13.3
Impact Factor
Theranostics 2023; 13(1):417-437. doi:10.7150/thno.77694 This issue Cite
Research Paper
Targeting WWP1 ameliorates cardiac ischemic injury by suppressing KLF15-ubiquitination mediated myocardial inflammation
1. Department of Cardiology, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200233, China.
2. Xiamen Cardiovascular Hospital, Xiamen University, Xiamen 361004, China.
3. Key Laboratory of Targeted Intervention of Cardiovascular Disease, Collaborative Innovation Center for Cardiovascular Disease Translational Medicine, Nanjing Medical University, Nanjing 211166, Jiangsu, China.
4. Fujian Provincial Key Laboratory of Innovative Drug Target Research, School of Pharmaceutical Sciences, Xiamen University, Xiamen 361102, China.
5. Key Laboratory of Tissue Microenvironment and Tumor, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, University of Chinese Academy of Sciences, Chinese Academy of Sciences (CAS), Shanghai, China.
6. The first clinical medical college, Southern Medical University, Guangzhou 510000, China.
#These authors contributed equally to this work.
Abstract
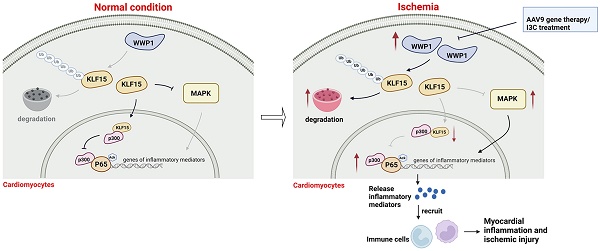
Rationale: Previous studies have suggested that myocardial inflammation plays a critical role after ischemic myocardial infarction (MI); however, the underlying mechanisms still need to be fully elucidated. WW domain-containing ubiquitin E3 ligase 1 (WWP1) is considered as an important therapeutic target for cardiovascular diseases due to its crucial function in non-ischemic cardiomyopathy, though it remains unknown whether targeting WWP1 can alleviate myocardial inflammation and ischemic injury post-MI.
Methods: Recombinant adeno-associated virus 9 (rAAV9)-cTnT-mediated WWP1 or Kruppel-like factor 15 (KLF15) gene transfer and a natural WWP1 inhibitor Indole-3-carbinol (I3C) were used to determine the WWP1 function in cardiomyocytes. Cardiac function, tissue injury, myocardial inflammation, and signaling changes in the left ventricular tissues were analyzed after MI. The mechanisms underlying WWP1 regulation of cardiomyocyte phenotypes in vitro were determined using the adenovirus system.
Results: We found that WWP1 expression was up-regulated in cardiomyocytes located in the infarct border at the early phase of MI and in hypoxia-treated neonatal rat cardiac myocytes (NRCMs). Cardiomyocyte-specific WWP1 overexpression augmented cardiomyocyte apoptosis, increased infarct size and deteriorated cardiac function. In contrast, inhibition of WWP1 in cardiomyocytes mitigated MI-induced cardiac ischemic injury. Mechanistically, WWP1 triggered excessive cardiomyocyte inflammation after MI by targeting KLF15 to catalyze K48-linked polyubiquitination and degradation. Ultimately, WWP1-mediated degradation of KLF15 contributed to the up-regulation of p65 acetylation, and activated the inflammatory signaling of MAPK in ischemic myocardium and hypoxia-treated cardiomyocytes. Thus, targeting of WWP1 by I3C protected against cardiac dysfunction and remodeling after MI.
Conclusions: Our study provides new insights into the previously unrecognized role of WWP1 in cardiomyocyte inflammation and progression of ischemic injury induced by MI. Our findings afford new therapeutic options for patients with ischemic cardiomyopathy.
Keywords: myocardial infarction, cardiomyocyte inflammation, WWP1, KLF15-Ubiquitination
 Global reach, higher impact
Global reach, higher impact