13.3
Impact Factor
Theranostics 2026; 16(5):2221-2248. doi:10.7150/thno.124843 This issue Cite
Review
Hydrogel adhesives for upper gastrointestinal wound healing
1. China-Japan Union Hospital of Jilin University, Jilin University, Changchun 130033, P. R. China.
2. International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun 130012, P. R. China.
Received 2025-9-8; Accepted 2025-11-18; Published 2026-1-1
Abstract
This review discusses the latest progress in hydrogel adhesives for upper gastrointestinal wound healing. The specific acidic environment of the upper gastrointestinal tract makes treating upper gastrointestinal wounds, especially gastric bleeding and perforation, quite challenging. Recent research on hydrogels in biomedical applications has demonstrated immense potential, particularly in wound dressing. With three-dimensional network structures, hydrogels provide an efficient and safe strategy for hemostasis and wound repair in the digestive tract. Moreover, hydrogels possess excellent properties, including biocompatibility, biodegradability, mechanical strength, cell/tissue adhesion, and controllable release, making them suitable for complex wound-healing processes. This review summarizes the latest advancements in hydrogels for various upper gastrointestinal conditions, including esophageal stenosis, gastric ulcers, gastric bleeding, gastric perforation, and endoscopic submucosal dissection. We also highlight the merits and demerits of current hydrogel dressings to provide a deep understanding of their working mechanisms. Finally, the challenges and prospects of hydrogels for upper gastrointestinal applications are reviewed, aiming to inspire researchers in different fields and encourage the development of multifunctional hydrogels for effective upper gastrointestinal wound healing.
Keywords: hydrogel, wound healing, hemostasis, combination therapy, gastrointestinal tract
1. Introduction
The gastrointestinal (GI) tract, comprising the esophagus, stomach, small intestine (duodenum, jejunum, and ileum), and large intestine (cecum, colon, and rectum), plays a crucial role in the transportation, digestion, and absorption of food [1]. GI wounds, such as esophageal stenosis, gastric hemorrhage, gastric perforation, and peptic ulcers, pose complex treatment challenges due to the unique physiological characteristics and dynamic microenvironment of the GI tract, particularly the presence of gastric acid. Frequent rebleeding, perforation, and infection remain the most difficult and prevalent clinical challenges. Although traditional treatment strategies, including suturing, clipping, and conventional hemostatic agents, have played an essential role in clinical practice, they often fail to provide prolonged adhesion and prevent healing in the acidic, dynamic gastric environment. Due to their strong wet adhesion and biocompatibility, hydrogel adhesives have emerged as promising candidates for GI wound management. However, conventional dressings are often inadequate or intolerable for wound sites, resulting in impaired wound healing [2]. When gastric perforation occurs, stomach acid, partially digested food, and bacteria can enter the abdominal cavity, significantly increasing the risk of infection and severe complications [3]. Therefore, innovative multifunctional dressings have been developed to repair, regenerate, or enhance dysfunctional organs or tissues in the GI tract [4, 5].
Given that strong interfacial adhesion to tissues is recognized as a critical factor influencing the overall robustness and reliability of therapeutic interventions, hydrogels have emerged as a key bridging material for the construction of adhesive dressings in the biomedical field. Hydrogels, built with a macromolecular backbone and side chains containing abundant reversible bonds, possess a typical three-dimensional (3D) network that can retain abundant water while exhibiting negligible changes in macroscopic solid-like rheological and adhesion behavior [6-8]. Compared with conventional dressings, hydrogels can adhere more readily to biological tissues due to their similarity to human tissues and organs, coupled with their remarkable toughness and stretchability [9]. In addition, hydrogels can prevent bacterial infections as physical barriers and concentrated platelets and coagulation factors by absorbing water, thereby shortening clotting time and accelerating the healing process [10]. Recently, hydrogels have also been used to load and deliver other therapeutic agents, enabling the achievement of additional functions, some of which are particularly required in many biomedical applications, such as gelatin hemostatic sponge and fibrin glue [11].
In particular, the introduction of non-covalent supramolecular interactions, including van der Waals forces, hydrogen bonding, hydrophobic interactions, and electrostatic interactions, provides a facile and effective method to endow hydrogels with reversible properties, such as degradability, self-healing, and stimuli-responsive abilities. These intriguing characteristics position hydrogels as promising materials for assisting hemostasis and wound healing in the GI tract [11]. Over the past few decades, significant advances have occurred in the study of viscous hydrogels, with substantial progress in their structural design and biomedical applications. For instance, Artzi et al. [12] reported a sprayable hydrogel that facilitates continuous adhesion to GI lesions, enabling the biomaterial to instantly gel and adhere to tissues, thereby forming a protective barrier. Gastric acid, with a pH<1.5, is an intractable obstacle to gastric wound therapy. Most drugs and conventional hemostatic dressings are inevitably damaged by the highly acidic microenvironment, leading to severely weakened adhesiveness. Recently, acid-resistant hydrogels have been developed and proven to be a boon to these patients. For example, Liu et al. [13] introduced an acid-resistant hydrogel bioadhesive (AtGel) comprising an acid-resistant hydrogel substrate and an adhesive polymer brush layer. The hydrogel demonstrates effective acid resistance and maintains strong interfacial toughness and robust bioadhesion, even after immersion in a simulated gastric fluid medium for 240 hours.
Although the research progress of hydrogels has been summarized in several interesting reviews, for instance, the research status of self-healing hydrogels in wound management [11], the applications of antioxidant hydrogels in oxidative stress-related diseases [14], and the application of polysaccharide-based hydrogels in different types of GI wounds [15], a thorough review entirely focusing on the hydrogels for upper GI tissue engineering applications has not been found in the literature, especially given that various hydrogels have emerged in the last five years. Existing clinical treatments for upper GI wound healing include suturing, clips, stents, and fibrin glue. These methods often face challenges, including complexity, patient discomfort, and an increased risk of infection or recurrence. Hydrogel adhesives offer advantages over traditional methods, such as strong wet adhesion, acid resistance, controllable degradation, injectability, and localized drug delivery. However, potential disadvantages, such as cost and durability, remain compared with conventional treatment approaches.
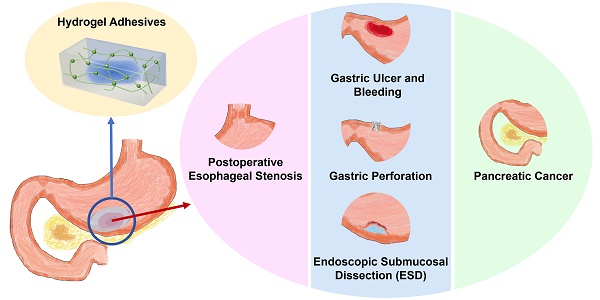
In this review, we aim to advance the development of hydrogel adhesives in the treatment and management of upper GI tissue injuries. First, we discussed the classification of advanced hydrogels by function, with an emphasis on their design, synthesis, and potential applications (Scheme 1). Then, we systematically summarized the applications of various advanced hydrogels in common GI diseases associated with tissue damage and their corresponding microenvironmental characteristics, including postoperative esophageal stenosis, gastric ulcer and bleeding, gastric perforation, endoscopic surgery incision, and pancreatic cancer (Scheme 1, Tables 1-3). Finally, the challenges and prospects in this research field are discussed from both fundamental research and translational medicine perspectives.
2. Classification of hydrogels
Hydrogel synthesis and structural design directly determine their properties and clinical applications, for example, acid-resistant groups and dense crosslinking enhance gastric stability, while thermosensitivity, shear-thinning properties, or rapid photocrosslinking enable injectability or sprayability for endoscopic delivery.
2.1 Classification of hydrogels by cross-linking methods
Hydrogels can be categorized based on the interactions between polymer chains into three types: chemical hydrogels, physical hydrogels, and hybrid hydrogels. Non-covalent physical crosslinking methods, such as van der Waals forces, hydrophobic interactions, and electrostatic interactions, endow hydrogels with reversibility and self-healing, but exhibit limited structural stability [16]. These physically crosslinked hydrogels are prone to degradation under gastric acid, restricting their durability in GI environments.
In contrast, chemical crosslinked hydrogels, constructed through radical polymerization, interpenetrating polymer networks, radiation polymerization, and complementary groups, exhibit greater mechanical strength and environmental stability. However, their slow degradation rates may raise long-term safety concerns, warranting further optimization [17]. Hybrid crosslinking strategies that integrate physical and chemical methods offer a rational design approach. Such hybrid hydrogels combine reversibility and stability, resulting in improved performance in complex gastrointestinal environments and providing meaningful insights for the design of advanced hydrogels.
The functional classification and specific applications of hydrogels, showing the types and characteristics of hydrogels used for postoperative esophageal stenosis, gastric ulcers and bleeding, gastric perforation, endoscopic submucosal dissection (ESD), and pancreatic cancer.

2.2 Classification of hydrogels by components
Hydrogels can be divided into simple hydrogels and hybrid hydrogels based on their components. Generally, simple hydrogels possess only a porous skeleton structure, providing only basic adhesion and hydration, while their therapeutic functionality is often limited. In contrast, hybrid hydrogels incorporate nanoparticles, peptides, DNA, drugs, cells, or bacteria, endowing them with enhanced mechanical robustness and multifunctional therapeutic potential.
2.2.1 Simple hydrogels
Simple hydrogels are easier to prepare. They not only have their own excellent adhesion and mechanical properties, but also can be used as a skeleton and carrier to participate in the composition of other materials. For instance, Xie et al. [18] introduced a simple hydrogel composed of carboxymethyl chitosan (CMCS), sodium alginate (SA), and oxidized dextran (ODE), which can achieve rapid hemostasis by adhering to the injured tissue and prevent infection induced by S. aureus due to the antibacterial effect of CMCS. Furthermore, hydrogels can mimic the 3D structure of the extracellular matrix and accelerate tissue repair in mice.
2.2.2 Hybrid hydrogels
Hydrogels with nanoparticles. Recently, with the rapid development of nanotechnology, the potential applications of nanomaterials in medicine have advanced significantly. Hybrid hydrogels with diverse functions can be developed by incorporating nanoparticles into the hydrogel matrix. Lu et al. [19] introduced a pH-sensitive hydrogel comprising chitosan (CS), SA, and tilapia collagen peptide to protect against gastric mucosal injury induced by alcohol, demonstrating continuous release of TCP, strong mucosal adhesion, and superior biodegradability (Figure 1A). The hydrogel used CS-NAC-encapsulating CaCO3 nanoparticles to achieve gastric acid-responsiveness. The SEM image of pure nano-CaCO3 particles was almost cubic (Figure 1B), while CS-NAC (CaCO3) particles were almost circular (Figure 1C). When gastric acid gradually decomposes CaCO3, releasing Ca2+, Ca2+ and SA form a classic eggshell structure, indicating the formation of a hydrogel. Moreover, Chen et al. [20] loaded hydrogels with nanoparticles with magneto-thermal function and promoted the gel-sol transition of hydrogels via magneto-thermal treatment to fill gastric ulcers.
Hydrogels containing nanoparticles or DNA. (A) Schematic diagram displaying the synthesis of CS-NAC/SA/TCP hydrogel for alcohol-induced gastric mucosal injury. Adapted with permission from [19], copyright 2022, Elsevier. (B) SEM of pure nano-CaCO3 particles. (C) SEM of CS-NAC (CaCO3) particles. (D) Schematic diagram showing the design and preparation of DGL sealant hydrogel through highly base complementary pairing of DNA. Adapted with permission from [21], copyright 2023, Elsevier.

Hydrogels with deoxyribonucleic acid (DNA). Bases are essential components of genetic material, forming stable structures of DNA and ribonucleic acid (RNA) through hydrogen bonding between purines and pyrimidines. Due to their precise base-pair recognition, DNA macromolecules can be used to construct dynamic hydrogel materials. For example, Xie et al. [21] applied gelatin, Laponite nanoclay, and DNA to build a nano-enabled DNA supramolecular hydrogel sealant (DGL) (Figure 1D), which exhibits excellent tissue adhesion, injectability, and self-healing properties, and can rapidly stop bleeding and prevent postoperative adhesions. DNA with highly complementary base pairing is the primary building block for constructing a physical interconnection network, enabling the DGL hydrogel to be dynamically reversible through multiple hydrogen bonds.
Hydrogels with drugs. Hydrogels can realize controlled and continuous delivery of various drugs. For example, Dave et al. [22] added paclitaxel and 6-aminocaproic acid to a self-assembled small molecule hydrogel based on diglycerol monostearate for anticancer and hemostatic effects, which slowly degraded in the presence of lipase. Parsa et al. [23] added tetracycline to a CS hydrogel to construct a hybrid hydrogel suitable for wound dressings and drug delivery. The hydrogel slowly released a therapeutic dose of tetracycline within five days. In addition, modifications to drug molecules can contribute to the formation of a hydrogel crosslinking network. For example, Ouyang et al. [14] used amino-modified montmorillonite to form amide linkages with the carboxyl of CMCS and oxidized sodium alginate (OSA), so that montmorillonite was evenly dispersed in the hydrogel.
Hydrogels with cells. Adult stem cells, such as endothelial progenitor cells, mesenchymal matrix stem cells, and adipose tissue-derived stem cells, have been used in applications for injured tissue healing [24]. Due to the swallowing and wriggling of food in the GI tract, stem cells used for treatment will be lost in the early stages of treatment. This problem can be solved by loading stem cells into hydrogels. For example, Chung et al. [25] loaded fat-derived stem cells into a hydrogel to prepare a hybrid hydrogel for postoperative esophageal stricture, thereby regulating the regenerative process through the paracrine effects of stem cells. In addition to stem cells, extracellular vesicles also contribute to wound healing and regulate homeostasis through cell recruitment, proliferation, migration, and differentiation [26].
Hydrogels with bacteria. Probiotic therapy can also be used for bacterial interference and immune regulation [27]. Applying hydrogel to the base of probiotic therapy can effectively prevent the escape of probiotics; for example, Ming et al. [28] wrapped Lactobacillus reuteri into hydrogel microspheres via emulsion polymerization and formed a hydrogel at the wound site under light irradiation through covalent crosslinking of materials. Lactobacillus reuteri can decrease local pH and produce antibacterial agents to repress the growth of pathogenic bacteria. The experiments showed that the wound treated by live bacterial hydrogel had a lighter inflammatory response and faster healing. However, this technology has not yet been applied to the GI tract.
Overall, chemical bonding and physical interactions synergistically influence the adhesion between hydrogels and GI tissues. Catechol hydrogel adhesives usually interact with various tissue components, such as mucin and collagen, through covalent bonds or coordination, generally possessing good acid resistance, durability, and mechanical stability. Schiff-base hydrogels are dynamically covalently bonded and exhibit moderate stability under mild acidic conditions. Non-covalent supramolecular interactions, including hydrogen bonding, electrostatic interactions, hydrophobic anchoring, and van der Waals forces, promote rapid initial adhesion. However, due to their susceptibility to microenvironmental fluctuations, supramolecular hydrogels are better suited for short-term adhesion.
