
237. Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430-9
238. Paolillo M, Schinelli S. Extracellular matrix alterations in metastatic processes. Int J Mol Sci. 2019;20:4947
239. Na TY, Schecterson L, Mendonsa AM, Gumbiner BM. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc Natl Acad Sci U S A. 2020;117:5931-7
240. Sinkevicius KW, Bellaria KJ, Barrios J, Pessina P, Gupta M, Brainson CF. et al. E-cadherin loss accelerates tumor progression and metastasis in a mouse model of lung adenocarcinoma. Am J Respir Cell Mol Biol. 2018;59:237-45
241. Stephens DC, Harris DA. Organizing 'elements': facilitating exocytosis and promoting metastasis. Trends Cancer. 2020;6:273-6
242. Buommino E, Baroni A, Canozo N, Petrazzuolo M, Nicoletti R, Vozza A. et al. Artemisinin reduces human melanoma cell migration by down-regulating alpha V beta 3 integrin and reducing metalloproteinase 2 production. Invest New Drugs. 2009;27:412-8
243. Rao Q, Yu H, Li R, He B, Wang Y, Guo X. et al. Dihydroartemisinin inhibits angiogenesis in breast cancer via regulating VEGF and MMP-2/-9. Fundam Clin Pharmacol. 2024;38:113-25
244. Wang SJ, Sun B, Cheng ZX, Zhou HX, Gao Y, Kong R. et al. Dihydroartemisinin inhibits angiogenesis in pancreatic cancer by targeting the NF-κB pathway. Cancer Chemother Pharmacol. 2011;68:1421-30
245. Tong Y, Liu Y, Zheng H, Zheng L, Liu W, Wu J. et al. Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget. 2016;7:31413-28
246. Rasheed SAK, Efferth T, Asangani IA, Allgayer H. First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int J Cancer. 2010;127:1475-85
247. Chen X, He LY, Lai S, He Y. Dihydroartemisinin inhibits the migration of esophageal cancer cells by inducing autophagy. Oncol Lett. 2020;20:94
248. Li Y, Lu J, Chen Q, Han S, Shao H, Chen P. et al. Artemisinin suppresses hepatocellular carcinoma cell growth, migration and invasion by targeting cellular bioenergetics and Hippo-YAP signaling. Arch Toxicol. 2019;93:3367-83
249. Li LN, Zhang HD, Yuan SJ, Tian ZY, Wang L, Sun ZX. Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/beta-catenin pathway. Int J Cancer. 2007;121:1360-5
250. Rui R, Zhou L, He S. Cancer immunotherapies: advances and bottlenecks. Front Immunol. 2023;14:1212476
251. Baxevanis CN, Perez SA, Papamichail M. Cancer immunotherapy. Crit Rev Clin Lab Sci. 2009;46:167-89
252. Oliveira G, Wu CJ. Dynamics and specificities of T cells in cancer immunotherapy. Nat Rev Cancer. 2023;23:295-316
253. Larsen ES, Joensen UN, Poulsen AM, Goletti D, Johansen IS. Bacillus Calmette-Guérin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. APMIS. 2020;128:92-103
254. Monjazeb AM, Hsiao HH, Sckisel GD, Murphy WJ. The role of antigen-specific and non-specific immunotherapy in the treatment of cancer. J Immunotoxicol. 2012;9:248-58
255. Bakhtiyaridovvombaygi M, Yazdanparast S, Mikanik F, Izadpanah A, Parkhideh S, Shahbaz Ghasabeh A. et al. Cytokine-induced memory-like NK cells: emerging strategy for AML immunotherapy. Biomed Pharmacother. 2023;168:115718
256. Efferth T, Oesch F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med Res Rev. 2021;41:3023-61
257. Bai L, Li H, Li J, Song J, Zhou Y, Liu B. et al. Immunosuppressive effect of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via regulating the differentiation of CD4+ T cell subsets in rats. Int Immunopharmacol. 2019;70:313-23
258. Han X, Chen Z, Yuan J, Wang G, Han X, Wu H. et al. Artemisia annua water extract attenuates DNCB-induced atopic dermatitis by restraining Th2 cell mediated inflammatory responses in BALB/c mice. J Ethnopharmacol. 2022;291:115160
259. Hu C, Wu D, Yu J, Xu J, Liu L, Zhang M. et al. Dihydroarteannuin ameliorates collagen-induced arthritis via inhibiting B cell activation by activating the FcγRIIb/Lyn/SHP-1 pathway. Front Pharmacol. 2022;13:883835
260. Sun W, Han X, Wu S, Wu J, Yang C, Li X. Unexpected mechanism of colitis amelioration by artesunate, a natural product from Artemisia annua L. Inflammopharmacology. 2020;28:851-68
261. Gao X, Lin X, Wang Q, Chen J. Artemisinins: promising drug candidates for the treatment of autoimmune diseases. Med Res Rev. 2024;44:867-91
262. Yin N, Li X, Zhang X, Xue S, Cao Y, Niedermann G. et al. Development of pharmacological immunoregulatory anti-cancer therapeutics: current mechanistic studies and clinical opportunities. Signal Transduct Target Ther. 2024;9:126
263. Mashati P, Esmaeili S, Dehghan-Nayeri N, Bashash D, Darvishi M, Gharehbaghian A. Methanolic extract from aerial parts of Artemisia Annua L. induces cytotoxicity and enhances vincristine-induced anticancer effect in pre-B acute lymphoblastic leukemia cells. Int J Hematol-Oncol Stem Cell Res. 2019;13:132-9
264. Abate G, Zhang L, Pucci M, Morbini G, Mac Sweeney E, Maccarinelli G. et al. Phytochemical analysis and anti-inflammatory activity of different ethanolic phyto-extracts of Artemisia annua L. Biomolecules. 2021;11:975
