13.3
Impact Factor
Theranostics 2023; 13(1):250-266. doi:10.7150/thno.79186 This issue Cite
Research Paper
Intravital imaging and cavitation monitoring of antivascular ultrasound in tumor microvasculature
1. Department of Medical Biophysics, University of Toronto, Canada.
2. Sunnybrook Research Institute, Toronto, Canada.
*These authors contributed equally to this work.
Abstract
Rationale: Focused ultrasound-stimulated microbubbles have been shown to be capable of inducing blood flow shutdown and necrosis in a range of tissue types in an approach termed antivascular ultrasound or nonthermal ablation. In oncology, this approach has demonstrated tumor growth inhibition, and profound synergistic antitumor effects when combined with traditional platforms of chemo-, radiation- and immune-therapies. However, the exposure schemes employed have been broad and underlying mechanisms remain unclear with fundamental questions about exposures, vessel types and sizes involved, and the nature of bubble behaviors and their acoustic emissions resulting in vascular damage - impeding the establishment of standard protocols.
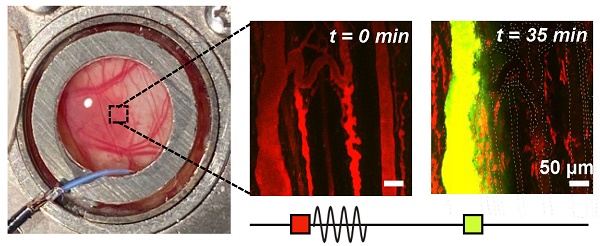
Methods: Here, ultrasound transmitters and receivers are integrated into a murine dorsal window chamber tumor model for intravital microscopy studies capable of real-time visual and acoustic monitoring during antivascular ultrasound. Vessel type (normal and tumor-affected), caliber, and viability are assessed under higher pressure conditions (1, 2, and 3 MPa), and cavitation signatures are linked to the biological effects.
Results: Vascular events occurred preferentially in tumor-affected vessels with greater incidence in smaller vessels and with more severity as a function of increasing pressure. Vascular blood flow shutdown was found to be due to a combination of focal disruption events and network-related flow changes. Acoustic emissions displayed elevated broadband noise and distinct sub- and ultra-harmonics and their associated third-order peaks with increasing pressure.
Conclusions: The observed vascular events taken collectively with identified cavitation signatures provide an improved mechanistic understanding of antivascular ultrasound at the microscale, with implications for establishing a specific treatment protocol and control platform.
Keywords: antivascular ultrasound, cavitation, mechanical ablation, microbubble, intravital imaging
 Global reach, higher impact
Global reach, higher impact