2.3 Design principles for acid-resistant and tissue-adhesive hydrogels
The adhesion and stability of hydrogel adhesives are usually affected by multiple covalent and non-covalent interactions. Functional groups, spatial architecture, and incorporated components directly determine their adhesion durability and anti-swelling capacity in highly acidic, enzyme-rich, and peristaltic environments. Covalent interactions form the primary network of hydrogel adhesives, such as catechol coupling, thiourea-catechol protection, Schiff-base linkages, and photo-crosslinking [29, 30]. These interactions can resist acid hydrolysis and prevent premature degradation. Dynamic interactions, including imine exchange and boronate ester linkages, can repeatedly break and reform in response to acidic fluctuations and peristaltic stress, endowing hydrogels with self-repairing and adaptive abilities [31].
Non-covalent interactions act as secondary modulators to further reinforce adhesion of hydrogels, including hydrogen bonding, hydrophobic anchoring, cation-π interactions, and electrostatic bridging. Their hydrophobic groups can expel water molecules from the wound site, promoting the hydrogel's close adherence to the tissue and reducing acid penetration [32]. In addition, hydrogels can bind to gastric mucins or ECM residues through electrostatic interactions [33]. The combined contribution of these reversible interactions significantly enhances the wet adhesion performance on gastric mucosa.
The network architecture and functional fillers of hydrogel adhesives can reduce acid-induced swelling, delay pepsin infiltration, and enhance stability under gastric peristalsis. In particular, the chemical-physical cross-linked network, which integrates permanent bonds and sacrificial bonds, exhibits excellent self-repairing capability [21]. Moreover, the incorporation of functional components such as DNA, peptides, polysaccharides, or nanomaterials can also regulate the adhesion, rheology, and degradation properties of hydrogels. In summary, the acid resistance, tissue adhesion, and dynamic stability of hydrogel adhesives suitable for the upper GI environment depend on the synergistic contribution of multiple interactions. Rational design and regulation of these interactions will yield effective strategies for developing next-generation hydrogel adhesives.
3. Applications of hydrogels
Selecting appropriate hydrogels based on clinical needs is crucial for treatment. Generally, injectable, sprayable, and photo-crosslinked hydrogels are mainly applied in endoscopic procedures, such as hemostasis, ulcer repair, and submucosal injection during ESD, due to their excellent in situ gelation and controlled fluidity. In contrast, prefabricated, mechanically reinforced, or powder-type hydrogels are mainly suitable for open surgical repairs because they require durable mechanical support. Therefore, we highlight recent advances in the clinical applications of hydrogels for various gastric diseases. Moreover, corresponding key performance parameters of hydrogel adhesives for different gastric disease models were summarized in Table 4.
3.1 Postoperative esophageal stricture
Esophageal cancer (EC) is the seventh leading cause of cancer death in the world [34]. Traditional endoscopic approaches, such as repeated dilation and stenting, are limited by mechanical trauma and insufficient promotion of tissue repair or epithelial regeneration. Hydrogel adhesives, particularly when combined with thermoresponsive or drug-loading properties, provide sustained support and promote epithelial regeneration. Nonetheless, challenges such as long-term biostability and controlled degradation remain to be addressed [35]. Nevertheless, postoperative stenosis is a common perioperative complication in endoscopic submucosal dissection (ESD) of the esophagus, with a particularly high incidence when the mucosal resection reaches three-quarters of the esophageal circumference [36-41].
Recently, several new biomedical materials have emerged for preventing esophageal stenosis. Lee and Park developed a hydrogel-impregnated robust interlocking nano connector (HiRINC) on a self-expandable metallic stent to connect the hydrogel and the extra-luminal stent strut surface. HiRINC has increased bonding sites and enhances mechanical interlocking and hydrogel bonding with hydrogel, thanks to its network-like porous structure. In the rat and porcine esophagus, HiRINC remains long-term anchored at the target esophageal stricture area by absorbing and distributing mechanical forces during peristalsis, offering new avenues to achieving a durable anti-restenosis approach.
A variety of methods have been investigated to prevent stenosis after ESD, including physical dilation, glucocorticoid application, anti-inflammatory or anti-fibrotic drug use, and autologous tissue transplantation [42]. The preponderance of evidence suggests that corticosteroid therapy reduces the incidence of postoperative esophageal stenosis. Recently, advances in biomedical materials research have led to several new methods for preventing esophageal stenosis. For example, Coffin et al. [26] combined extracellular vesicles (EV) of stromal cells with a temperature-responsive hydrogel to prevent stenosis occurrence after endoscopic surgery in pig models. The experimental results confirmed that the rate of esophageal stenosis was markedly reduced, the average fibrosis area was reduced, and the regenerated mucosal muscle layer was larger in the EV+gel group. Chung et al. [25] designed a cell transplantation platform based on an ascidian-inspired hyaluronic acid hydrogel loaded with adipose-derived stem cells to alleviate esophageal stenosis. Excessive fibroblast differentiation and long-term inflammation are important factors in the development of esophageal stenosis; inhibiting fibroblast proliferation and accelerating early wound healing can effectively prevent its development [43]. Wang et al. [44] reported a thermosensitive hydrogel containing CS for triamcinolone delivery, which can be injected into esophageal wounds. Pan et al. [45] reported a double-crosslinked hydrogel containing SA, gelatin powder, aqueous transglutaminase, and Ca2+ to prevent esophageal stenosis after ESD. Effective delivery of dressings to the ESD wound site is also important. It is also crucial to update the structure of the endoscope to adapt to the treatment methods of injection or spraying drugs. Fujiyabu et al. [46] combined polytetrafluoroethylene tubes with five different structures of catheter tip nozzles manufactured using 3D printers to develop a powder applicator for endoscopic powder delivery, achieving lateral powder delivery at the pointed end of endoscopic catheters, thereby enabling effective and efficient delivery.
3.2 Gastric diseases
Gastric diseases, such as gastric ulcer, gastric perforation, and endoscopic submucosal dissection (ESD), are often accompanied by symptoms such as mucosal erosion and bleeding, which may be caused by excessive acid, infection, or drug use. Conventional treatment methods such as proton pump inhibitors and endoscopic clipping provide temporary relief but often fail to achieve long-term mucosal protection, resulting in recurrent bleeding and delayed healing. Hydrogel adhesives exhibit strong wet adhesion, drug delivery capacity, and in situ protective barrier capability, providing a promising alternative for gastric ulcer treatment. However, long-term stability and controlled degradation in the dynamic gastric environment remain critical challenges.
3.2.1 Gastric ulcer and bleeding
Gastrointestinal bleeding (GIB) is a medical emergency and the most common cause of hospitalization for digestive system diseases in the majority of countries [47]. GIB can be caused by inflammation, mechanical damage, vascular disease, tumors, and other factors within the digestive tract itself, as well as by lesions in adjacent organs and systemic diseases affecting the digestive tract [48]. Among these, peptic ulcers are the most common [49]. Although the incidence of upper GIB has been declining worldwide, it still has an unnegligible high incidence and mortality rate [50]. Endoscopy is the gold standard for GI hemostasis, and acute hemostasis can be achieved in most cases [51, 52]. However, in certain exceptional cases, such as diffuse bleeding, bleeding in areas difficult to reach by endoscopy, tumor bleeding, and recurrent bleeding, the therapeutic effect of endoscopic hemostasis is limited [53]. Therefore, it is very crucial to develop a safe, effective, and stable hemostatic agent.
CS is composed of a copolymer of N-acetylglucosamine and glucosamine linked by β(1-4) [54, 55], first published by Schorigin and colleagues in 1935 [56]. Currently, hydrogels based on CS are widely used in multiple biomedical fields, including tissue engineering, controlled drug delivery, and wound repair. [57, 58]. CS can also improve the biocompatibility of materials. Ni et al. [53] introduced CS into polyethyleneimine/polyacrylic acid (PEI/PAA) hydrogel and developed PEI/PAA/CS multi-functional hydrogel to reduce the risk of gastrorrhagia. The mixing of CS strengthened electrostatic interactions and hydrogen bonds in the original hydrogel, increased its crosslinking density, and enhanced its tissue adhesion. Meanwhile, due to the superior biocompatibility and hemostatic functions of CS, the capabilities of PEI/PAA/CS hydrogels have been improved. The powder of this hydrogel, which was produced by lyophilization and grinding, can absorb water from the surface of the tissue to quickly form a hydrogel. And it has strong tissue adhesion, which can adhere to the ulcer wound to avoid mucosal bleeding and promote tissue healing (Figure 2). In addition, incorporating CS into other hydrogels can also significantly enhance the material's water absorption capacity and accelerate blood coagulation, achieving effective hemostasis of gastric bleeding within 20 seconds [59].
Compared with CS, CMCS has excellent hydrophilicity, appropriate viscosity, and injection pressure, and can combine with the bacterial cytoderm and destroy its structure, inducing bacterial death [60]. Therefore, CMCS has been widely used as a hydrogel. CMCS/LAP/PVA hydrogel was formed via hydrogen bonds and electrostatic interactions between CMCS, lithium magnesium sodium salt (LAP), and poly(vinyl alcohol) (PVA), which has excellent hemostasis and antibacterial ability (Figure 3A) [60]. It exhibited superior resistance against S. aureus and E. coli (Figure 3B-C). Other hydrogels based on CS also show excellent performance; for example, Liu et al. [61] introduced a chitosan hydrogel powder (HP) that forms hydrogels via electrostatic interactions within 5 seconds of contact with liquid. Washing with sodium bicarbonate solution can selectively remove the hydrogel.
Schematic diagram showing the synthesis of PEI/PAA/CS hydrogel and the formation through hydrogen bonds and electrostatic interactions, as well as the adhesion mechanism of PEI/PAA/CS hydrogel in the gastric mucosa, which prevents bleeding and promotes wound repair. Adapted with permission from [53], copyright 2023, The Royal Society of Chemistry.

The antibacterial hydrogel based on CMCS for gastric ulcer and bleeding. (A) Schematic diagram displaying the synthesis of CMCS/LAP/PVA hydrogel cross-linking through electrostatic interactions and hydrogen bonds, and the hemostatic and antimicrobial properties of CMCS/LAP/PVA hydrogel. Colony growth of E. coli. (B) and S. aureus (C). Adapted with permission from [60], copyright 2023, American Chemical Society.

Hydrogels based on thiourea-catechol reactions are widely used because of their strong mechanical properties and extremely short gel time [29, 62-64]. In nature, mussels achieve strong underwater adhesion by secreting catechol-rich mussel adhesive protein [65]. By mimicking mussel adhesive protein, many catechol-related (CAT-related) hydrogels exhibit strong adhesion to the surfaces of most objects [66-68]. However, when CAT is exposed to oxidants (such as O2), it is inevitably oxidized to quinones, leading to loss of adhesion. As an excellent nucleophile, the thiourea group (NCSN) forms crosslinks with the conjugated catechol structure under acidic conditions to improve the preservation of the catechol structure, resulting in significantly enhanced adhesion [64]. Xu et al. introduced a biomimetic hydrogel based on thiourea-CAT coupling, which showed ultrafast gelation independent of pH value [62]. The hydrogel can be transported to the designated position via the endoscope, and a low-concentration oxidant can be sprayed onto the hydrogel precursor, which quickly forms a stable, viscous hydrogel in situ and remains in place for at least 48 hours. The introduction of the second crosslinked network can optimize the hemostatic application of this hydrogel. The research group introduced 4-arm thiolated polyethylene glycol (PEG) into HA-CAT-NCSN hydrogel to obtain a hybrid hydrogel (HACN-PEG hydrogel), which has stronger mechanical properties and shorter gelation time in relation to fibrin hydrogels or hydrogels with a single network (Figure 4A) [29]. In addition, Zhang et al. mixed catechol motif-modified methacrylated gelatin (GelMAc) and polyvinyl methacrylate (PVAMA) and mesoporous polydopamine (MPDA) nanoparticles to form GelMAc/PVAMA/MPDA@Cur hydrogel, in which MPDA is used as a drug carrier and loaded with curcumin (Cur) through π-π stacking and hydrogen bonds (Figure 4B). This hydrogel was gelatinized under ultraviolet light and demonstrated unique stability in gastric tissue under acidic and oxidative stress conditions (H2O2). Additionally, it can promote macrophage polarization, thereby regulating inflammation and facilitating tissue repair [69].
Catechol-related hydrogels for gastric ulcer and bleeding. (A) Schematic illustration showing the chemical structure, utilization process, the hemostasis and adhesion mechanisms of the HACN-PEG hydrogel. Adapted with permission from [29], copyright 2021, Wiley-VCH GmbH. (B) Schematic illustration showing the presentation of the GelMAc/PVAMA/MPDA@Cur hydrogel, which loads Cur into MPDA NPs through π-π stacking and hydrogen bonds for promoting gastric ulcer repair through regulating immune response. Adapted with permission from [69], copyright 2022, Elsevier.

Hydrogels based on some other materials also show excellent performance, for example, the hydrogels based on acryloyl showed superior injectability, self-healing ability, and stable adhesion behavior under stomach conditions, demonstrating their hemostasis function and promoting wound repair [70]. However, its gelation time can reach 9 minutes, and the initial in vitro pre-polymerization step of 3 minutes increases the complexity of clinical procedures, thereby limiting its transformation potential. The polyurethane/small intestinal submucosa (PU/SIS) hydrogel has distinctive pH-sensitive properties [71]. It can rapidly form a dense film in acidic conditions to protect GES-1 cells and accelerate ulcer healing. The hydrogel made of methacryloylhaluronic acid (MA-HA), 6-acrylamidohexanoic acid (AA), and ginsenoside Rg1 as raw materials is also pH-sensitive, which can shrink and tighten the wound in an acidic environment, while releasing Rg1 with anti-inflammatory effect [30]. Artzi et al. [12] designed a sprayable bioadhesive hydrogel, GastroShield, which consists of two low-viscosity precursors. Due to the reduced viscosity of the precursors, their delivery will be more convenient via a catheter inserted into an endoscope channel. The precursor solution rapidly reacts upon contact with mucosal tissue, producing cohesive tissue shields. In most cases, the treatment of gastric ulcers and bleeding is guided by endoscopy. The injectability of hydrogel provides significant convenience for endoscopic therapy, which is the main future direction for hydrogel materials in the GI tract. However, keratin-based hydrogels can be administered orally without endoscopic guidance to form a high-viscosity gel at the surface of gastric wounds, especially at ulcer sites [72]. This hydrogel can effectively improve the compliance in patients with mild gastric ulcers. Strong adhesion also plays a significant role in hemostasis. Ying et al. [73] reported an electroadhesive hydrogel for the treatment of recurrent GI bleeding. Under electrical stimulation, the hydrogel cationic polymer interacts with multiple proteins in deep mucosal tissue, increasing adhesion energy by 30 times. This technology allows the hydrogel to remain in the body for up to 30 days. Overall, the hemostatic performance of hydrogel adhesives used for gastric ulcers mainly depends on rapid interfacial water absorption, electrostatic bridging with mucosal proteins, and pH-triggered contraction that stabilizes adhesion under strong acidity. It is worth noting that the appropriate hemostatic material should be selected based on the bleeding pathology.