265. Nikitin E, Fitsev I, Egorova A, Logvinenko L, Terenzhev D, Bekmuratova F. et al. Five different Artemisia L. species ethanol extracts' phytochemical composition and their antimicrobial and nematocide activity. Int J Mol Sci. 2023;24:14372
266. Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670-95
267. Huang M, Lu JJ, Ding J. Natural products in cancer therapy: past, present and future. Nat Prod Bioprospecting. 2021;11:5-13
268. Zhou X, Suo F, Haslinger K, Quax WJ. Artemisinin-type drugs in tumor cell death: mechanisms, combination treatment with biologics and nanoparticle delivery. Pharmaceutics. 2022;14:395
269. Nakase I, Gallis B, Takatani-Nakase T, Oh S, Lacoste E, Singh NP. et al. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009;274:290-8
270. Oh S, Kim BJ, Singh NP, Lai H, Sasaki T. Synthesis and anti-cancer activity of covalent conjugates of artemisinin and a transferrin-receptor targeting peptide. Cancer Lett. 2009;274:33-9
271. Yang Y, Zhang X, Wang X, Zhao X, Ren T, Wang F. et al. Enhanced delivery of artemisinin and its analogues to cancer cells by their adducts with human serum transferrin. Int J Pharm. 2014;467:113-22
272. Chen C, Liu P, Duan X, Cheng M, Xu LX. Deferoxamine-induced high expression of TfR1 and DMT1 enhanced iron uptake in triple-negative breast cancer cells by activating IL-6/PI3K/AKT pathway. OncoTargets Ther. 2019;12:4359-77
273. Corte-Rodríguez M, Blanco-González E, Bettmer J, Montes-Bayón M. Quantitative analysis of transferrin receptor 1 (TfR1) in individual breast cancer cells by means of labeled antibodies and elemental (ICP-MS) detection. Anal Chem. 2019;91:15532-8
274. Villalobos-Manzo R, Ríos-Castro E, Hernández-Hernández JM, Oza G, Medina MA, Tapia-Ramírez J. Identification of transferrin receptor 1 (TfR1) overexpressed in lung cancer cells, and internalization of magnetic Au-CoFe2O4 core-shell nanoparticles functionalized with its ligand in a cellular model of small cell lung cancer (SCLC). Pharmaceutics. 2022;14:1715
275. Wang Z, Yao X, Wang K, Wang B. TFR1-mediated iron metabolism orchestrates tumor ferroptosis and immunity in non-small cell lung cancer. J Environ Pathol Toxicol Oncol. 2024;43:1-12
276. Osmola M, Gierej B, Mleczko-Sanecka K, Jończy A, Ciepiela O, Kraj L. et al. Anemia, iron deficiency, and iron regulators in pancreatic ductal adenocarcinoma patients: a comprehensive analysis. Curr Oncol. 2023;30:7722-39
277. Yang H, Said AM, Huang H, Papa APD, Jin G, Wu S. et al. Chlorogenic acid depresses cellular bioenergetics to suppress pancreatic carcinoma through modulating c-Myc-TFR1 axis. Phytother Res. 2021;35:2200-10
278. Zhou X, Soto-Gamez A, Nijdam F, Setroikromo R, Quax WJ. Dihydroartemisinin-transferrin adducts enhance TRAIL-induced apoptosis in triple-negative breast cancer in a P53-independent and ROS-dependent manner. Front Oncol. 2021;11:789336
279. Kim SH, Kang SH, Kang BS. Therapeutic effects of dihydroartemisinin and transferrin against glioblastoma. Nutr Res Pract. 2016;10:393-7
280. von Hagens C, Walter-Sack I, Goeckenjan M, Storch-Hagenlocher B, Sertel S, Elsässer M. et al. Long-term add-on therapy (compassionate use) with oral artesunate in patients with metastatic breast cancer after participating in a phase I study (ARTIC M33/2). Phytomedicine. 2019;54:140-8
281. Chen J, Bai Y, He X, Xiao W, Chen L, Wong YK. et al. The spatiotemporal transcriptional profiling of murine brain during cerebral malaria progression and after artemisinin treatment. Nat Commun. 2025;16:1540
282. Jung EJ, Paramanantham A, Kim HJ, Shin SC, Kim GS, Jung JM. et al. Identification of growth factors, cytokines and mediators regulated by Artemisia annua L. polyphenols (pKAL) in HCT116 colorectal cancer cells: TGF-β1 and NGF-β attenuate pKAL-induced anticancer effects via NF-κB p65 upregulation. Int J Mol Sci. 2022;23:1598
283. Yang Z, Zhou Z, Meng Q, Chen Z, Yun L, Jiang J. et al. Dihydroartemisinin sensitizes lung cancer cells to cisplatin treatment by upregulating ZIP14 expression and inducing ferroptosis. Cancer Med. 2024;13:e70271
284. Zhang X, Ai Z, Zhang Z, Dong R, Wang L, Jin S. et al. Dihydroartemisinin triggers ferroptosis in multidrug-resistant leukemia cells. DNA Cell Biol. 2022;41:705-15
285. Yang Y, Gao Y, Gong Y, Lu J, Li S, Xiong Y. et al. Dihydroartemisinin breaks the immunosuppressive tumor niche during cisplatin treatment in hepatocellular carcinoma. Acta Histochem. 2024;126:152171
286. Hu LJ, Jiang T, Wang FJ, Huang SH, Cheng XM, Jia YQ. Effects of artesunate combined with bortezomib on apoptosis and autophagy of acute myeloid leukemia cells in vitro and its mechanism. Zhonghua Xue Ye Xue Za Zhi. 2019;40:204-8
287. Li S, Xue F, Cheng Z, Yang X, Wang S, Geng F. et al. Effect of artesunate on inhibiting proliferation and inducing apoptosis of SP2/0 myeloma cells through affecting NFkappaB p65. Int J Hematol. 2009;90:513-21
288. Li Z, Ding X, Wu H, Liu C. Artemisinin inhibits angiogenesis by regulating p38 MAPK/CREB/TSP-1 signaling pathway in osteosarcoma. J Cell Biochem. 2019;120:11462-70
