13.3
Impact Factor
Theranostics 2023; 13(15):5561-5583. doi:10.7150/thno.85390 This issue Cite
Research Paper
Tat-NTS peptide protects neurons against cerebral ischemia-reperfusion injury via ANXA1 SUMOylation in microglia
1. Department of Neurobiology, School of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China.
2. The Institute for Brain Research, Collaborative Innovation Center for Brain Science, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China.
3. Key Laboratory of Neurological Diseases, Ministry of Education, Wuhan, Hubei 430030, China.
4. Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China.
5. Department of Clinical Laboratory, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450052, China.
6. Department of Pathophysiology, School of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China.
Abstract
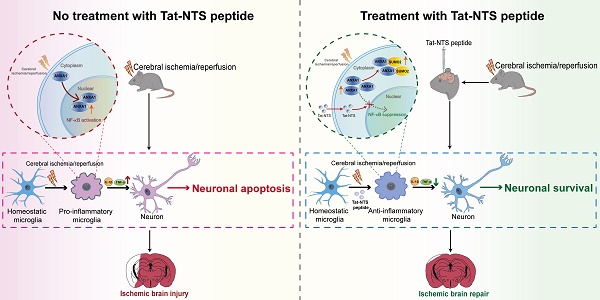
Rationale: Recent studies indicate that microglial activation and the resulting inflammatory response could be potential targets of adjuvant therapy for ischemic stroke. Many studies have emphasized a well-established function of Annexin-A1 (ANXA1) in the immune system, including the regulation of microglial activation. Nevertheless, few therapeutic interventions targeting ANXA1 in microglia for ischemic stroke have been conducted. In the present study, Tat-NTS, a small peptide developed to prevent ANXA1 from entering the nucleus, was utilized. We discovered the underlying mechanism that Tat-NTS peptide targets microglial ANXA1 to protect against ischemic brain injury.
Methods: Preclinical studies of ischemic stroke were performed using an oxygen-glucose deprivation and reperfusion (OGD/R) cell model in vitro and the middle cerebral artery occlusion (MCAO) animal model of ischemic stroke in vivo. Confocal imaging and 3D reconstruction analyses for detecting the protein expression and subcellular localization of microglia in vivo. Co-immunoprecipitation (Co-IP), immunoblotting, ELISA, quantitative real-time PCR (qRT-PCR), Luciferase reporter assay for determining the precise molecular mechanism. Measurement on the cytotoxicity of Tat-NTS peptide for microglia was assessed by CCK-8 and LDH assay. TUNEL staining was used to detect the microglia conditioned medium-mediated neuronal apoptosis. Adeno-associated viruses (AAVs) were injected into the cerebral cortex, striatum and hippocampal CA1 region of adult male Cx3cr1-Cre mice, to further verify the neurofunctional outcome and mechanism of Tat-NTS peptide by TTC staining, the modified Neurological Severity Score (mNSS) test, the open field test (OFT), the novel object recognition task (NORT), the Morris water maze (MWM) test, the long-term potentiation (LTP) and the Transmission electron microscopy (TEM).
Results: It was observed that administration of Tat-NTS led to a shift of subcellular localization of ANXA1 in microglia from the nucleus to the cytoplasm in response to ischemic injury. Notably, this shift was accompanied by an increase in ANXA1 SUMOylation in microglia and a transformation of microglia towards an anti-inflammatory phenotype. We confirmed that Tat-NTS-induced ANXA1 SUMOylation in microglia mediated IKKα degradation via NBR1-dependent selective autophagy, then blocking the activation of the NF-κB pathway. As a result, the expression and release of the pro-inflammatory factors IL-1β and TNF-α were reduced in both in vitro and in vivo experiments. Furthermore, we found that Tat-NTS peptide's protective effect on microglia relieved ischemic neuron apoptosis. Finally, we demonstrated that Tat-NTS peptide administration, through induction of ANXA1 SUMOylation in microglia, reduced infarct volume, improved neurological function and facilitated behavioral recovery in MCAO mice.
Conclusions: Our study provides evidence for a novel mechanism of Tat-NTS peptide in regulating microglial ANXA1 function and its substantial neuroprotective effect on neurons with ischemic injuries. These findings suggest that Tat-NTS peptides have a high potential for clinical application and may be a promising therapeutic candidate for treating cerebral ischemia.
Keywords: Tat-NTS peptide, Annexin-A1, cerebral ischemia-reperfusion injury, microglia, SUMOylation
 Global reach, higher impact
Global reach, higher impact