3.2.2 Gastric perforation
Gastric perforation is one of the most serious diseases of the digestive system, which refers to the complete damage to all four layers of the stomach wall and penetration into the abdominal cavity [74, 75]. After gastric perforation, the entry of gastric contents into the abdominal cavity can lead to gastric fluid leakage into the cavum abdominis, abdominal adhesions, bacterial peritonitis, and even death [3, 76]. Surgical suturing is a common method to treat gastric perforation, but it can cause secondary damage to the gastric wall [77]. Therefore, it is crucial to design an adhesive material that avoids suturing for repairing gastric perforation. Research has shown that the mortality rate of rabbits with gastric perforation treated with sutures and cyanoacrylate was 66%, while all rabbits treated with the light-controlled adhesive patch survived [78]. To secure normal gastric peristalsis and avert contraction at the anastomotic site, the optimal adhesive for treating gastric perforation should exhibit strong elasticity, adhesiveness, fatigue resistance, and flexibility [79].
Traditional hydrogel adhesives are typically in a gel state with a wet surface, and wet hydrogels are inconvenient for storage and transportation. If hydrogel adhesives are made into a powder, this problem can be resolved. A hybrid dry powder (HDP) strategy is reported that features rapid gelation of the powder upon contact with water and achieves tissue adhesion, enabling rapid sealing of wet tissue [80]. Peng et al. [81] reported a hydrogel powder consisting of PEI and PAA, which can rapidly gelatinize in situ by adsorbing interfacial water within 2 seconds via physical interactions between the polymers (Figure 5A). Owing to the slow healing of gastric perforation, secondary perforation occurs very easily [74], and the retention time of hydrogel adhesives at the site of gastric perforation is particularly important. The PEI/PAA hydrogel remained stable for 30 days after implantation in the stomach, with no noticeable degradation, which indicates that it can provide long-term protection for the perforated part of the stomach until the complete healing of the perforated part (Figure 5B-D). Another type of hemostatic powder, such as carboxymethyl chitosan/poly γ-glutamic acid/oxidized dextran (CPO) powder, can also be used to block gastric perforation (Figure 6) [82]. When gastric perforation occurs, CPO powder is sprayed onto the affected area. This powder can absorb interstitial fluid from the blood near the wound, thereby concentrating coagulation factors and platelets and achieving rapid hemostasis. Meanwhile, Schiff base and amide bonds were formed, and the hydrogel was gelatinized within 15 seconds. In addition, the abundant -NH2 groups in the tissue react with the -CHO group of ODE via a Schiff base reaction to form a strong bond, creating a physical seal that prevents bleeding.
Owing to the special anatomical position of the GI tract, the hydrogel used in the GI tract must be delivered to the wound site via endoscopy, so injectability is essential. Wang et al. [83] proposed an injectable hydrogel-related supramolecular assembly of ABA triblock copolymer for repairing gastric perforation in rats (Figure 7). The ABA triblock copolymer consists of an intermediate hydrophilic PEG block and a terminal temperature-reactive poly(N-isopropylacrylamide) (PNI-PAM) block, with pH-sensitive acryloyl-6-aminocaproic acid randomly incorporated [83]. The hydrogel dressing is liquid at 4 ℃ and forms a hydrogel at physiological temperature, allowing it to be readily injected into the required position.
Hydrogel powder for gastric perforation. (A) Schematic illustration of PEI/PAA powder and PEI/PAA hydrogel for gastric perforation. (B) Photos of a PEIFITC/PAA hydrogel on the serosa of the stomach on day 0 and (C) day 30 after applying PEIFITC/PAA powder. (D) t-test suggesting the hydrogel remains stable during the tested period. FITC: fluorescein isothiocyanate used in hydrogel staining. Adapted with permission from [81], copyright 2021, American Association for the Advancement of Science.

Schematic diagram of CPO hemostatic powder for the gastric perforation model in rats and its chemical structure. Adapted with permission from [82], copyright 2024, Elsevier.

Schematic diagram of the gastric environment-adaptive supramolecular hydrogel dressing for gastric perforation and chemical structure of the hydrogel and supramolecular hydrogel network structure. Adapted with permission from [83], copyright 2021, American Chemical Society.

Due to hydrophobic interactions and hydrogen bonds between the materials, this hydrogel has the ability to rapidly self-heal, allowing it to repeatedly repair damage to the hydrogel dressing caused by gastric peristalsis [83]. Besides, the supramolecular hydrogel dressing can prevent biological contamination, thereby reducing the incidence of infection after gastric perforation and accelerating its repair [83]. Compared with a single syringe, a double syringe can separate the polymer from the crosslinking agent and control in situ gelation of the hydrogel at the desired location. Chen et al. [84] proposed an injectable dual-network hydrogel formed in situ using a double syringe to separate the polymer and crosslinking agent. The hydrogel can take shape after extrusion from the micro mixer, which is connected to the double syringes. It has strong adhesion, toughness, adaptability, degradability, and biological activity, which can effectively seal GP and promote the regeneration of gastric mucosa. Other injectable hydrogels are also reported, such as a hydrogel based on cation-π interactions between protonated amines and aromatic rings [85], hydrogel adhesive AN@CD-PEG&TQ with therapeutic and biological imaging functions [86] and so on.
Patients with severe gastric perforations often require surgical suturing for repair, which can cause secondary tissue damage. Xing et al. [87] designed a sutureless hydrogel for gastric surgery, which is composed of gelatin methylate (GelMA) and tannic acid (TA). TA provides abundant hydrogen bonds in this hydrogel, resulting in excellent adhesion performance, ultimate stress, compression modulus, and elongation. Inspired by the shape of a mushroom cap-stick, Liu et al. [88] designed a medium-CBA cross-linked hydrogel (MCH) with silica nanoparticles (SNPs) coating, which remained at the defect for 4 weeks and enhanced mucosal and vascular generation via loading acidic fibroblast growth factor (AFGF). Moreover, a built-in wireless pH capsule maintained intragastric pH above 4 for >83% of the time, minimizing rebleeding risk (Figure 8A). Similarly, inspired by cardiac occluders, Linghu et al. prepared a double umbrella hydrogel occluder (EHO) composed of caffeic acid (CA) grafted CS and polyacrylamide, which can be used for endoscopic delivery and effectively seal gastric perforation through hydrogen bonding and chelation between tissue and polymer, and expansion of hydrogel (Figure 8B) [89]. Under the action of gastric acid, the catechol groups in CS and caffeic acid undergo nucleophilic reactions, endowing EHO with the ability to resist gastric acid and enzyme invasion, thereby promoting gastric perforation healing. At the same time, the EHO group had higher M2 macrophages and lower M1 macrophages at the site of gastric perforation, and the expression of bFGFp and pro-angiogenic VEGF was enhanced. This material has excellent anti-inflammatory effects.
Hyperboloid hydrogel occluders for gastric perforation. (A) Schematic diagram of a compressible hyperboloid hydrogel inspired by a compressible mushroom-cap-inspired device for the treatment of gastric perforation. Adapted with permission from [88], copyright 2022, American Chemical Society. (B) Schematic diagram of the preparation of EHO and its application in endoscopic repair of gastric perforation. Adapted with permission from [89], copyright 2025, American Chemical Society.

The introduction of hydrophobic substances into hydrogel adhesives can effectively attenuate the hydration barrier between hydrogels and hydrated tissue, thereby ensuring a relatively tight connection between them [33]. Taguchi et al. [90] increased the hydrophobicity of the hydrogel by modifying the long alkyl chain and developed a hydrogel adhesion consisting of dodecyl-modified Alaska pollock gelatin (ApGltn) and 4S-PEG. The amino residue of ApGltn is modified by a dodecyl group to form a hydrophobic group, which enhances its sealing effect on wet tissues [90, 91]. Animal experiments have shown that a C12-ApGltn-based hydrogel injected into the subcutaneous tissue of rats degrades within 28 days without eliciting an inflammatory response [90]. Consequently, C12-ApGltn-based sealant has excellent potential for application in various surgeries. Due to its underwater adhesion stability, the research group also used hydrophobically-modified ApGltn to treat GI tract wounds after ESD (Figure 9A) [91]. Contact angle measurements indicate that the surface hydrophobicity of particles modified with long alkyl chains is significantly increased, with C10-MPs having the highest hydrophobicity. Based on hydrophobically modified ApGltn, the research team made spray-hydrophobized microparticles (hMPs) as a wound dressing via coagulation, freeze-drying, and thermal crosslinking, which exhibit high tissue adhesion under wet conditions (Figure 9B) [32]. The sprayable tissue adhesive hMPs can close perforations after ESD or prevent delayed perforation after ESD. However, hydrophobic designs alone do not yield optimal wet adhesion because, even with hydrophobic pairs, contact remains incomplete due to small puddles formed by rough nanoscale channels [92]. Therefore, rapid dehydration and subsequent removal of residual water are critical to achieve strong noncovalent adhesion to the wet surface. A half-dry adhesive was reported that used PAA to form a covalent cross-linking network and silk fibroin (SF) to create a secondary semi-interpenetrating network [33]. When encountering wet tissue, the adhesion can quickly repel liquids and then adsorb residues under external pressure, thereby eliminating the influence of liquids on adhesion. Due to the excellent adhesion to wet tissue, hydrophobically modified hydrogels can achieve local sustained release of anti-cancer drugs when loaded with them, thereby removing residual cancer tissue after endoscopic surgery [93].
The above conventional double-sided biological adhesives have a significant clinical limitation: their uncontrolled adhesion can lead to postoperative adhesions, severely limiting their clinical application in wound repair, especially in abdominal surgery. The formation of postoperative adhesions will lead to serious consequences, including chronic pelvic inflammation, intestinal obstruction, and infertility, typically requiring readmission or surgery [94, 95]. Accordingly, designing and synthesizing a single-sided adhesive is particularly important. The Janus hydrogel patch is a single-sided adhesive hydrogel that can prevent postoperative adhesion. One side has strong adhesion, which can be used to paste the injured tissue to promote healing, and the other side has no adhesion to prevent postoperative adhesions [77, 96]. The carboxyl groups of polymeric N-acryloyl aspartic acid (PAASP) not only form hydrogen bonds with polar groups at the tissue surface, demonstrating strong adhesion to injured tissues, but also bind with metal ions to form non-adhesive interfaces, avoiding peritoneal adhesion during tissue repair in vivo (Figure 10) [77]. The authors achieved the function through paper-based Fe3+ transfer printing technology. The experiment showed that the Janus hydrogel patch can simultaneously achieve tissue repair and prevent postoperative adhesion. Moreover, the negatively charged carboxyl groups in the hydrogel can be electrostatically bound to the cationic oligosaccharide via unilateral impregnation to form a new Janus hydrogel, whose two sides show significantly different adhesiveness [96]. However, such hydrogels are challenging to use in minimally invasive surgeries because they cannot be administered through injection. In contrast, Wu et al. proposed a photocurable injectable hydrogel (HAD) that grafts catechol and methacrylate onto hyaluronic acid, using LAP as a photo-initiator. The HAD precursor solution contains plentiful catechol groups, which can form hydrogen bonds with protein residues of the injured tissue, thereby allowing the precursor solution to adhere to the tissue surface [97]. Under UV curing, the external surface of HAD hydrogel shows antiadhesion due to the formation of 2-aminoethyl methacrylate hydrochloride (AEMA) network that restricts the free circulation of catechol groups, so as to prohibit the interaction between the catechol groups on the HAD hydrogel and the amino groups on the tissue. In addition, the precursor solution of HAD hydrogel exhibits good shear-thinning properties, enabling endoscopic injection and adaptation to complex, irregular tissue surfaces. However, due to the low viscosity of HAD precursors, they cannot remain stably on the wound surface. Therefore, the group added hyaluronic acid grafting phenylboronic acid (PBA) groups to the HAD hydrogel, which ensures the adherence of the hydrogel to irregular or inclined surfaces upon delivery while preserving its injectability (Figure 11) [31]. There are other types of Janus hydrogels, e.g., Liang et al. [98] designed an asymmetric adhesion hydrogel induced by microparticle deposition composed of acrylic acid, chitosan, tannin, and Al3+, whose asymmetric adhesion is effectuated by the deposition of calcium alginate microspheres on the bottom of the hydrogel. Chen et al. [99] constructed a double-sided tissue-adhesive hydrogel, which integrates onto the bottom surface of the acellular dermal matrix. Li et al. [100] developed an adhesion-triggered hydrogel system consisting of polyacrylamide-alginate (PAM/SA) hydrogel and tannic acid (TA). TA contains rich polyphenol structures and acts as an adhesion-trigger molecule, which can form plentiful hydrogen bonds at the tissue interface, achieving rapid and strong adhesion to the surface of wound tissue. Besides, Liu et al. prepared the Tempo FPF hydrogel based on dynamic iminoborate and borate bonds, which can not only seal gastric perforation, but also prevent organ adhesion [101].
When gastric acid is high, the hydrogel swells readily, leading to a mismatched adhesion between the hydrogel adhesives and the gastric mucosa, further impacting the adhesive effect [102]. To solve the issue, Yuan et al. [103] proposed a double-network hydrogel strategy based on ionic nanoreservoirs (INR) that can persist prolonged adhesion. The first polyacrylamide network cross-links on gastric tissue within 5 seconds after exposure to blue light and improves adhesive properties through mechanical interlocking [103]. As an INR, nanohydroxyapatite can gradually release Ca2+ in an acidic environment and form a second network with SA to restrict the expansion of hydrogels in gastric juice [103]. In addition, an acid-resistant hydrogel (ATGel) adhesive was developed, achieving immediate, effective sealing of a gastric perforation within 2 seconds [13]. Compared with hydrogel adhesives used for hemostasis of gastric ulcers, the hydrogel used for perforation repair relies more on mechanical strengthening mechanisms. For instance, postoperative tissue adhesion is prevented through strategies such as dual-network structures, hydrophobic interlocking, and asymmetric adhesion.