289. Scott RE. Differentiation, differentiation/gene therapy and cancer. Pharmacol Ther. 1997;73:51-65
290. Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004;51:1-28
291. Li Y, Shan NN, Sui XH. Research progress on artemisinin and its derivatives against hematological malignancies. Chin J Integr Med. 2020;26:947-55
292. Mancuso RI, Foglio MA, Olalla Saad ST. Artemisinin-type drugs for the treatment of hematological malignancies. Cancer Chemother Pharmacol. 2021;87:1-22
293. Wang Q, Wu LM, Li AY, Zhao Y, Wang NP. Experimental studies of antitumor effect of artesunate on liver cancer. Zhongguo Zhong Yao Za Zhi. 2001;26:707-8 720
294. Xing R, Gao J, Cui Q, Wang Q. Strategies to improve the antitumor effect of immunotherapy for hepatocellular carcinoma. Front Immunol. 2021;12:783236
295. Yin L, Liu Y, Peng Y, Peng Y, Yu X, Gao Y. et al. PARP inhibitor veliparib and HDAC inhibitor SAHA synergistically co-target the UHRF1/BRCA1 DNA damage repair complex in prostate cancer cells. J Exp Clin Cancer Res. 2018;37:153
296. Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-44
297. Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA. et al. Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy. 2014;10:1391-402
298. Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159-69
299. Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853-61
300. Yoshida K, Toden S, Ravindranathan P, Han H, Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis. 2017;38:1036-46
301. Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res. 2015;6:45-62
302. Huang J, Liu D, Wang Y, Liu L, Li J, Yuan J. et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022;71:734-45
303. Chen ST, Lee TY, Tsai TH, Lin YC, Lin CP, Shieh HR. et al. The traditional Chinese medicine DangguiBuxue Tang sensitizes colorectal cancer cells to chemoradiotherapy. Molecules. 2016;21:1677
304. Gao C, Zhou Y, Jiang Z, Zhao Y, Zhang D, Cong X. et al. Cytotoxic and chemosensitization effects of Scutellarin from traditional Chinese herb Scutellaria altissima L. in human prostate cancer cells. Oncol Rep. 2017;38:1491-9
305. Han X, Wei Q, Lv Y, Weng L, Huang H, Wei Q. et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30:327-40
306. Huang MY, Chen YC, Lyu WY, He XY, Ye ZH, Huang CY. et al. Ginsenoside Rh2 augmented anti-PD-L1 immunotherapy by reinvigorating CD8+ T cells via increasing intratumoral CXCL10. Pharmacol Res. 2023;198:106988
307. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48-58
308. Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52-79
309. Wang R, Huang R, Yuan Y, Wang Z, Shen K. Two-carbon tethered artemisinin-isatin hybrids: design, synthesis, anti-breast cancer potential, and in silico study. Front Mol Biosci. 2023;10:1293763
310. Wang J, Zhang J, Guo Z, Hua H, Zhang H, Liu Y. et al. Targeting HSP70 chaperones by rhein sensitizes liver cancer to artemisinin derivatives. Phytomedicine. 2024;122:155156
311. Lu X, Yang F, Chen D, Zhao Q, Chen D, Ping H. et al. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int J Biol Sci. 2020;16:1121-34
312. Ma F, Li Y, Cai M, Yang W, Wu Z, Dong J. et al. ML162 derivatives incorporating a naphthoquinone unit as ferroptosis/apoptosis inducers: design, synthesis, anti-cancer activity, and drug-resistance reversal evaluation. Eur J Med Chem. 2024;270:116387
313. Shen Q, Qiu L. Reversal of P-glycoprotein-mediated multidrug resistance by doxorubicin and quinine co-loaded liposomes in tumor cells. J Liposome Res. 2017;27:293-301
314. Yuan Y, Chiba P, Cai T, Callaghan R, Bai L, Cole SPC. et al. Fabrication of psoralen-loaded lipid-polymer hybrid nanoparticles and their reversal effect on drug resistance of cancer cells. Oncol Rep. 2018;40:1055-63
315. Yu KH, Wu IT, Yu CP, Wang WC, Chi CH, Tsai KC. et al. Discovery of oral chemotherapeutic reversal agents for treating multidrug resistance cancer. Eur J Pharmacol. 2024;977:176682
316. Zhao Y, Tang C, Huang J, Zhang H, Shi J, Xu S. et al. Screening multidrug resistance reversal agents in traditional Chinese medicines by efflux kinetics of D-luciferin in MCF-7/DOXFluc cells. ACS Omega. 2023;8:4853-61
317. Zhuo Z, Zhang D, Lu W, Wu X, Cui Y, Zhang W. et al. Reversal of tamoxifen resistance by artemisinin in ER+ breast cancer: bioinformatics analysis and experimental validation. Oncol Res. 2024;32:1093-107
318. Cao D, Chen D, Xia JN, Wang WY, Zhu GY, Chen LW. et al. Artesunate promoted anti-tumor immunity and overcame EGFR-TKI resistance in non-small-cell lung cancer by enhancing oncogenic TAZ degradation. Biomed Pharmacother. 2022;155:113705
319. Cui Z, Wen J, Yang G, Wei L, Chen H, Zhao Q. et al. Artesunate enhances sensitivity of renal cancer cells to sunitnib by mediating tripartite motif containing 24-induced ubiquitination of paired Box 6. Chem Biol Drug Des. 2025;105:e70116
320. Ma Z, Chen W, Liu Y, Yu L, Mao X, Guo X. et al. Artesunate sensitizes human hepatocellular carcinoma to sorafenib via exacerbating AFAP1L2-SRC-FUNDC1 axis-dependent mitophagy. Autophagy. 2024;20:541-56