Hydrogel with hydrophobic properties. (A) Structure and preparation process of ApGltn hydrogel. Adapted with permission from [91], copyright 2019, Elsevier. (B) A coacervation method for the formation of hm-ApGltn modified with various alkyl chains, and the preparation of hMPs by freeze drying and thermal crosslinking. (I) Cancer growing onto the mucosa and submucosa. (II) Perforation due to inflammation in post-ESD. (III) hMPs are sprayed on the wound tissue. (IV) hMPs swell and form the hydrogel to close the perforation. (V) hMP hydrogels degradation and wound healing. Adapted with permission from [32], copyright 2021, Elsevier.

Schematic diagram of PAASP hydrogel adhesion to tissue via hydrogen bond crosslinked network and Janus hydrogel patch prepared by paper-based Fe3+ transfer printing for gastric perforation repair [77].

Schematic diagram showing the disadvantages of surgical sutures and fibrin as materials for closing gastric perforations and the realization of asymmetric adhesion of HADP hydrogel via the dynamic reversible boronate ester bonds and irreversible crosslinking after UV light. Adapted with permission from [31], copyright 2024, Wiley-VCH GmbH.

3.2.3 Endoscopic submucosal dissection (ESD)
Endoscopic resection includes endoscopic mucosal resection (EMR), ESD, endoscopic submucosal tunnel resection, and trap-assisted endoscopic resection [104, 105]. With the evolution of endoscopic technology, an increasing number of GI diseases can be treated through endoscopy, including early gastric cancer, gastric polyps, and intragastric masses [106-109]. Nevertheless, endoscopic surgery also has some disadvantages, such as incomplete resection, perforation, and the risk of bleeding [110, 111]. The key method to reduce the risk of endoscopic resection is to inject a solution under the mucosa to form a submucosal fluid cushion [112]. Submucosal fluid cushions can elevate lesions, creating sufficient operating space between the mucosa and intrinsic muscle layer, thereby reducing iatrogenic risks [113]. Physiological saline is one of the most ordinary fluids used as a submucosal injection, but it is easily absorbed by surrounding tissues, resulting in a submucosal fluid cushion that can only last for a short period of time [114, 115]. The best submucosal injection material should have injectable properties and form a thick, durable submucosal cushion after injection, supporting the diseased mucosa and adhering to the wound to accelerate wound healing after the diseased mucosa is removed. Hydrogels are suitable as submucosal injection materials because of their excellent biocompatibility, appropriate hardness, and low diffusivity. To achieve low viscosity and injectability during injection, while forming a solid gel after injection, some hydrogels with special properties are designed, including temperature-sensitive hydrogels, photo-crosslinked hydrogels, hydrogels with a 2-step injection system, and shear-thinning hydrogels.
By mixing sodium glycerophosphate (GP) with the CS solution, a CS/GP hydrogel was developed [116]. It is a viscous liquid at lower temperatures, such as room temperature or below, and can be converted into solid hydrogels at physiological temperatures near 37 ºC, which means that it has clear injectability at an appropriate temperature. The outcomes of animal experiments indicated that the CS/GP system was an effective submucosal injection, and the required amount in ESD surgery was much less than that of physiological saline [117]. Shan et al. [118] introduced collagen into the CS/GP hydrogel to construct the chitosan/β-glyceryl phosphate/collagen (CS/GP/Col) system, which rapidly gelled at 37 ºC and kept flowing for a long time at low temperature, and could effectively stop bleeding. Hydroxypropyl cellulose (HPC) has excellent biological adhesion properties [119]. Tang et al. added HPC to the CS/GP/Col hydrogel to strengthen the adhesion and biocompatibility of the hydrogel, which made the hydrogel system more suitable for ESD [120]. Nevertheless, the application of CS/GP/Col hydrogel for submucosal injection is challenging because of its reduced fluidity at low temperatures [121]. To address this disadvantage, Ni et al. [121] utilized lactic acid (LA) to modify chitosan, thereby enhancing the fluidity of CS/GP/Col hydrogel at low temperatures and creating a new hydrogel system. Liu et al. [122] made the hydrogel into a lyophilized powder. Compared with the hydrogel, the powder has better low-temperature fluidity and significantly increased injectability [122, 123]. The concentration of the chitosan solution affects the injectability of the hydrogel precursor solution and gelation time, making it difficult to achieve a balance. Liu et al. [124] prepared a chitosan solution with a high pH value (HpHCS; pH 6.19), which can maintain rapid gelation at a low concentration and improve injectability. However, as the concentration of HpHCS decreased, the precursor solution of the hydrogel became more and more turbid, and the hydrogel became more and more brittle. Subsequently, the team added polyvinylpyrrolidone (PVP) to the system to form a strong hydrogen bond with CS, making the hydrogel more elastic [125]. Experiments showed that HpHCS-5%-PVP-GP hydrogel effectively improved the height of the submucous fluid pad [125]. In addition, other types of temperature-sensitive hydrogels are also used for ESD, such as a FS hydrogel based on a mixture of polyethylene-polypropylene glycol (F-127) and SA [126] and a hydrogen-bond-driven conductive thermosensitive hydrogel based on polystyrene sulfonate and F-127 [127].
In addition to thermo-sensitive hydrogels, photocrosslinked hydrogels can also be used as submucosal liquid cushions for ESD. Chitosan hydrogel containing azide and lactose (Az-CH-LA) can be coagulated by ultraviolet irradiation, injected into the submucosa before ESD, and then irradiated with ultraviolet light, producing significant mucosal bulges and significantly reducing bleeding after mucosal resection [128]. Compared with hypertonic saline, photocrosslinked chitosan hydrogel (PTH) can make more durable submucosal bulges with clearer edges, which are more conducive to the accurate development of ESD. According to histological analysis, PCH can undergo complete biodegradation within 8 weeks without adverse effects on biological systems [129].
The two-step injection method is also effective for obtaining a high-mucosal protrusion liquid pad. In a two-step injection system, two low-viscosity solutions are injected separately into the submucosa, where they crosslink to produce a high-viscosity submucosal pad [130]. Fan et al. reported a naturally derived gelatin-oxidized alginate saline gel (G-OALG), which used an endoscopic double-needle/two-step injection needle to inject gelatin and alginate oxide into the esophageal submucosa, and via the formation of Schiff base, a good submucosal liquid pad was obtained [113].
Temperature-sensitive hydrogels can clog endoscopic needles, and photocrosslinkable hydrogel curing requires an ultraviolet light source, which is challenging to use within an endoscope. Furthermore, the two-step injection method is complicated, making it an unnecessary burden. Therefore, shear-thinning hydrogels began to be used as submucosal fluid cushions. When shear force is applied to the shear-thinning hydrogel, it can rapidly transform into a low-viscosity sol, reducing the injection pressure. After injection, the shear-thinning hydrogel can reform a cross-linking network, and completely restore the solid hydrogel morphology [131]. Gellan glue hydrogel (GGH) formed through double-spiral aggregation at a cross-linking point exhibits shear-thinning hydrogel properties [131]. Its injection pressure is equivalent to the injection pressure of a small molecule solution when injected through the endoscopic injection needle. Ma et al. [132] prepared an injectable shear-thinning hydrogel for ESD, composed of thiolated sodium alginate (SA-GSH) and OSA (Figure 12A). The shear-thinning property enables this hydrogel to be injected at low pressure. When this hydrogel is injected into the submucosa, the cross-linked network composed of disulfide bonds and thioacetals again forms, and the submucosa elevation generated by the hydrogel is 13% -18% higher than that of commercial ESD injection solution [132]. Ren et al. [133] used a range of aromatic dipeptides, such as 9-fluorenylmethoxycarbonyl (Fmoc)-conjugated phenylalanine-leucine (FL), tyrosine-leucine (YL), leucine-leucine (LL), and tyrosine-alanine (YA), to prepare self-healing and shear-thinning hydrogels, explored their self-assembly behaviors and regulated the mechanical strength (Figure 12B). Compared to commercial ESD fillers such as physiological saline and commercial polymer PF-127, Fmoc YL hydrogel is more suitable for ESD as a filler [133]. In addition, hydrogel composed of the anionic sodium carboxymethyl starch (CMS) and cationic LAP prepared by Wang et al. [134], the CS hydrogel with Ag nanoparticles [135], synergistic drug-loaded shear-thinning hydrogel [136], thermogel generated by the “block blend” strategy [137], alginate/Laponite hydrogel [138], the polyglycerol stearate hydrogel (PGSH) [139] and the hydrogel based on maleimide-based OSA and sulfhydryl carboxymethyl-chitosan [140], all can effectuate persistent improvement of the mucosal cushion by endoscopic injection. Additionally, Liu et al. [141] prepared succinylated hydroxybutyl chitosan (HBC) hydrogel with excellent temperature sensitivity, in situ gelation at 37 °C, and can also be injected through the endoscope injection needle after hydrogel formation, effectively avoiding the risk of clogging the needle tube. Therefore, unlike hydrogel adhesives used for gastric ulcer and perforation therapy, submucosal injection hydrogels primarily form stable mucosal pads via mechanisms such as shear-thinning behavior, temperature- or light-triggered sol-gel transitions, and controllable osmotic swelling. These hydrogels usually exhibit excellent injectability and shape retention rather than strong interfacial adhesion.
In conclusion, for gastric bleeding, rapid gelation and acid-resistant hydrogels can quickly adhere to bleeding sites and provide strong interfacial stability. For gastric perforation repair, hydrogel adhesives are needed to provide sufficient mechanical strength to withstand gastric peristalsis and maintain long-lasting sealing. In ESD surgery, hydrogels are typically used as submucosal cushions, requiring excellent injectability and shape retention. Postoperative esophageal stenosis is often accompanied by inflammation. Therefore, hydrogel adhesives loaded with cells or exosomes can be used to regulate inflammatory responses and inhibit fibrosis. Notably, due to individual differences among patients and the dynamic fluctuations in the microenvironment, real-time monitoring and timely adjustment of hydrogel adhesive selection are often required in clinical treatment.
3.3 Pancreatic cancer
Pancreatic cancer is the sixth most common cause of cancer-related deaths worldwide. It is estimated that 510,566 patients were newly diagnosed with pancreatic cancer and 467,005 patients died of pancreatic carcinoma in 2022 worldwide [34]. Because of the absence of typical clinical symptoms in patients with early pancreatic cancer, the majority of patients were already in the advanced stage when diagnosed, and the five-year relative survival rate was only 10% [142]. Currently, radiotherapy is considered the standard therapy for patients with pancreatic cancer, particularly those with localized advanced cancer who have responded to chemotherapy for the first six months, are in a stable condition, have experienced unacceptable chemotherapy-related toxicity or have deteriorated due to chemotherapy toxicity [143]. Recent research has shown that increasing the dose of radiotherapy can improve overall survival in patients [144]. However, the main problem with an increased radiation dose is the toxicity to contiguous organs at risk (OARs), especially the duodenum, which is close to the pancreas [145]. Ding et al. [146, 147] used an absorbable radiopaque hydrogel spacer for spacing the OARs from the head of the pancreas through endoscopic ultrasound guidance in a cadaveric model. Subsequently, the group demonstrated the feasibility and safety of injecting the hydrogel spacer in the porcine model, human trial and multi-site feasibility cohort study [147-149] and proposed a dose prediction model to identify patients who require placement of a hydrogel spacer before radiotherapy to meet predefined dose constraints [150]. The team developed a duodenal spacer simulator platform and systematically studied the dosimetric effects of spacer positions [145].
4. Challenges and perspectives
Although hydrogel adhesives show great promise for GI wound healing, several challenges remain, including durability in acidic environments, mechanical robustness under peristalsis, and long-term safety. Additionally, delivery issues such as blocking of injection needles or spray nozzles during application still pose practical difficulties. The integration of hydrogel materials with nanomaterials and controlled drug-release systems offers promising opportunities to address the above issues. Overcoming these obstacles will be crucial for the clinical translation of hydrogel adhesives in digestive tract wound healing.
Shear-thinning hydrogels for ESD. (A) Schematic diagram displaying the synthesis mechanism and application of the injectable sodium alginate hydrogels (ISAHs). Adapted with permission from [132], copyright 2022, Elsevier. (B) Schematic illustration displaying Fmoc-dipeptide self-assembly hydrogels with shear-thinning and self-healing properties. Adapted with permission from [133], copyright 2020, American Chemical Society.

Hydrogel adhesives for gastric ulcers and gastric hemorrhages
| Types of Hydrogels | Materials | Crosslinking Methods | Advantages | Refs. |
|---|---|---|---|---|
| CS-NAC/SA/TCP | CS-NAC SA TCP | Electrostatic interaction Hydrogen bonds Ionic crosslink | pH responsiveness Anti-inflammatory | [19] |
| PEI/PAA/CS | PEI PAA CS | Electrostatic interaction Hydrogen bonds | Ultrafast gelation (<3 s) Excellent tissue adhesion Gastric fluid resistance Hemostasis | [53] |
| CMCS/Lap/PVA | CMCS PVA Lap | Electrostatic interaction Hydrogen bonds | Injectability Antibacterial properties Hemostasis | [60] |
| HP powders | HTCC PA | Electrostatic interaction | Fast hemostatic capability Strong acid resistance | [61] |
| HA-CAT-NCSN | HA-Cat HA-NCSN | Thiourea-catechol coupling | Ultrafast gelation In situ gelation | [62] |
| HACN-PEG | HA-Cat HA-NCSN PEG | Thiourea-catechol coupling Disulfide bonds | Ultrafast gelation In situ gelation Hemostasis | [29] |
| GelMAc/PVAMA/MPDA@Cur | GelMAc PVAMA MPDA NPs Cur | π-π stacking Hydrogen bonds | Anti-inflammatory Stable under acidic/oxidative stress Promotes macrophage polarization and tissue repair | [69] |
| ISFIHs | AA AA-NHS | Covalent crosslink | Injectability pH-responsiveness | [70] |
| PU/SIS | PU SIS | Ionic bonds | pH-responsiveness | [71] |
| Oral keratin hydrogel | Keratins | Disulfide bonds | No endoscopic guidance | [72] |
| e-GLUE | SA PAM Cations | Electrostatic interaction | Strong electroadhesive | [73] |
| GastroShield | oxidized dextran PluPEI | Schiff base bonds | Sprayable Ultrafast gelation Strong tissue adhesion | [12] |
| MA-HA/AA10/Rg1 | MA-HA AA ginsenoside Rg1 | Photocrosslinking | Anti-inflammatory Hemostasis pH responsiveness | [30] |
CS-NAC: N-acetylcysteine grafted chitosan; TCP: tilapia collagen peptide; PEI: polyethyleneimine; PAA: polyacrylic acid; CS: chitosan; CMCS: carboxymethyl chitosan; PVA: poly(vinyl alcohol); Lap: lithium magnesium sodium salt; HA-Cat: hyaluronic acid- catechol; HA-NCSN: hyaluronic acid-thiourea; PEG: polyethylene glycol; GelMAc: catechol motif-modified methacrylated gelatin; PVAMA: methacrylated poly (vinyl alcohol); MPDA NPs: mesoporous polydopamine nanoparticles; Cur: curcumin; AA: acryloyl-6-aminocaproic acid; AA-NHS: AA-g-N-hydroxysuccinimid; PU: Polyurethane; BIS: N,N'-methylenebisacrylamide; SIS: small intestinal submucosa; PAM: poly-acrylamide; PluPEI: amine-modified block-copolymer; MA-HA: methacryloylhaluronic acid.
Hydrogel adhesives for gastric perforation
| Types of Hydrogels | Materials | Crosslinking Methods | Advantages | Refs. |
|---|---|---|---|---|
| PEI/PAA powder | PEI PAA | Electrostatic interaction; Hydrogen bonds | Ultrafast self-gelling (< 2 s) In situ gelation Absorb interfacial water | [81] |
| CPO powder | CMCS γ-PGA OD | Amide bonds; Schiff base bonds | Fast self-gelling In situ gelation Absorb interfacial water Hemostasis Antibacterial properties | [82] |
| ABA triblock copolymer | PEG PNI-PAM A6ACA | Hydrophobic interactions; Hydrogen bonds | Rapid self-healing Injectability | [83] |
| DGL | GelMA Laponite nanoclay DNA | Electrostatic interaction; Covalent bonds | Dynamic reversibility Low swelling Wet adhesive properties Injectability Hemostasis | [21] |
| PTH | THMA gelatin collagenase type II APS | TG crosslinking of gelatin; Covalent bonds | In situ gelation Injectability | [84] |
| PTOPT | N-(2-aminoethyl)-N-[3-oxo-3-(phenethylamino)propyl]acrylamide | Cation-π interactions; π-π stacking | Injectability Self-healing Antibacterial properties | [85] |
| AN@CD-PEG&TQ | PEG-SC AN@CD nanoprobe anti-microbial peptide Tet213 pro-angiogenic peptide QK | Host/guest complexation; Amide bonds | Injectability Antibacterial properties Facilitating angiogenesis | [86] |
| GelMA-TA | GelMA TA | Hydrogen bonds | Anti-tensile healing Gastric fluid resistance | [87] |
| MCH | DMA Ca2+ SA VF AFGF | Covalent bonds; Ionic crosslink | Effective closure Easy operation Noninvasive delivery | [88] |
| EHO | CA-CS PAM | Photocrosslinking | Customizable Endoscopy delivery | [89] |
| hm-ApGltn MPs | Hydrophobically-modified ApGltn | Hydrophobic interactions | Anti-inflammatory Anti-fibrotic | [91] |
| hMPs | Hydrophobically-modified ApGltn | Hydrophobic interactions | Strong burst pressure Stable adhesion Sprayable | [32] |
| PSA | SF PAA | Hydrogen bonds; Electrostatic interactions; Chain entanglement derived from SF | Reliable adhesion via silk fibroin chain entanglement Strong wet adhesion by rapid dehydration effect | [33] |
| Janus hydrogel | PAASP Fe3+ | Hydrogen bonds | Asymmetric adhesion Excellent stretchability Fatigue resistance Strong adhesion to bio-tissues Hemostasis Anti-postoperative tissue adhesion | [77] |
| Janus hydrogel | PAGG COS | Hydrogen bonds; Electrostatic interactions | Asymmetric adhesion Tissue adhesion Replacement of surgical suture Anti-postoperative tissue adhesion | [96] |
| Janus hydrogel | Catechol-grafted HA | Photocrosslinking | Asymmetric adhesion Anti-postoperative tissue adhesion Injectability | [97] |
| HADP | HA DA PBA | Boronate ester bonds; Photocrosslinking | Asymmetric adhesion Sprayable Anti-postoperative tissue adhesion | [31] |
| Acrylic acid/chitosan/tannic acid/Al3+ | AA CS TA Al3+ SA Ca2+ | Hydrogen bonds | Asymmetric adhesion High adhesive strength Anti-postoperative tissue adhesion | [98] |
| DQA | PDA QCS Acrylic acid ADM | Electrostatic interactions; Hydrogen bonds | Asymmetric adhesion Anti-postoperative tissue adhesion Antibacterial properties High burst pressure Replacement of surgical suture | [99] |
| PAM/TA | PAM SA TA | Hydrogen bonds | Asymmetric adhesion Anti-inflammatory | [100] |
| PAM-SA-NHA | PAM SA NHA | Covalent crosslink; Ionic crosslink | Low swelling properties Excellent mechanical and adhesion properties | [103] |
| ATGel | Poly(HEMA-NVP) Poly(AA-NHSAE) | Covalent crosslink | Gastric fluid resistance Instant and tough adhesion to gastric tissues Long-term adhesion | [13] |
PEI: polyethyleneimine; PAA: polyacrylic acid; CMCS: carboxymethyl chitosan; γ-PGA: poly-γ-glutamic acid; OD: oxidized dextran; PEG: polyethylene glycol; PNI-PAM: poly(N-isopropylacrylamide); A6ACA : acryloyl-6-aminocaproic acid; GelMA: methacrylate gelatin; DNA: deoxyribonucleic acid; THMA: N-[Tris(hydroxymethyl)methyl]acrylamide; APS: ammonium persulfate; TA: tannic acid; EHO: endoscopy-deliverable hydrogel occlude; CA-CS: caffeic acid-grafted chitosan; DMA: dimethylacrylamide; CBA: N,N′-cystaminebis(acrylamide); SA: sodium alginate; VF: vonoprazan fumarate; AFGF: acidic fibroblast growth factor; ApGltn: Alaska pollock gelatin; SF: silk fibroin; PAASP: Polymeric N-acryloyl aspartic acid; PAGG: poly(N-acryloyl 2-glycine); COS: chitooligosaccharide; HA: hyaluronic acid; DA: dopamine; AA: acrylic acid; CS: chitosan; PDA: polydopamine; QCS: quaternary ammonium chitosan; PBA: phenylboronic acid; ADM: acellular dermal matrix; PAM: poly-acrylamide; NHA: nano-hydroxyapatite; HEMA-NVP: 2-hydroxyethyl methacrylate-co-N-vinylpyrrolidone; AA-NHSAE: acrylic acid-co-N-hydroxysuccinimide acrylate ester.
For hydrogel adhesives used for gastric bleeding and ulcers, the ingredients that play a therapeutic role are relatively simple and cannot address serious complications. Hydrogels used in submucosal injection materials usually have good physical properties that can provide an ideal height of mucosal bulge, but their therapeutic functions have been largely overlooked. Key issues, such as accelerating postoperative wound healing and preventing postoperative infections, have not been addressed. The introduction of nanomaterials, small-molecule drugs, peptides, and proteins to optimize the therapeutic function of the hydrogel adhesive is one of the most direct means; they have a significant therapeutic effect on gastric wound healing and its complications. Therefore, a reasonable combination of the above therapeutic ingredients and hydrogels to achieve controlled drug release and functional synergy between drugs and hydrogels is a significant future development direction for hydrogel adhesives. Lin et al. [151] uniformly coat covalent organic frameworks (COFs) on oxidized carbon nanotubes (OCNTs) and coordinate with Fe3+ to obtain nanocomposites O@CF. O@CF can participate in a Schiff base reaction via its surface residues to crosslink with oxidized dextran and with chitosan grafted with caffeic acid, yielding a hybrid hydrogel (O@CF@G). The hybrid hydrogel can improve the mechanical properties of the original hydrogel and exhibit multifunctionality through O@CF, including peroxidase (POD) and catalase (CAT) activities and photothermal properties. Therefore, it can effectively eliminate drug-resistant biofilms, kill bacteria, alleviate hypoxia in diabetic wounds, enhance transmission of intercellular electrical signals, and ultimately accelerate wound healing. In addition, many other materials, such as metal-organic frameworks (MOFs) [152-154], metal nanozymes [155, 156], and probiotics [157] are used to mix with hydrogels to form composite materials that play a role in antibacterial and angiogenesis, or used for diabetic skin wounds. These materials have excellent performance and significant effects; however, they have not yet been applied to esophageal or gastric wounds. By selecting different types of nanomaterials and appropriate hydrogels, hybrid hydrogels with enhanced functionality can be obtained, achieving specific properties far beyond those of traditional hydrogels.
Submucosal injection hydrogels for tissue lifting and indirect wound healing support
| Types of Hydrogels | Materials | Crosslinking Methods | Advantages | Treatment | Refs. |
|---|---|---|---|---|---|
| CS/GP/Col | CS GP Col | Hydrogen bonds | Temperature sensitivity Hemostasis | Hemostasis | [118] |
| CS/GP/HPC/Col | CS GP Col HPC | Hydrogen bonds | Temperature sensitivity Stable adhesion | Hemostasis | [120] |
| CSLA/CS/GP | CSLA CS GP | Hydrogen bonds | Temperature sensitivity Injectability | Promoting wound healing | [121] |
| HpHCS-PVP-GP | HpHCS PVP GP | Hydrogen bonds | Temperature sensitivity Injectability High mechanical strength | Non | [125] |
| FS | SA F-127 | Hydrogen bonds | Temperature sensitivity Injectability | Antibacterial Promoting wound healing | [126] |
| Az-CH-LA | Az-CH-LA | Photocrosslinking | Crosslinking through ultraviolet irradiation Hemostasis | Non | [128] |
| PSS/F127 | Polystyrene sulfonate F-127 | Hydrogen bonds | Conductive thermosensitive | Reducing tissue burns and bleeding | [127] |
| G-OALG | Gelatin OSA | Schiff base bonds | Controllable gelation viscosity and degradation rate Low cost | Non | [113] |
| GGH | GGH | Double-helix aggregation | Easy endoscopic injectability Good hemostatic property | Hemostasis | [131] |
| ISAHs | OSA SA-GSH | Hemi-thioacetal bonds | Gel stability Self-healing properties Injectability | Non | [132] |
| Dipeptide self-assembled hydrogels | FL YL LL YA | π-π stacking Hydrophobic effect | Self-healing properties Injectability | Non | [133] |
| CMS/Lap | CMS Lap | Electrostatic interaction | Self-healing properties Injectability | Hemostasis | [134] |
| CCS@Ag | Catechol Chitosan Ag | Schiff base bonds | Self-healing properties Injectability Antibacterial properties | Antibacterial | [135] |
| HBC-SA | HBC SA | Hydrogen bonds Hydrophobic interaction | Temperature sensitivity Injectability | Hemostasis | [141] |
| βCP-TET-ISO | β-CD PEGMA | Radical polymerization | Self-healing properties Injectability | Hemostasis Antibacterial Drug delivery | [136] |
| PLGA-b-PEG-b-PLGA | PLGA PEG | Block blend | Temperature sensitivity Injectability | Non | [137] |
| EISH | SA Laponite | Electrostatic interaction | Self-healing properties Injectability | Non | [138] |
| PGSH | PGS | Self-assembly | Self-healing properties Injectability Hemostasis | Hemostasis | [139] |
| Two-component in-situ hydrogel | CMCS SA | Schiff base bonds Thioether bonds | Injectability Hemostasis | Hemostasis Promoting wound healing | [140] |
CS: chitosan; GP: β-glycerophosphate; Col: collagen; HPC: hydroxypropyl cellulose; CSLA: lactobionic acid-modified chitosan; HpHCS: chitosan solution with high pH value; PVP: polyvinylpyrrolidone; SA: sodium alginate; F-127: polyethylene-polypropylene glycol; GGH: gellan gum hydrogel; OSA: oxidized sodium alginate; SA-GSH: glutathione reduced-modified sodium alginate; FL: 9-fluorenylmethoxycarbonyl (Fmoc)-conjugated phenylalanine-leucine; YL: tyrosine-leucine; LL: leucine-leucine; YA: tyrosine-alanine; CMS: carboxymethyl starch; Lap: Laponite; HBC: hydroxybutyl chitosan.
Key performance parameters of hydrogel adhesives for different upper gastrointestinal wound healing models
| Applications | Design and synthesis | Key performance parameters | Refs. | ||||
|---|---|---|---|---|---|---|---|
| Gelation time | Adhesion strength | Acid resistance time | Hemostasis time | Others | |||
| Gastric bleeding and ulcer | CS-NAC/SA/TCP | In situ gelation | > 2 h | Degradation rate: 39.51 ± 2.01% (pH 1.2, 3 days) | [19] | ||
| PEI/PAA/CS | < 3 s | < 15.7 kPa (porcine stomach tissue after 1 h in acidic buffer) | 28 days | 33 s (rat liver model), 86 s (rabbit gastric artery model), 38.5 s (rabbit liver model) | Degradation rate: 46.4% (pH 1, 28 days), 35.4% (pH 4, 28 days) | [53] | |
| CMCS/Lap/PVA | 4.69 ± 1.01 min | 3.90 ± 0.25 kPa | 14 days | < 90 s (rat liver model) | Swelling rate: 296.7 ± 142.5% Degradation rate: < 70% | [60] | |
| HP | < 5 s | < 3.6 kPa (porcine stomach at pH 1, HTCC/PA = 3:1) | 10 days | 208.3 s (rat tail bleeding model), 142.0 s (rat liver injury model) | Degradation rate: < 64.7% (10 days) | [61] | |
| HACN-PEG | 1.7 s | < 10.4 kPa (chicken skin) | Overnight | 59 ± 7.94 s (rat femoral artery model), < 2 min (porcine upper GI hemorrhage model) | Swelling rate: < 189% | [29] | |
| ISFIHs | < 9 min | 6.63 kPa (porcine stomach tissue) | Immediate hemostasis | Swelling rate: 430%-670% (pH 7.4), 30%-90% (pH 2.0) Adhesion duration: 28 days | [70] | ||
| e-GLUE | 40 - 80 s | 30 days | Immediate hemostasis | Adhesion duration: 4-8 days (colon) | [73] | ||
| GastroShield | 2 - 17 s | ≥ 1 week (pH 2) | Immediate hemostasis | Adhesion duration: 7 days Swelling rate: ~ 50% Degradation rate: < 50% (pH 2, 7 days) | [12] | ||
| Gastric perforation repair | PEI/PAA powder | < 2 s | 74.6 kPa (wet chicken skin) | ≥ 2 weeks | Adhesion duration: ≥ 30 days | [81] | |
| CPO powder | 14.7 ± 3.1 s | 55.4 ± 4.5 kPa (wet porcine skin) | 36.7 ± 4.5 s | Swelling rate: 1530%-1840% Degradation rate: 78.9 ± 3.9% | [82] | ||
| PTOPT | 15.3 kPa (stomach), 20.5 kPa (skin) | 60 h | Immediate hemostasis | Adhesion duration: 10 days Degradation rate: 83.28% (pH 2.5) | [85] | ||
| MCH | 81 ± 5 kPa (glass) | < 10 s (≈ 5 s) (rabbit liver hemorrhage model) | Adhesion duration: ≥ 24 h Swelling rate: 3500% | [88] | |||
| EHO | ≥ 24 h (pH 2.0) | Swelling rate: 500% (pH 7.4), 580% (pH 2.0) | [89] | ||||
| PAASP/Fe3+ Janus hydrogel | 20 min | The adhesive side: 106 kPa (porcine skin), the non-adhesive side: 7.9 kPa | ≥ 6 h | ≤ 10 s | Swelling rate: ~120% (pH 3) | [77] | |
| HAD Janus hydrogel | < 3 s | ~8.5 kPa (porcine stomach) | ≥ 6 h | Immediate hemostasis | Swelling rate: 400% (pH 1.2) | [97] | |
| HADP | Within seconds | ≥ 6 h | Swelling rate: 171 ± 17% (pH 7.4, 2 days) Degradation rate: 90.8 ± 10.3% (pH 7.4, 7 days) | [31] | |||
| DQA | ≤ 1 h | 59.1 kPa (porcine skin), 30.4 kPa (stomach tissue), 23.7 kPa (intestine tissue) | 14 days | Adhesion duration: ≥ 14 days (pH 1.21) Swelling rate: 22.8 ± 4.5% (pH 1.21, 14 days), 48.1 ± 9.1% (pH 1.21, 14 days) | [99] | ||
| PAM-SA-NHA | 5 s | 108 J/m² (porcine skin) | Immediate hemostasis | Adhesion duration: ≥ 12 h | [103] | ||
| ATGel | Within seconds | 40 - 60 kPa | 10 days | Immediate hemostasis | Adhesion duration: ≥ 240 h (pH 2.0) Swelling rate: 82 % (pH 2.0, 24 h) | [13] | |
Metal-coordination hydrogels have immense potential for medical use [158]. Some hydrogels based on Fe3+ exhibit extremely fast self-assembly (about 1 minute), a stronger hydrogel network, and a slower degradation rate, and show vigorous antibacterial activity against E. coli and S. aureus [159].