321. Riganti C, Doublier S, Viarisio D, Miraglia E, Pescarmona G, Ghigo D. et al. Artemisinin induces doxorubicin resistance in human colon cancer cells via calcium-dependent activation of HIF-1alpha and P-glycoprotein overexpression. Br J Pharmacol. 2009;156:1054-66
322. Zhong H, Zhao X, Zuo Z, Sun J, Yao Y, Wang T. et al. Combating P-glycoprotein-mediated multidrug resistance with 10-O-phenyl dihydroartemisinin ethers in MCF-7 cells. Eur J Med Chem. 2016;108:720-9
323. Haynes RK, Cheu KW, N'Da D, Coghi P, Monti D. Considerations on the mechanism of action of artemisinin antimalarials: part 1-The 'carbon radical' and 'heme' hypotheses. Infect Disord Drug Targets. 2013;13:217-77
324. Chen J, Gao P, Xiao W, Cheng G, Krishna S, Wang J. et al. Multi-omics dissection of stage-specific artemisinin tolerance mechanisms in Kelch13-mutant Plasmodium falciparum. Drug Resist Updat. 2023;70:100978
325. Liu H, Wang Q, Guo J, Feng K, Ruan Y, Zhang Z. et al. Prodrug-based strategy with a two-in-one liposome for Cerenkov-induced photodynamic therapy and chemotherapy. J Control Release. 2023;364:206-15
326. Taubenschmid-Stowers J, Orthofer M, Laemmerer A, Krauditsch C, Rózsová M, Studer C. et al. A whole-genome scan for artemisinin cytotoxicity reveals a novel therapy for human brain tumors. EMBO Mol Med. 2023;15:e16959
327. Kumar DN, Chaudhuri A, Dehari D, Gamper AM, Kumar D, Agrawal AK. Oral delivery of dihydroartemisinin for the treatment of melanoma via bovine milk exosomes. Drug Deliv Transl Res. 2025
328. Ma J, Liao Z, Li J, Li X, Guo H, Zhong Q. et al. A cRGD-modified liposome for targeted delivery of artesunate to inhibit angiogenesis in endometriosis. Biomater Sci. 2025;13:1045-58
329. Shahbazi R, Mirjafary Z, Zarghami N, Saeidian H. Efficient PEGylated magnetic nanoniosomes for co-delivery of artemisinin and metformin: a new frontier in chemotherapeutic efficacy and cancer therapy. Sci Rep. 2024;14:27380
330. Zheng B, Pan F, Shi M, He C, He B, Wang R. et al. 2-Monoacylglycerol mimetic liposomes to promote intestinal lymphatic transport for improving oral bioavailability of dihydroartemisinin. Int J Nanomedicine. 2024;19:5273-95
331. Xu F, Shan X, Li J, Li J, Yuan J, Zou D. et al. The plant matrix of Artemisia annua L. for the treatment of malaria: pharmacodynamic and pharmacokinetic studies. PloS One. 2025;20:e0322835
332. Zhang J, Chen Q, Gao X, Suo Z, Wu D, Zhou Y. et al. Study on a TCM evaluation method based on an MIP-modified MOF sensor with highly selective electrocatalytic activity-an Artemisia annua L. perspective. Anal Methods. 2025;17:3436-45
333. Adjei GO, Goka BQ, Kurtzhals JAL. Neurotoxicity of artemisinin derivatives. Clin Infect Dis. 2006;43:1618
334. Kamchonwongpaisan S, McKeever P, Hossler P, Ziffer H, Meshnick SR. Artemisinin neurotoxicity: neuropathology in rats and mechanistic studies in vitro. Am J Trop Med Hyg. 1997;56:7-12
335. Mohapatra MK, Srinivas D, Kar AK, Murmu M. Anaphylactic reaction to intravenous artesunate. J Assoc Physicians India. 2009;57:183-4
336. Elbadawi M, Boulos JC, Dawood M, Zhou M, Gul W, ElSohly MA. et al. The novel artemisinin dimer isoniazide ELI-XXIII-98-2 induces c-MYC inhibition, DNA damage, and autophagy in leukemia cells. Pharmaceutics. 2023;15:1107
337. Drenberg CD, Buaboonnam J, Orwick SJ, Hu S, Li L, Fan Y. et al. Evaluation of artemisinins for the treatment of acute myeloid leukemia. Cancer Chemother Pharmacol. 2016;77:1231-43
338. Wang T, Wang J, Ren W, Liu ZL, Cheng YF, Zhang XM. Combination treatment with artemisinin and oxaliplatin inhibits tumorigenesis in esophageal cancer EC109 cell through Wnt/β-catenin signaling pathway. Thorac Cancer. 2020;11:2316-24
339. Wang M, Chen H, He X, Zhao X, Zhang H, Wang Y. et al. Artemisinin inhibits the development of esophageal cancer by targeting HIF-1α to reduce glycolysis levels. J Gastrointest Oncol. 2022;13:2144-53
340. Zare P, Shahbazfar AA, Alem M, Varmaghani S. Effects of coadministration of artemisinin and iron on histopathologicalalterations in the AGS gastric adenocarcinoma cell line. Turk J Med Sci. 2017;47:364-7
341. Zhang HT, Wang YL, Zhang J, Zhang QX. Artemisinin inhibits gastric cancer cell proliferation through upregulation of p53. Tumour Biol. 2014;35:1403-9
342. Fröhlich T, Ndreshkjana B, Muenzner JK, Reiter C, Hofmeister E, Mederer S. et al. Synthesis of novel hybrids of thymoquinone and artemisinin with high activity and selectivity against colon cancer. ChemMedChem. 2017;12:226-34
343. Abdulkareem SJ, Jafari-Gharabaghlou D, Farhoudi-Sefidan-Jadid M, Salmani-Javan E, Toroghi F, Zarghami N. Co-delivery of artemisinin and metformin via PEGylated niosomal nanoparticles: potential anti-cancer effect in treatment of lung cancer cells. Daru. 2024;32:133-44
344. Shakeel I, Haider S, Khan S, Ahmed S, Hussain A, Alajmi MF. et al. Thymoquinone, artemisinin, and thymol attenuate proliferation of lung cancer cells as Sphingosine kinase 1 inhibitors. Biomed Pharmacother. 2024;177:117123