Nowadays, the intelligence of hydrogels is mainly reflected in pH response or temperature sensitivity, rather than in monitoring wound recovery. It is a new trend to combine hydrogel adhesives with capsule robots or fluorescence imaging technology to monitor wound healing and changes in gastric acid [160-167]. Suppose the healing of gastric perforation can be monitored using hydrogel adhesives, it will not only effectively prevent secondary perforations but also be beneficial to the clinical research on gastric perforation healing. In addition, researchers continue to pursue good mechanical and hemostatic properties. Addressing the above limitations will effectively advance the research on hydrogel adhesives for clinical GI wound care.
Currently, some hydrogels used for hemostasis in the digestive tract have entered clinical trials. For example, UI-EWD, a new hemostatic powder, consists of aldehyde-glucan and succinic acid-modified ε-polyglucan [168]. When it comes into contact with water, the powder will immediately transform into the high-adhesive hydrogel. The initial hemostasis rate of UI-EWD reached 94%, and only 19% of patients experienced rebleeding.
Integration with emerging technologies offers new opportunities for developing hydrogel adhesives. For example, 3D printing can design portable hydrogel preparations. Hydrogel structures for patients, targeted drug-delivery technologies can enhance localized treatment; imaging-guided hydrogels enable real-time monitoring of wound-healing progress. These technologies are critical to promoting the next generation of hydrogel adhesives in the upper GI field.
Biological safety is the key prerequisite for the application of hydrogel adhesives in upper GI wound management, and is mainly reflected in the following three aspects:
First, biocompatibility and mucosal tolerance should be ensured. Current studies mainly focus on short-term safety, while the risks associated with chronic exposure or repeated administration remain insufficiently studied. Therefore, it is necessary to assess the mucosal irritation, effects on epithelial barrier integrity, and the cytotoxicity and tissue compatibility of the hydrogel adhesives.
Second, degradation behavior and the toxicity of metabolic by-products require careful attention. There is a general lack of in-depth research on the correlation among their degradation kinetics, metabolic products and biosafety. Therefore, the degradation pathways of hydrogels and the mucosal toxicity and metabolic pathways of the degradation products should be elucidated.
Third, local immune responses and systemic toxicity must be considered. Since research on the chronic immune response, absorption, and accumulation risks of hydrogel adhesives remain limited, it is necessary to assess the local immune responses that hydrogels may induce, including macrophage polarization and activation of mucosal inflammatory pathways, and to reveal the correlation between the components of hydrogels and their immune activation.
Overall, biosafety evaluation of hydrogel adhesives is crucial for advancing their preclinical validation and translation into upper gastrointestinal tract therapies, and it also supports the development of safer, more controllable adhesive materials.
5. Conclusions
Upper GI disorders, including bleeding, ulceration, perforation, and postoperative defects, are very prevalent worldwide and seriously affect the quality of survival of most patients. Moreover, the acidic environment of the stomach makes gastric wound healing difficult. Hydrogel adhesives provide a unique combination of strong wet adhesion, acid resistance, mechanical compliance, injectability, and drug-loading capability, making them highly suitable for GI wound management. This review summarizes the significant progress of hydrogel adhesives across major clinical upper GI wounds, including hemostasis and ulcer sealing, perforation repair, assistance in ESD, and protection during radiotherapy via hydrogel spacers. We highlighted the key design principles and representative materials and therapeutic strategies. The challenges and perspectives of the hydrogel adhesives for upper GI wound healing are also discussed. Overall, hydrogel adhesives are promising candidates for upper GI wound treatment materials.
Abbreviations
GI: gastrointestinal; 3D: three-dimensional; CMCS: carboxymethyl chitosan; SA: sodium alginate; ODE: oxidized dextran; DNA: deoxyribonucleic acid; RNA: ribonucleic acid; EC: esophageal cancer; ESD: endoscopic submucosal dissection; EV: extracellular vesicles; GIB: gastrointestinal bleeding; CS: chitosan; CS-NAC: N-acetylcysteine grafted chitosan; TCP: tilapia collagen peptide; PEI: polyethyleneimine; PAA: polyacrylic acid; Lap: lithium magnesium sodium salt; PVA: poly(vinyl alcohol); HA-Cat: hyaluronic acid-catechol; HA-NCSN: hyaluronic acid-thiourea; PEG: polyethylene glycol; PVAMA: methacrylated poly(vinyl alcohol); GelMAc: catechol motif-modified methacrylated gelatin; MPDA NPs: mesoporous polydopamine nanoparticles; Cur: curcumin; AA-NHS: AA-g-N-hydroxysuccinimid; PU: Polyurethane; BIS: N,N′-methylenebisacrylamide; SIS: small intestinal submucosa; PluPEI: amine-modified block-copolymer; γ-PGA: poly-γ-glutamic acid; OD: oxidized dextran; PNI-PAM: poly(N-isopropylacrylamide); A6ACA: acryloyl-6-aminocaproic acid; GelMA: methacrylate gelatin; TA: tannic acid; DMA: dimethylacrylamide; CBA: N,N′-cystaminebis(acrylamide); AFGF: acidic fibroblast growth factor; VF: vonoprazan fumarate; SF: silk fibroin; PAASP: Polymeric N-acryloyl aspartic acid; HA: hyaluronic acid; DA: dopamine; AA: acrylic acid; PDA: polydopamine; QCS: quaternary ammonium chitosan; PBA: phenylboronic acid; ADM: acellular dermal matrix; PAM: poly-acrylamide; NHA: nano-hydroxyapatite; HPC: hydroxypropyl cellulose; Col: collagen; GP: β-glycerophosphate; CSLA: lactobionic acid-modified chitosan; HpHCS: chitosan solution with high pH value; PVP: polyvinylpyrrolidone; F-127: polyethylene-polypropylene glycol; GGH: gellan gum hydrogel; OSA: oxidized sodium alginate; SA-GSH: glutathione reduced-modified sodium alginate; FL: 9-fluorenylmethoxycarbonyl (Fmoc)-conjugated phenylalanine-leucine; YA: tyrosine-alanine; LL: leucine-leucine; YL: tyrosine-leucine; CMS: carboxymethyl starch; HBC: hydroxybutyl chitosan; OARs: organs at risk; COFs: covalent organic frameworks; OCNTs: oxidized carbon nanotubes; POD: peroxidase; CAT: catalase; MOF: metal-organic framework.
Acknowledgements
This work was financially supported by the Special Foundation for Health Research Talent of Jilin Province (2023SCZ44), the Spring Bud Plan of China-Japan Union Hospital of Jilin University (2024CL02), and the Fundamental Research Funds for the Central Universities (2024-JCXK-15). This review was completed without the assistance of artificial intelligence (AI) tools.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hu X, Grinstaff MW. Advances in hydrogel adhesives for gastrointestinal wound closure and repair. Gels. 2023;9:282
2. Goswami SK, Rand AA, Wan D, Yang J, Inceoglu B, Thomas M. et al. Pharmacological inhibition of soluble epoxide hydrolase or genetic deletion reduces diclofenac-induced gastric ulcers. Life Sci. 2017;180:114-22
3. Sonambekar A, Desai D. Gastric perforation by stiff guidewire in Danis stent assembly. Hepatology. 2017;66:1698
4. Holm JS, Toyserkani NM, Sorensen JA. Adipose-derived stem cells for treatment of chronic ulcers: current status. Stem Cell Res Ther. 2018;9:142
5. Sanpinit S, Chonsut P, Punsawad C, Wetchakul P. Gastroprotective and antioxidative effects of the traditional Thai polyherbal formula Phy-Blica-D against ethanol-induced gastric ulcers in rats. Nutrients. 2022;14:172
6. Guo Y, Wang M, Liu Q, Liu G, Wang S, Li J. Recent advances in the medical applications of hemostatic materials. Theranostics. 2023;13:161-96
7. Kumar R, Parashar A. Atomistic simulations of pristine and nanoparticle reinforced hydrogels: A review. Wires Comput Mol Sci. 2023;13:e1655
8. Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res. 2015;6:105-21
9. Han ZL, Wang P, Lu YC, Jia Z, Qu SX, Yang W. A versatile hydrogel network-repairing strategy achieved by the covalent-like hydrogen bond interaction. Sci Adv. 2022;8:eabl5066
10. Morales A, Labidi J, Gullón P, Astray G. Synthesis of advanced biobased green materials from renewable biopolymers. Curr Opin Green Sustainable Chem. 2021;29:100436
11. Zhang A, Liu Y, Qin D, Sun M, Wang T, Chen X. Research status of self-healing hydrogel for wound management: A review. Int J Biol Macromol. 2020;164:2108-23
12. Taboada GM, Dahis D, Dosta P, Edelman E, Artzi N. Sprayable hydrogel sealant for gastrointestinal wound shielding. Adv Mater. 2024;36:2311798
13. Chen XM, Zhang J, Chen GD, Xue Y, Zhang JJ, Liang XY. et al. Hydrogel bioadhesives with extreme acid-tolerance for gastric perforation repairing. Adv Funct Mater. 2022;32:2202285
14. Hu B, Ouyang YL, Zhao T, Wang ZY, Yan QL, Qian QY. et al. Antioxidant hydrogels: antioxidant mechanisms, design strategies, and applications in the treatment of oxidative stress-related diseases. Adv Healthcare Mater. 2024;13:2303817
15. Ding CB, Liu XL, Zhang S, Sun SW, Yang JL, Chai GD. et al. Multifunctional hydrogel bioscaffolds based on polysaccharide to promote wound healing: A review. Int J Biol Macromol. 2024;259:129356
16. Lin J, Zheng SY, Xiao R, Yin J, Wu ZL, Zheng Q. et al. Constitutive behaviors of tough physical hydrogels with dynamic metal-coordinated bonds. J Mech Phys Solids. 2020;139:103935
17. Bashir S, Hina M, Iqbal J, Rajpar AH, Mujtaba MA, Alghamdi NA. et al. Fundamental concepts of hydrogels: synthesis, properties, and their applications. Polymers (Basel). 2020;12:2702
18. Xie MM, Zeng YB, Wu H, Wang SG, Zhao JL. Multifunctional carboxymethyl chitosan/oxidized dextran/sodium alginate hydrogels as dressing for hemostasis and closure of infected wounds. Int J Biol Macromol. 2022;219:1337-50
19. Lu S, Kong S, Wang Y, Hu Z, Zhang L, Liao M. Gastric acid-response chitosan/alginate/tilapia collagen peptide composite hydrogel: Protection effects on alcohol-induced gastric mucosal injury. Carbohydr Polym. 2022;277:118816
20. Chen ZH, Chen H, Fang KW, Liu N, Yu JF. Magneto-thermal hydrogel swarms for targeted lesion sealing. Adv Healthcare Mater. 2025;14:2403076
21. Xie M, Chen Y, Yang Q, Li Q, Zhang R, Bi W. et al. Nano-enabled DNA supramolecular sealant for soft tissue surgical applications. Nano Today. 2023;50:101825
22. Dave H, Vithalani H, Singh H, Yadav I, Jain A, Pal A. et al. Amphiphilic gelator-based shear-thinning hydrogel for minimally invasive delivery via endoscopy catheter to remove gastrointestinal polyps. Small. 2025;21:2405508
23. Parsa P, Paydayesh A, Davachi SM. Investigating the effect of tetracycline addition on nanocomposite hydrogels based on polyvinyl alcohol and chitosan nanoparticles for specific medical applications. Int J Biol Macromol. 2019;121:1061-9
24. Kanji S, Das H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediat Inflamm. 2017;2017:5217967
25. Chung H, An S, Han SY, Jeon J, Cho SW, Lee YC. Endoscopically injectable and self-crosslinkable hydrogel-mediated stem cell transplantation for alleviating esophageal stricture after endoscopic submucosal dissection. Bioeng Transl Med. 2023;8:e10521
26. Coffin E, Grangier A, Perrod G, Piffoux M, Marangon I, Boucenna I. et al. Extracellular vesicles from adipose stromal cells combined with a thermoresponsive hydrogel prevent esophageal stricture after extensive endoscopic submucosal dissection in a porcine model. Nanoscale. 2021;13:14866-78
27. O'Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057
28. Ming ZZ, Han L, Bao MY, Zhu HH, Qiang SJ, Xue SB. et al. Living bacterial hydrogels for accelerated infected wound healing. Adv Sci. 2021;8:2102545
29. Xia XF, Xu XY, Wang B, Zhou D, Zhang WL, Xie X. et al. Adhesive hemostatic hydrogel with ultrafast gelation arrests acute upper gastrointestinal hemorrhage in pigs. Adv Funct Mater. 2022;32:2109332
30. Li YX, Li TY, Feng JT, Liu BH, Wang ZY, He JH. et al. Acid-responsive contractile hyaluronic acid-based hydrogel loaded with ginsenoside Rg1 for hemostasis and promotion of gastric wound healing. Biomaterials. 2025;321:123320
31. Wang ZH, Xu J, Wu XQ, Han MY, Peng RJ, Zhao R. et al. A sprayable Janus hydrogel as an effective bioadhesive for gastrointestinal perforation repair. Adv Funct Mater. 2024;34:2408479
32. Ito S, Nishiguchi A, Sasaki F, Maeda H, Kabayama M, Ido A. et al. Robust closure of post-endoscopic submucosal dissection perforation by microparticle-based wound dressing. Mater Sci Eng C Mater Biol Appl. 2021;123:111993
33. Liu X, Yang Y, Yu H, Wang L, Sheng Y, Huang Z. et al. Instant and tough adhesives for rapid gastric perforation and traumatic pneumothorax sealing. Adv Healthcare Mater. 2022;11:e2201798
34. Bray F, Laversanne M, Sung HYA, Ferlay J, Siegel RL, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-cancer J Clin. 2024;74:229-63
35. Semenkovich TR, Hudson JL, Subramanian M, Mullady DK, Meyers BF, Puri V. et al. Trends in treatment of T1N0 esophageal cancer. Annals of Surgical. 2019;270:434-43
36. Terheggen G, Horn EM, Vieth M, Gabbert H, Enderle M, Neugebauer A. et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett's neoplasia. Gut. 2017;66:783-93
37. Kudo S, Chinda D, Shimoyama T, Yasuda K, Akitaya K, Arai T. et al. Influence of esophageal endoscopic submucosal dissection on the changes of energy metabolism during the perioperative period. Cancers (Basel). 2022;14:2015
38. Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen T. et al. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy. 2014;46:640-4
39. Bhatt A, Mehta NA. Stricture prevention after esophageal endoscopic submucosal dissection. Gastrointest Endosc. 2020;92:1187-9
40. Shi KD, Ji F. Prophylactic stenting for esophageal stricture prevention after endoscopic submucosal dissection. World J Gastroenterol. 2017;23:931-4
41. Jain D, Singhal S. Esophageal stricture prevention after endoscopic submucosal dissection. Clin Endosc. 2016;49:241-56
42. Abe S, Iyer PG, Oda I, Kanai N, Saito Y. Approaches for stricture prevention after esophageal endoscopic resection. Gastrointest Endosc. 2017;86:779-91
43. Wu J, Guo CG, Zhao Y, Wang J, Liu J, Li P. Hydrogels for preventing post-endoscopic submucosal dissection stenosis in early esophageal cancer: A comprehensive literature review. Therap Adv Gastroenterol. 2025;18:17562848251348971
44. Wang Y, Su Y, Zhu YC, Ni PXZ, Yu T, Yuan T. et al. Research on triamcinolone-loaded thermosensitive chitosan hydrogels for preventing esophageal stricture induced by endoscopic submucosal dissection. Int J Biol Macromol. 2024;261:129679
45. Pan Q, Tsuji Y, Sreedevi Madhavikutty A, Ohta S, Fujisawa A, Inagaki NF. et al. Prevention of esophageal stenosis via in situ cross-linkable alginate/gelatin powder in a new submucosal exfoliation model in rats. Biomater Sci. 2023;11:6781-9
46. Fujiyabu T, Qi P, Yoshie K, Fujisawa A, Tsuji Y, Chandel AKS. et al. Development of applicator to deliver hydrogel precursor powder for esophageal stricture prevention after endoscopic submucosal dissection. Chem Eng J. 2024;500:156742
47. Lanas A, Dumonceau JM, Hunt RH, Fujishiro M, Scheiman JM, Gralnek IM. et al. Non-variceal upper gastrointestinal bleeding. Nat Rev Dis Primers. 2018;4:1-21
48. Kamboj AK, Hoversten P, Leggett CL. Upper gastrointestinal bleeding: etiologies and management. Mayo Clin Proc. 2019;94:697-703
49. Cook D, Guyatt G. Prophylaxis against upper gastrointestinal bleeding in hospitalized patients. N Engl J Med. 2018;378:2506-16
50. Tielleman T, Bujanda D, Cryer B. Epidemiology and risk factors for upper gastrointestinal bleeding. Gastrointest Endosc Clin N Am. 2015;25:415-28
51. Bauder M, Schmidt A, Caca K. Endoscopic full-thickness resection of duodenal lesions-a retrospective analysis of 20 FTRD cases. United European Gastroenterol J. 2018;6:1015-21
52. Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG clinical guideline: upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. 2021;116:899-917
53. Ni P, Ye S, Xiong S, Zhong M, Shan J, Yuan T. et al. A chitosan-optimized polyethyleneimine/polyacrylic acid multifunctional hydrogel for reducing the risk of ulcerative arterial bleeding. J Mater Chem B. 2023;11:5207-22
54. Andersen T, Bleher S, Eide Flaten G, Tho I, Mattsson S, Škalko-Basnet N. Chitosan in mucoadhesive drug delivery: focus on local vaginal therapy. Mar Drugs. 2015;13:222-36
55. Bhardwaj N, Nguyen QT, Chen AC, Kaplan DL, Sah RL, Kundu SC. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials. 2011;32:5773-81
56. Malette WG, Quigley HJ, Gaines RD, Johnson ND, Rainer WG. Chitosan: a new hemostatic. Ann Thorac Surg. 1983;36:55-8
57. Said MM, Rehan M, El-Sheikh SM, Zahran MK, Abdel-Aziz MS, Bechelany M. et al. Multifunctional hydroxyapatite/silver nanoparticles/cotton gauze for antimicrobial and biomedical applications. Nanomaterials (Basel). 2021;11:429
58. Zhang X, Liang Y, Huang S, Guo B. Chitosan-based self-healing hydrogel dressing for wound healing. Adv Colloid Interface Sci. 2024;332:103267
59. Peng W, Jiang YF, Zhang Y, Lai YJ, Zhu YM, Wang HX. et al. AA/MA/AMA copolymer and chitosan as self-gelling powders for rapid hemostasis and robust tissue adhesion. Int J Biol Macromol. 2025;310:143443
60. Guo H, Shen H, Ma J, Wang P, Yao Z, Zhang W. et al. Versatile injectable carboxymethyl chitosan hydrogel for immediate hemostasis, robust tissue adhesion barrier, and antibacterial applications. ACS Appl Mater Interfaces. 2023;15:52290-304
61. Liu AS, Huang ZM, Cui SY, Xiao Y, Guo XS, Pan GK. et al. Ionically assembled hemostatic powders with rapid self-gelation, strong acid resistance, and on-demand removability for upper gastrointestinal bleeding. Materials Horizons. 2024;11:5983-96
62. Xu X, Xia X, Zhang K, Rai A, Li Z, Zhao P. et al. Bioadhesive hydrogels demonstrating pH-independent and ultrafast gelation promote gastric ulcer healing in pigs. Sci Transl Med. 2020;12:eaba8014
63. Wei K, Senturk B, Matter MT, Wu X, Herrmann IK, Rottmar M. et al. Mussel-inspired injectable hydrogel adhesive formed under mild conditions features near-native tissue properties. ACS Appl Mater Interfaces. 2019;11:47707-19
64. Xu YJ, Wei K, Zhao P, Feng Q, Choi CK, Bian L. Preserving the adhesion of catechol-conjugated hydrogels by thiourea-quinone coupling. Biomater Sci. 2016;4:1726-30
65. Waite JH, Tanzer ML. Polyphenolic substance of mytilus edulis: novel adhesive containing L-Dopa and hydroxyproline. Science. 1981;212:1038-40
66. Wang R, Li JZ, Chen W, Xu TT, Yun SF, Xu Z. et al. A biomimetic mussel-inspired ε-poly-ι-lysine hydrogel with robust tissue-anchor and anti-infection capacity. Adv Funct Mater. 2017;27:1604894
67. Zhou S, Chang Q, Lu F, Xing M. Injectable mussel-inspired immobilization of platelet-rich plasma on microspheres bridging adipose micro-tissues to improve autologous fat transplantation by controlling release of PDGF and VEGF, angiogenesis, stem cell migration. Adv Healthcare Mater. 2017;6:1700131
68. Shin J, Choi EJ, Cho JH, Cho AN, Jin Y, Yang K. et al. Three-dimensional electroconductive hyaluronic acid hydrogels incorporated with carbon nanotubes and polypyrrole by catechol-mediated dispersion enhance neurogenesis of human neural stem cells. Biomacromolecules. 2017;18:3060-72
69. Zhang X, He Y, Li X, Lin CC, Yuan Z, Dai LL. et al. Biocompatible, adhesive and stable GelMAc/PVAMA/MPDA@Cur hydrogels regulate immune response to improve endoscopic submucosal dissection-induced gastric ulcer healing in vivo. Appl Mater Today. 2022;28:101539
70. He J, Zhang Z, Yang Y, Ren F, Li J, Zhu S. et al. Injectable self-healing adhesive pH-responsive hydrogels accelerate gastric hemostasis and wound healing. Nano-Micro Lett. 2021;13:80
71. Zhao LM, Gong M, Wang R, Yuan QJ, Zhang Y, Pi JK. et al. Accelerating ESD-induced gastric ulcer healing using a pH-responsive polyurethane/small intestinal submucosa hydrogel delivered by endoscopic catheter. Regen Biomater. 2021;8:rbaa056
72. Cheng Z, Qing R, Hao S, Ding Y, Yin H, Zha G. et al. Fabrication of ulcer-adhesive oral keratin hydrogel for gastric ulcer healing in a rat. Regenerative Biomaterials. 2021;8:rbab008
73. Ying BB, Nan KW, Zhu Q, Khuu T, Ro H, Qin SP. et al. An electroadhesive hydrogel interface prolongs porcine gastrointestinal mucosal theranostics. Sci Transl Med. 2025;17:eadq1975
74. Weledji EP. An overview of gastroduodenal perforation. Front Surg. 2020;7:573901
75. Baldaque-Silva F, Marques M, Andrade AP, Sousa N, Lopes J, Carneiro F. et al. Endoscopic submucosal dissection of gastrointestinal lesions on an outpatient basis. United European Gastroenterol J. 2019;7:326-34
76. Luan Z, Liu S, Wang W, Xu K, Ye S, Dan R. et al. Aligned nanofibrous collagen membranes from fish swim bladder as a tough and acid-resistant suture for pH-regulated stomach perforation and tendon rupture. Biomater Res. 2022;26:60
77. Yu J, Qin Y, Yang Y, Zhao X, Zhang Z, Zhang Q. et al. Robust hydrogel adhesives for emergency rescue and gastric perforation repair. Bioact Mater. 2023;19:703-16
78. Zhu Q, Hong Y, Huang Y, Zhang Y, Xie C, Liang R. et al. Polyglutamic acid-based elastic and tough adhesive patch promotes tissue regeneration through in situ macrophage modulation. Adv Sci. 2022;9:e2106115
79. Vuocolo T, Haddad R, Edwards GA, Lyons RE, Liyou NE, Werkmeister JA. et al. A highly elastic and adhesive gelatin tissue sealant for gastrointestinal surgery and colon anastomosis. J Gastrointest Surg. 2012;16:744-52
80. Guo J, Ye L, Gao Y, Li S, Zhang L, Liu W. et al. Hybrid dry powders for rapid sealing of gastric perforations under an endoscope. ACS Nano. 2023;17:9521-8
81. Peng X, Xia X, Xu X, Yang X, Yang B, Zhao P. et al. Ultrafast self-gelling powder mediates robust wet adhesion to promote healing of gastrointestinal perforations. Sci Adv. 2021;7:eabe8739
82. Bian D, Chen Z, Ouyang Y, Wang S, Wang M, Chen W. Ultrafast self-gelling, sprayable, and adhesive carboxymethyl chitosan/poly-γ-glutamic acid/oxidized dextran powder for effective gastric perforation hemostasis and wound healing. Int J Biol Macromol. 2024;254:127960
83. Wang W, Zeng Z, Xiang L, Liu C, Diaz-Dussan D, Du Z. et al. Injectable self-healing hydrogel via biological environment-adaptive supramolecular assembly for gastric perforation healing. ACS Nano. 2021;15:9913-23
84. Chen J, Caserto JS, Ang I, Shariati K, Webb J, Wang B. et al. An adhesive and resilient hydrogel for the sealing and treatment of gastric perforation. Bioact Mater. 2022;14:52-60
85. He C, Bi S, Liu R, Zhao H, Chen C, Zhao X. et al. Cation-π interaction-enhanced self-healing injectable hydrogels for gastric perforation repair. ACS Appl Mater Interfaces. 2024;16:35887-97
86. Sun LH, Ouyang J, She ZP, Li R, Zeng F, Yao ZC. et al. Injectable-hydrogel-based tissue sealant for hemostasis, bacteria inhibition, and pro-angiogenesis in organ bleeding wounds and therapeutic outcome monitoring via NIR-II optical imaging. Adv Healthcare Mater. 2024;13:2303997
87. Liu BC, Wang Y, Miao Y, Zhang XY, Fan ZX, Singh G. et al. Hydrogen bonds autonomously powered gelatin methacrylate hydrogels with super-elasticity, self-heal and underwater self-adhesion for sutureless skin and stomach surgery and E-skin. Biomaterials. 2018;171:83-96
88. Liu S, Luan ZH, Wang TC, Xu KG, Luo Q, Ye SS. et al. Endoscopy deliverable and mushroom-cap-inspired hyperboloid-shaped drug-laden bioadhesive hydrogel for stomach perforation repair. ACS Nano. 2023;17:111-26
89. Li HY, Chai NL, Yang YY, Liu ZY, Liu ZY, Liu XM. et al. Endoscopic delivery of a double-umbrella-shaped hydrogel occluder with instant mechanical interlock and robust wet adhesion for gastric perforation repair. ACS Appl Mater Interfaces. 2025;17:23642-55
90. Mizuno Y, Mizuta R, Hashizume M, Taguchi T. Enhanced sealing strength of a hydrophobically-modified Alaska pollock gelatin-based sealant. Biomater Sci. 2017;5:982-9
91. Nishiguchi A, Kurihara Y, Taguchi T. Underwater-adhesive microparticle dressing composed of hydrophobically-modified Alaska pollock gelatin for gastrointestinal tract wound healing. Acta Biomater. 2019;99:387-96
92. Sun M, Kumar N, Dhinojwala A, King H. Attractive forces slow contact formation between deformable bodies underwater. Proc Natl Acad Sci USA. 2021;118:e2104975118
93. Ito S, Komatsu H, Minamisakamoto S, Palai D, Nishiguchi A, Taguchi T. Local administration of an anti-cancer drug using tissue adhesive microparticles based on hydrophobically modified Alaska pollock gelatin for post-surgical cancer chemotherapy. Colloids Surf B. 2025;254:114825
94. Liu KS, Kao CW, Tseng YY, Chen SK, Lin YT, Lu CJ. et al. Assessment of antimicrobial agents, analgesics, and epidermal growth factors-embedded anti-adhesive poly(lactic-co-glycolic acid) nanofibrous membranes: in vitro and in vivo studies. Int J Nanomed. 2021;16:4471-80
95. Cho H, Jammalamadaka U, Tappa K, Egbulefu C, Prior J, Tang R. et al. 3D printing of poloxamer 407 nanogel discs and their applications in adjuvant ovarian cancer therapy. Mol Pharm. 2019;16:552-60
96. Cui CY, Wu TL, Chen XY, Liu Y, Li Y, Xu ZY. et al. A Janus hydrogel wet adhesive for internal tissue repair and anti-postoperative adhesion. Adv Funct Mater. 2020;30:2005689
97. Wu X, Wang Z, Xu J, Yu L, Qin M, Li J. et al. Photocurable injectable Janus hydrogel with minimally invasive delivery for all-in-one treatment of gastric perforations and postoperative adhesions. Theranostics. 2023;13:5365-85
98. Liang L, Wang HD, Li LF, Lin D, Guo BY, Yao MM. et al. Microparticle deposition induced asymmetric adhesive hydrogel for suture-less gastric trauma treatment. Chem Eng J. 2024;485:150086
99. Chen CW, Tang QQ, Wu L, Gu GS, Huang XX, Chen K. et al. Hybrid double-sided tape with asymmetrical adhesion and burst pressure tolerance for abdominal injury treatment. ACS Appl Mater Interfaces. 2024;16:30430-42
100. Li L, An JH, Lin ZD, Liu LS, Liu Q. A rapid and robust organ repair polyacrylamide/alginate adhesive hydrogel mediated via interfacial adhesion-trigger molecules. Int J Biol Macromol. 2024;281:135681
101. Liu JY, Shen KX, Chu DK, Cheng YL. Tempo-functionalized Janus hydrogel crosslinked by tandem dynamic covalent bonds for internal organ injury repair and deep second-degree burn wound healing. Adv Funct Mater. 2025: 2505194.