345. Xiao FL, Gao WJ, Liu CY, Wang XP, Chen TS. Artemisinin induces caspase-8/9-mediated and Bax/Bak-independent apoptosis in human lung adenocarcinoma (ASTC-a-1) cells. J Xray Sci Technol. 2011;19:545-55
346. Blazquez AG, Fernandez-Dolon M, Sanchez-Vicente L, Maestre AD, Gomez-San Miguel AB, Alvarez M. et al. Novel artemisinin derivatives with potential usefulness against liver/colon cancer and viral hepatitis. Bioorg Med Chem. 2013;21:4432-41
347. Nandi D, Cheema PS, Singal A, Bharti H, Nag A. Artemisinin mediates its tumor-suppressive activity in hepatocellular carcinoma through targeted inhibition of FoxM1. Front Oncol. 2021;11:751271
348. Zhang M, Wang L, Liu W, Wang T, De Sanctis F, Zhu L. et al. Targeting inhibition of accumulation and function of myeloid-derived suppressor cells by artemisinin via PI3K/AKT, mTOR, and MAPK pathways enhances anti-PD-L1 immunotherapy in melanoma and liver tumors. J Immunol Res. 2022;2022:2253436
349. Noori S, Hassan ZM, Farsam V. Artemisinin as a Chinese medicine, selectively induces apoptosis in pancreatic tumor cell line. Chin J Integr Med. 2014;20:618-23
350. Dong J, Chen Y, Yang W, Zhang X, Li L. Antitumor and anti-angiogenic effects of artemisinin on breast tumor xenografts in nude mice. Res Vet Sci. 2020;129:66-9
351. Gharib A, Faezizadeh Z, Mesbah-Namin SAR, Saravani R. Experimental treatment of breast cancer-bearing BALB/c mice by artemisinin and transferrin-loaded magnetic nanoliposomes. Pharmacogn Mag. 2015;11:S117-122
352. Gong Y, Gallis BM, Goodlett DR, Yang Y, Lu H, Lacoste E. et al. Effects of transferrin conjugates of artemisinin and artemisinin dimer on breast cancer cell lines. Anticancer Res. 2013;33:123-32
353. Morrissey C, Gallis B, Solazzi JW, Kim BJ, Gulati R, Vakar-Lopez F. et al. Effect of artemisinin derivatives on apoptosis and cell cycle in prostate cancer cells. Anticancer Drugs. 2010;21:423-32
354. Cao JT, Mo HM, Wang Y, Zhao K, Zhang TT, Wang CQ. et al. Dihydroartemisinin-induced apoptosis in human acute monocytic leukemia cells. Oncol Lett. 2018;15:3178-84
355. Tang N, Liu X, Liu Y, Wang H, Zhao Y, Wang H. et al. Dihydroartemisinin induces ferroptosis in T cell acute lymphoblastic leukemia cells by downregulating SLC7A11 and activating the ATF4-CHOP signaling pathway. Oncol Lett. 2024;28:337
356. Phikulsod P, Kariya R, Panaampon J, Okada S. Dihydroartemisinin induced apoptosis and synergized with chemotherapy in pleural effusion lymphoma cells. Anticancer Res. 2023;43:1139-48
357. Wang Q, Wu S, Zhao X, Zhao C, Zhao H, Huo L. Mechanisms of dihydroartemisinin and dihydroartemisinin/holotransferrin cytotoxicity in T-cell lymphoma cells. PloS One. 2015;10:e0137331
358. Zhang Y, Ma LH, Deng LL, Zhang ZM. Antitumor effect of dihydroartemisinin on diffuse large B-cell lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30:1428-34
359. Fan HN, Zhu MY, Peng SQ, Zhu JS, Zhang J, Qu GQ. Dihydroartemisinin inhibits the growth and invasion of gastric cancer cells by regulating cyclin D1-CDK4-Rb signaling. Pathol Res Pract. 2020;216:152795
360. Li N, Zhang S, Luo Q, Yuan F, Feng R, Chen X. et al. The effect of dihydroartemisinin on the malignancy and Epithelial-Mesenchymal Transition of gastric cancer cells. Curr Pharm Biotechnol. 2019;20:719-26
361. Ma Y, Zhang P, Zhang Q, Wang X, Miao Q, Lyu X. et al. Dihydroartemisinin suppresses proliferation, migration, the Wnt/β-catenin pathway and EMT via TNKS in gastric cancer. Oncol Lett. 2021;22:688
362. Wang H, Ding Q, Zhou H, Huang C, Liu G, Zhao X. et al. Dihydroartemisinin inhibited vasculogenic mimicry in gastric cancer through the FGF2/FGFR1 signaling pathway. Phytomedicine. 2024;134:155962
363. Wang H, Lu C, Zhou H, Zhao X, Huang C, Cheng Z. et al. Synergistic effects of dihydroartemisinin and cisplatin on inducing ferroptosis in gastric cancer through GPX4 inhibition. Gastric Cancer. 2025;28:187-210
364. Bader S, Wilmers J, Ontikatze T, Ritter V, Jendrossek V, Rudner J. Loss of pro-apoptotic Bax and Bak increases resistance to dihydroartemisinin-mediated cytotoxicity in normoxia but not in hypoxia in HCT116 colorectal cancer cells. Free Radic Biol Med. 2021;174:157-70
365. Han W, Duan X, Ni K, Li Y, Chan C, Lin W. Co-delivery of dihydroartemisinin and pyropheophorbide-iron elicits ferroptosis to potentiate cancer immunotherapy. Biomaterials. 2022;280:121315
366. Wang Y, Yang Z, Zhu W, Chen Y, He X, Li J. et al. Dihydroartemisinin inhibited stem cell-like properties and enhanced oxaliplatin sensitivity of colorectal cancer via AKT/mTOR signaling. Drug Dev Res. 2023;84:988-98
367. Yi YC, Liang R, Chen XY, Fan HN, Chen M, Zhang J. et al. Dihydroartemisinin suppresses the tumorigenesis and cycle progression of colorectal cancer by targeting CDK1/CCNB1/PLK1 signaling. Front Oncol. 2021;11:768879
368. Han N, Yang ZY, Xie ZX, Xu HZ, Yu TT, Li QR. et al. Dihydroartemisinin elicits immunogenic death through ferroptosis-triggered ER stress and DNA damage for lung cancer immunotherapy. Phytomedicine. 2023;112:154682
369. Hu BQ, Huang JF, Niu K, Zhou J, Wang NN, Liu Y. et al. B7-H3 but not PD-L1 is involved in the antitumor effects of dihydroartemisinin in non-small cell lung cancer. Eur J Pharmacol. 2023;950:175746
370. Lai XY, Shi YM, Zhou MM. Dihydroartemisinin enhances gefitinib cytotoxicity against lung adenocarcinoma cells by inducing ROS-dependent apoptosis and ferroptosis. Kaohsiung J Med Sci. 2023;39:699-709
371. Ji J, Cheng Z, Zhang J, Wu J, Xu X, Guo C. et al. Dihydroartemisinin induces ferroptosis of hepatocellular carcinoma via inhibiting ATF4-xCT pathway. J Cell Mol Med. 2024;28:e18335
372. Peng Q, Li S, Shi X, Guo Y, Hao L, Zhang Z. et al. Dihydroartemisinin broke the tumor immunosuppressive microenvironment by inhibiting YAP1 expression to enhance anti-PD-1 efficacy. Phytother Res. 2023;37:1740-53
373. Wang Z, Li M, Liu Y, Qiao Z, Bai T, Yang L. et al. Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein response-induced upregulation of CHAC1 expression. Oncol Rep. 2021;46:240
374. Zhou Z, Lei J, Fang J, Chen P, Zhou J, Wang H. et al. Dihydroartemisinin remodels tumor micro-environment and improves cancer immunotherapy through inhibiting cyclin-dependent kinases. Int Immunopharmacol. 2024;139:112637
375. Aung W, Sogawa C, Furukawa T, Saga T. Anticancer effect of dihydroartemisinin (DHA) in a pancreatic tumor model evaluated by conventional methods and optical imaging. Anticancer Res. 2011;31:1549-58
376. Du J, Wang X, Li Y, Ren X, Zhou Y, Hu W. et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 2021;12:705
377. Hu H, Wang Z, Tan C, Liu X, Zhang H, Li K. Dihydroartemisinin/miR-29b combination therapy increases the pro-apoptotic effect of dihydroartemisinin on cholangiocarcinoma cell lines by regulating Mcl-1 expression. Adv Clin Exp Med. 2020;29:911-9
378. Noori S, Hassan ZM. Dihydroartemisinin shift the immune response towards Th1, inhibit the tumor growth in vitro and in vivo. Cell Immunol. 2011;271:67-72
379. Wang SJ, Gao Y, Chen H, Kong R, Jiang HC, Pan SH. et al. Dihydroartemisinin inactivates NF-kappaB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett. 2010;293:99-108
380. Wang S, Sun B, Pan S, Chen H, Kong R, Li J. et al. Experimental study of the function and mechanism combining dihydroartemisinin and gemcitabine in treating pancreatic cancer. Zhonghua Wai Ke Za Zhi. 2010;48:530-4
381. Wang Y, Chen F, Zhou H, Huang L, Ye J, Liu X. et al. Redox Dyshomeostasis with dual stimuli-activatable dihydroartemisinin nanoparticles to potentiate ferroptotic therapy of pancreatic cancer. Small Methods. 2023;7:e2200888
382. Li Y, Zhou X, Liu J, Gao N, Yang R, Wang Q. et al. Dihydroartemisinin inhibits the tumorigenesis and metastasis of breast cancer via downregulating CIZ1 expression associated with TGF-β1 signaling. Life Sci. 2020;248:117454
383. Zhang J, Li Y, Wang JG, Feng JY, Huang GD, Luo CG. Dihydroartemisinin affects STAT3/DDA1 signaling pathway and reverses breast cancer resistance to cisplatin. Am J Chin Med. 2023;51:445-59
384. Chen T, Li M, Zhang R, Wang H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J Cell Mol Med. 2009;13:1358-70
385. Liu Y, Gao S, Zhu J, Zheng Y, Zhang H, Sun H. Dihydroartemisinin induces apoptosis and inhibits proliferation, migration, and invasion in epithelial ovarian cancer via inhibition of the hedgehog signaling pathway. Cancer Med. 2018;7:5704-15
386. Yan Y, Yang X, Han N, Liu Y, Liang Q, Li LG. et al. Metal-organic framework-encapsulated dihydroartemisinin nanoparticles induces apoptotic cell death in ovarian cancer by blocking ROMO1-mediated ROS production. J Nanobiotechnology. 2023;21:204
387. Zheng J, Li X, Yang W, Zhang F. Dihydroartemisinin regulates apoptosis, migration, and invasion of ovarian cancer cells via mediating RECK. J Pharmacol Sci. 2021;146:71-81
388. He Q, Shi J, Shen XL, An J, Sun H, Wang L. et al. Dihydroartemisinin upregulates death receptor 5 expression and cooperates with TRAIL to induce apoptosis in human prostate cancer cells. Cancer Biol Ther. 2010;9:819-24
389. Xia T, Liu S, Xu G, Zhou S, Luo Z. Dihydroartemisinin induces cell apoptosis through repression of UHRF1 in prostate cancer cells. Anticancer Drugs. 2022;33:e113-24
390. Yang J, Xia T, Zhou S, Liu S, Pan T, Li Y. et al. Anticancer effect of dihydroartemisinin via dual control of ROS-induced apoptosis and protective autophagy in prostate cancer 22Rv1 cells. Curr Pharm Biotechnol. 2024;25:1321-32
391. Liu Y, Li H, Luo Z, Yu Y, Yang J, Zhang M. et al. Artesunate, a new antimalarial clinical drug, exhibits potent anti-AML activity by targeting the ROS/Bim and TFRC/Fe2+ pathways. Br J Pharmacol. 2023;180:701-20
392. Tao Y, Li W, Yang J, Xue T, Wang Y, Dong X. et al. Exploring underlying mechanism of artesunate in treatment of acute myeloid leukemia using network pharmacology and molecular docking. Clin Transl Oncol. 2023;25:2427-37
393. Chen Y, Tao H, Wang F, Wu P, Gao J, Zhang X. et al. Artesunate synergistically promotes sorafenib-induced apoptosis and ferroptosis in non-Hodgkin lymphoma cells through inhibition of the STAT3 pathway. Oncol Rep. 2023;50:147
394. Chen Y, Wang F, Wu P, Gong S, Gao J, Tao H. et al. Artesunate induces apoptosis, autophagy and ferroptosis in diffuse large B cell lymphoma cells by impairing STAT3 signaling. Cell Signal. 2021;88:110167
395. Våtsveen TK, Myhre MR, Steen CB, Wälchli S, Lingjærde OC, Bai B. et al. Artesunate shows potent anti-tumor activity in B-cell lymphoma. J Hematol Oncol. 2018;11:23
396. Wang ZC, Liu Y, Wang H, Han QK, Lu C. Research on the relationship between artesunate and Raji cell autophagy and apoptosis of Burkitt's lymphoma and its mechanism. Eur Rev Med Pharmacol Sci. 2017;21:2238-43
397. Xiong D, Geng C, Zeng L, Yao H, Tan J, Zhang L. et al. Artesunate induces ferroptosis by regulating MT1G and has an additive effect with doxorubicin in diffuse large B-cell lymphoma cells. Heliyon. 2024;10:e28584
398. Su T, Li F, Guan J, Liu L, Huang P, Wang Y. et al. Artemisinin and its derivatives prevent Helicobacter pylori-induced gastric carcinogenesis via inhibition of NF-κB signaling. Phytomedicine. 2019;63:152968
399. Wang L, Liu L, Wang J, Chen Y. Inhibitory effect of artesunate on growth and apoptosis of gastric cancer cells. Arch Med Res. 2017;48:623-30
400. Zhou X, Sun WJ, Wang WM, Chen K, Zheng JH, Lu MD. et al. Artesunate inhibits the growth of gastric cancer cells through the mechanism of promoting oncosis both in vitro and in vivo. Anticancer Drugs. 2013;24:920-7
401. Cui C, Feng H, Shi X, Wang Y, Feng Z, Liu J. et al. Artesunate down-regulates immunosuppression from colorectal cancer Colon26 and RKO cells in vitro by decreasing transforming growth factor β1 and interleukin-10. Int Immunopharmacol. 2015;27:110-21
402. Kumar VL, Verma S, Das P. Artesunate suppresses inflammation and oxidative stress in a rat model of colorectal cancer. Drug Dev Res. 2019;80:1089-97
403. Verma S, Das P, Kumar VL. Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chem Biol Interact. 2017;278:84-91
404. Fan R, Deng A, Lin R, Zhang S, Cheng C, Zhuang J. et al. A platinum(IV)-artesunate complex triggers ferroptosis by boosting cytoplasmic and mitochondrial lipid peroxidation to enhance tumor immunotherapy. MedComm. 2024;5:e570
405. Wang Q, Zhou J, Cheng A, Liu Y, Guo J, Li X. et al. Artesunate-binding FABP5 promotes apoptosis in lung cancer cells via the PPARγ-SCD pathway. Int Immunopharmacol. 2024;143:113381
406. Zhao Y, Liu J, Liu L. Artesunate inhibits lung cancer cells via regulation of mitochondrial membrane potential and induction of apoptosis. Mol Med Rep. 2020;22:3017-22
407. Chen W, Ma Z, Yu L, Mao X, Ma N, Guo X. et al. Preclinical investigation of artesunate as a therapeutic agent for hepatocellular carcinoma via impairment of glucosylceramidase-mediated autophagic degradation. Exp Mol Med. 2022;54:1536-48
408. Ilamathi M, Prabu PC, Ayyappa KA, Sivaramakrishnan V. Artesunate obliterates experimental hepatocellular carcinoma in rats through suppression of IL-6-JAK-STAT signalling. Biomed Pharmacother. 2016;82:72-9
409. Yao X, Zhao CR, Yin H, Wang K, Gao JJ. Synergistic antitumor activity of sorafenib and artesunate in hepatocellular carcinoma cells. Acta Pharmacol Sin. 2020;41:1609-20
410. Du JH, Zhang HD, Ma ZJ, Ji KM. Artesunate induces oncosis-like cell death in vitro and has antitumor activity against pancreatic cancer xenografts in vivo. Cancer Chemother Pharmacol. 2010;65:895-902
411. Liu Y, Cui YF. Synergism of cytotoxicity effects of triptolide and artesunate combination treatment in pancreatic cancer cell lines. Asian Pac J Cancer Prev. 2013;14:5243-8
412. Wang K, Zhang Z, Wang M, Cao X, Qi J, Wang D. et al. Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des Devel Ther. 2019;13:2135-44
413. Youns M, Efferth T, Reichling J, Fellenberg K, Bauer A, Hoheisel JD. Gene expression profiling identifies novel key players involved in the cytotoxic effect of artesunate on pancreatic cancer cells. Biochem Pharmacol. 2009;78:273-83
414. Dong HY, Wang ZF. Antitumor effects of artesunate on human breast carcinoma MCF-7 cells and IGF-IR expression in nude mice xenografts. Chin J Cancer Res. 2014;26:200-7
415. Greenshields AL, Fernando W, Hoskin DW. The anti-malarial drug artesunate causes cell cycle arrest and apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3 breast cancer cells. Exp Mol Pathol. 2019;107:10-22
416. Gu L, Zhang J, Liu D, Chen J, Liu S, Peng Q. et al. Development of artesunate intelligent prodrug liposomes based on mitochondrial targeting strategy. J Nanobiotechnology. 2022;20:376
417. Terragno M, Vetrova A, Semenov O, Sayan AE, Kriajevska M, Tulchinsky E. Mesenchymal-epithelial transition and AXL inhibitor TP-0903 sensitise triple-negative breast cancer cells to the antimalarial compound, artesunate. Sci Rep. 2024;14:425
418. Pirali M, Taheri M, Zarei S, Majidi M, Ghafouri H. Artesunate, as a HSP70 ATPase activity inhibitor, induces apoptosis in breast cancer cells. Int J Biol Macromol. 2020;164:3369-75
419. Chen X, Zhang XL, Zhang GH, Gao YF. Artesunate promotes Th1 differentiation from CD4+ T cells to enhance cell apoptosis in ovarian cancer via miR-142. Braz J Med Biol Res. 2019;52:e7992
420. Greenshields AL, Shepherd TG, Hoskin DW. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol Carcinog. 2017;56:75-93
421. Li G, Ling M, Yu K, Yang W, Liu Q, He L. et al. Synergetic delivery of artesunate and isosorbide 5-mononitrate with reduction-sensitive polymer nanoparticles for ovarian cancer chemotherapy. J Nanobiotechnology. 2022;20:471
422. Li J, Feng L, Yuan Y, He T, Zou X, Su B. et al. Inhibition of HOXC11 by artesunate induces ferroptosis and suppresses ovarian cancer progression through transcriptional regulation of the PROM2/PI3K/AKT pathway. World J Surg Oncol. 2024;22:268
423. Wei Y, Liu F, Zhu X, Liu X, Li H, Hou L. et al. Artesunate disrupts ribosome RNA biogenesis and inhibits ovarian cancer growth by targeting FANCA. Phytomedicine. 2025;136:156333
424. Huang X, Yuan D, Zhang C, Zhang X. Artesunate induces prostate cancer cell line PC-3 differentiation and cell cycle arrest. Zhong Xi Yi Jie He Xue Bao. 2008;6:591-4
425. Nunes JJ, Pandey SK, Yadav A, Goel S, Ateeq B. Targeting NF-kappa B signaling by artesunate restores sensitivity of castrate-resistant prostate cancer cells to antiandrogens. Neoplasia. 2017;19:333-45
426. Vakhrusheva O, Erb HHH, Bräunig V, Markowitsch SD, Schupp P, Baer PC. et al. Artesunate inhibits the growth behavior of docetaxel-resistant prostate cancer cells. Front Oncol. 2022;12:789284
427. Zhao X, Guo X, Yue W, Wang J, Yang J, Chen J. Artemether suppresses cell proliferation and induces apoptosis in diffuse large B cell lymphoma cells. Exp Ther Med. 2017;14:4083-90
428. Farsam V, Hassan ZM, Zavaran-Hosseini A, Noori S, Mahdavi M, Ranjbar M. Antitumor and immunomodulatory properties of artemether and its ability to reduce CD4+ CD25+ FoxP3+ T reg cells in vivo. Int Immunopharmacol. 2011;11:1802-8
429. Chen J, Huang X, Tao C, Xiao T, Li X, Zeng Q. et al. Artemether attenuates the progression of non-small cell lung cancer by inducing apoptosis, cell cycle arrest and promoting cellular senescence. Biol Pharm Bull. 2019;42:1720-5
430. Chen D, Li G, Luo L, Lin T, Chu X, Liu K. et al. Artemisitene induces apoptosis of breast cancer cells by targeting FDFT1 and inhibits the growth of breast cancer patient-derived organoids. Phytomedicine. 2024;135:156155
431. Lin X, Lin T, Liu M, Chen D, Chen J. Liensinine diperchlorate and artemisitene synergistically attenuate breast cancer progression through suppressing PI3K-AKT signaling and their efficiency in breast cancer patient-derived organoids. Biomed Pharmacother. 2024;176:116871
432. Ye Y, Yang Y, Yan L, Zhou L, Yu S, Du Q. et al. Integrating network pharmacology and experimental validation to explore the effects and mechanisms of Qinghao Biejia decoction and its active compound artemisinin B against non-small-cell lung cancer. Drug Des Devel Ther. 2023;17:2461-79
433. Jiwa H, Xie Z, Qu X, Xu J, Huang Y, Huang X. et al. Casticin induces ferroptosis in human osteosarcoma cells through Fe2+ overload and ROS production mediated by HMOX1 and LC3-NCOA4. Biochem Pharmacol. 2024;226:116346
434. Shih YL, Chou HM, Chou HC, Lu HF, Chu YL, Shang HS. et al. Casticin impairs cell migration and invasion of mouse melanoma B16F10 cells via PI3K/AKT and NF-κB signaling pathways. Environ Toxicol. 2017;32:2097-112
435. Yang X, Liu Z, Xu X, He M, Xiong H, Liu L. Casticin induces apoptosis and cytoprotective autophagy while inhibiting stemness involving Akt/mTOR and JAK2/STAT3 pathways in glioblastoma. Phytother Res. 2024;38:305-20
436. Freitas S, Costa S, Azevedo C, Carvalho G, Freire S, Barbosa P. et al. Flavonoids inhibit angiogenic cytokine production by human glioma cells. Phytother Res. 2011;25:916-21
437. Lang SJ, Schmiech M, Hafner S, Paetz C, Werner K, El Gaafary M. et al. Chrysosplenol D, a flavonol from Artemisia annua, induces ERK1/2-mediated apoptosis in triple negative human breast cancer cells. Int J Mol Sci. 2020;21:4090
438. Zhang H, You Z, Li Y, Gao C, Wang Y, Zhang X. Chrysosplenol D can inhibit the growth of prostate cancer by inducing reactive oxygen species and autophagy. Immun Inflamm Dis. 2023;11:e1061
439. Jung EJ, Lee WS, Paramanantham A, Kim HJ, Shin SC, Kim GS. et al. p53 enhances Artemisia annua L. polyphenols-induced cell death through upregulation of p53-dependent targets and cleavage of PARP1 and Lamin A/C in HCT116 colorectal cancer cells. Int J Mol Sci. 2020;21:9315
440. Jung EJ, Kim HJ, Shin SC, Kim GS, Jung JM, Hong SC. et al. Anticancer effect by combined treatment of Artemisia annua L. polyphenols and docetaxel in DU145 prostate cancer cells and HCT116 colorectal cancer cells. Curr Issues Mol Biol. 2024;46:1621-34
Author contact
![]() Corresponding authors: Xinbing Sui, suilabedu.cn, Jun He, hj1460799764com, or Shegan Gao, gsg112258com.
Corresponding authors: Xinbing Sui, suilabedu.cn, Jun He, hj1460799764com, or Shegan Gao, gsg112258com.
 Global reach, higher impact
Global reach, higher impact