102. Wang Q, Zhao X. A three-dimensional phase diagram of growth-induced surface instabilities. Sci Rep. 2015;5:1-10
103. Yuan YH, Wu H, Ren XY, Wang JW, Liu RQ, Hu BH. et al. Dual-network hydrogel based on ionic nano-reservoir for gastric perforation sealing. Sci China Mater. 2022;65:827-35
104. Khan S, Cui X, Nasir S, Rafiq SM, Qin B, Bai Q. Advances in endoscopic resection techniques of small gastric tumors originating from the muscularis propria. Front Oncol. 2022;12:1001112
105. Lee EK, Kim SY, Lee YJ, Kwak MH, Kim HJ, Choi IJ. et al. Improvement of diabetes and hypertension after gastrectomy: a nationwide cohort study. World J Gastroenterol. 2015;21:1173-81
106. Wang L, Liu Z, Kou H, He H, Zheng B, Zhou L. et al. Double contrast-enhanced ultrasonography in preoperative T staging of gastric cancer: a comparison with endoscopic ultrasonography. Front Oncol. 2019;9:66
107. Wang T, Wang DN, Liu WT, Zheng ZQ, Chen X, Fang WL. et al. Hemostatic effect of topical hemocoagulase spray in digestive endoscopy. World J Gastroenterol. 2016;22:5831-6
108. Esaki M, Ihara E, Sumida Y, Fujii H, Takahashi S, Haraguchi K. et al. Hybrid and conventional endoscopic submucosal dissection for early gastric neoplasms: a multi-center randomized controlled trial. Clin Gastroenterol H. 2023;21:1810-8
109. Jacobson BC, Bhatt A, Greer KB, Lee LS, Park WG, Sauer BG. et al. ACG clinical guideline: diagnosis and management of gastrointestinal subepithelial lesions. Am J Gastroenterol. 2023;118:46-58
110. Zhang Y, Wang Z, Jin T, Li KQ, Hao K, Zhang W. et al. Hyperechoic demarcation line between a tumor and the muscularis propria layer as a marker for deciding the endoscopic treatment of gastric submucosal tumor. J Zhejiang Univ Sci B. 2017;18:707-16
111. Du C, Chai N, Linghu E, Li H, Zhai Y, Li L. et al. Clinical outcomes of endoscopic resection for the treatment of gastric gastrointestinal stromal tumors originating from the muscularis propria: a 7-year experience from a large tertiary center in China. Surg Endosc. 2022;36:1544-53
112. Moles-Aranda C, Calpena-Campmany AC, Halbaut-Bellowa L, Díaz-Tomé V, Otero-Espinar FJ, Morales-Molina JA. et al. Novel polymeric formulation for removal of gastrointestinal polyps by digestive endoscopy. Pharmaceutics. 2020;12:322
113. Fan C, Xu K, Huang Y, Liu S, Wang T, Wang W. et al. Viscosity and degradation controlled injectable hydrogel for esophageal endoscopic submucosal dissection. Bioact Mater. 2021;6:1150-62
114. Zhu Y, Xu JX, Cheng J, Zhang Z, Zhu BQ, Chen TY. et al. A novel injectable thermo-sensitive binary hydrogels system for facilitating endoscopic submucosal dissection procedure. United European Gastroenterol J. 2019;7:782-9
115. Kuwai T, Tamaru Y, Kusunoki R, Ishaq S. Submucosal injection solutions for ESD: separating the winners from the losers. Dig Dis Sci. 2019;64:2699-700
116. Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD. et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155-61
117. Jeon HJ, Choi HS, Bang EJ, Lee KW, Kim SH, Lee JM. et al. Efficacy and safety of a thermosensitive hydrogel for endoscopic submucosal dissection: An in vivo swine study. PLoS One. 2021;16:e0260458
118. Shan J, Tang B, Liu L, Sun X, Shi W, Yuan T. et al. Development of chitosan/glycerophosphate/collagen thermo-sensitive hydrogel for endoscopic treatment of mucosectomy-induced ulcer. Mater Sci Eng C Mater Biol Appl. 2019;103:109870
119. Repka MA, McGinity JW. Bioadhesive properties of hydroxypropylcellulose topical films produced by hot-melt extrusion. J Control Release. 2001;70:341-51
120. Tang B, Shan J, Yuan T, Xiao Y, Liang J, Fan Y. et al. Hydroxypropylcellulose enhanced high viscosity endoscopic mucosal dissection intraoperative chitosan thermosensitive hydrogel. Carbohydr Polym. 2019;209:198-206
121. Ni P, Li R, Ye S, Shan J, Yuan T, Liang J. et al. Lactobionic acid-modified chitosan thermosensitive hydrogels that lift lesions and promote repair in endoscopic submucosal dissection. Carbohydr Polym. 2021;263:118001
122. Liu J, Ni P, Wang Y, Zhou Z, Li J, Chen T. et al. Design and validation of performance-oriented injectable chitosan thermosensitive hydrogels for endoscopic submucosal dissection. Biomater Sci. 2023;146:213286
123. Ni P, Ye S, Li R, Shan J, Yuan T, Liang J. et al. Chitosan thermosensitive hydrogels based on lyophilizate powders demonstrate significant potential for clinical use in endoscopic submucosal dissection procedures. Int J Biol Macromol. 2021;184:593-603
124. Liu Y, Lang C, Ding YP, Sun SY, Sun GW. Chitosan with enhanced deprotonation for accelerated thermosensitive gelation with β-glycerophosphate. Eur Polym J. 2023;196:112229
125. Liu Y, Lang C, Zhang K, Feng L, Li J, Wang T. et al. Injectable chitosan-polyvinylpyrrolidone composite thermosensitive hydrogels with sustained submucosal lifting for endoscopic submucosal dissection. Int J Biol Macromol. 2024;276:133165
126. Xu RF, Yang XY, Yi T, Tan T, Li ZQ, Feng XY. et al. Injectable temperature-sensitive hydrogel facilitating endoscopic submucosal dissection. Front Bioeng Biotechnol. 2024;12:1395731
127. Di ZN, Xiang Y, Pan YY, Shao YY, Fan X, Hua Y. et al. Hydrogen Bond-Driven Conductive Thermosensitive Hydrogel for Advancing Endoscopic Electrosurgery. Adv Healthcare Mater. 2025;14:2501495
128. Ono K, Saito Y, Yura H, Ishikawa K, Kurita A, Akaike T. et al. Photocrosslinkable chitosan as a biological adhesive. J Biomed Mater Res Part A. 2000;49:289-95
129. Ishizuka T, Ishihara M, Aiko S, Nogami Y, Nakamura S, Kanatani Y. et al. Experimental evaluation of photocrosslinkable chitosan hydrogel as injection solution for endoscopic resection. Endoscopy. 2009;41:25-8
130. Hirose R, Nakaya T, Naito Y, Yoshida T, Bandou R, Daidoji T. et al. An innovative next-generation endoscopic submucosal injection material with a 2-step injection system (with video). Gastrointest Endosc. 2021;93:503-13.e5
131. Tang Y, Hu M, Tang F, Huang R, Wang H, Wu D. et al. Easily-injectable shear-thinning hydrogel provides long-lasting submucosal barrier for gastrointestinal endoscopic surgery. Bioact Mater. 2022;15:44-52
132. Ma J, Wang P, Tang C, Liao H, Zhang W, Yang R. et al. Injectable shear-thinning sodium alginate hydrogels with sustained submucosal lift for endoscopic submucosal dissection. Int J Biol Macromol. 2022;223:939-49
133. Ren P, Li JT, Zhao LY, Wang AH, Wang MY, Li JL. et al. Dipeptide self-assembled hydrogels with shear-thinning and instantaneous self-healing properties determined by peptide sequences. ACS Appl Mater Interfaces. 2020;12:21433-40
134. Wang PH, Li RX, Ma JP, Zhang WJ, Shen HF, Ren YH. et al. Facilitating safe and sustained submucosal lift through an endoscopically injectable shear-thinning carboxymethyl starch sodium hydrogel. Carbohydr Polym. 2024;336:122128
135. Feng WJ, Wu YH, Liu XY, Wang ZK. Shear-thinning catechol-modified chitosan hydrogel loaded with silver nanoparticles for endoscopic submucosal dissection. Chin J Polym Sci. 2024;42:1147-55
136. Zhang Y, Miao DT, Su ML, Tang YX, Zhou MH, Yu Y. et al. Synergistic drug-loaded shear-thinning star polymer hydrogel facilitates gastrointestinal lesion resection and promotes wound healing. Adv Sci. 2024;11:2309586
137. Cui SQ, Wei YM, Bian Q, Zhu Y, Chen XB, Zhuang YP. et al. Injectable thermogel generated by the "block blend" strategy as a biomaterial for endoscopic submucosal dissection. ACS Appl Mater Interfaces. 2021;13:19778-92
138. Pang Y, Liu JY, Moussa ZL, Collins JE, McDonnell S, Hayward AM. et al. Endoscopically injectable shear-thinning hydrogels facilitating polyp removal. Adv Sci. 2019;6:1901041
139. Kuddushi M, Vithalani H, Singh H, Dave H, Jain A, Pal A. et al. Easily injectable, organic solvent-free self-assembled hydrogel platform for endoscope mediated gastrointestinal polypectomy. Adv Healthcare Mater. 2025;14:2403915
140. Lei XX, Hu JJ, Zou CY, Jiang YL, Zhao LM, Zhang XZ. et al. Multifunctional two-component in-situ hydrogel for esophageal submucosal dissection for mucosa uplift, postoperative wound closure and rapid healing. Bioact Mater. 2023;27:461-73
141. Liu SR, Ju RB, Zhang ZG, Jiang Z, Cui JZ, Liu WS. et al. Temperature-sensitive injectable chitosan-based hydrogel for endoscopic submucosal dissection. Int J Biol Macromol. 2024;282:136566
142. Stoffel EM, Brand RE, Goggins M. Pancreatic cancer: changing epidemiology and new approaches to risk assessment, early detection, and prevention. Gastroenterology. 2023;164:752-65
143. Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH. et al. Locally advanced, unresectable pancreatic cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34:2654-68
144. Krishnan S, Chadha AS, Suh Y, Chen HC, Rao A, Das P. et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:755-65
145. Hooshangnejad H, Youssefian S, Narang A, Shin EJ, Rao AD, Han-Oh S. et al. Finite element-based personalized simulation of duodenal hydrogel spacer: spacer location dependent duodenal sparing and a decision support system for spacer-enabled pancreatic cancer radiation therapy. Front Oncol. 2022;12:833231
146. Rao AD, Feng Z, Shin EJ, He J, Waters KM, Coquia S. et al. A novel absorbable aadiopaque hydrogel spacer to separate the head of the pancreas and duodenum in radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2017;99:1111-20
147. Bhutani MS, Narang AK, Ding K, Casey B, Krishnan K, Koay EJ. et al. EUS-guided hydrogel injection to separate pancreatic head carcinoma from duodenum for enhanced radiotherapy: Multi-site feasibility study. Endosc Int Open. 2024;12:E861-7
148. Rao AD, Shin EJ, Beck SE, Garrett C, Kim SH, Lee NJ. et al. Demonstration of safety and feasibility of hydrogel marking of the pancreas-duodenum interface for image guided radiation therapy (IGRT) in a porcine model: implications in IGRT for pancreatic cancer patients. Int J Radiat Oncol Biol Phys. 2018;101:640-5
149. Rao AD, Shin EJ, Meyer J, Thompson EL, Fu W, Hu C. et al. Evaluation of a novel absorbable radiopaque hydrogel in patients undergoing image guided radiation therapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Pract Radiat Oncol. 2020;10:e508-13
150. Feng Z, Rao AD, Cheng Z, Shin EJ, Moore J, Su L. et al. Dose prediction model for duodenum sparing with a biodegradable hydrogel spacer for pancreatic cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102:651-9
151. Lin XJ, Zhang M, Lv WX, Li J, Huang R, Wang Y. Engineering carbon nanotube-based photoactive COF to synergistically arm a multifunctional antibacterial hydrogel. Adv Funct Mater. 2024;34:2310845
152. Reddy YN, De AAA, Paul S, Pujari AK, Bhaumik J. In situ nanoarchitectonics of a MOF hydrogel: a self-adhesive and pH-responsive smart platform for phototherapeutic delivery. Biomacromolecules. 2023;24:1717-30
153. Deng LL, Huang YJ, Chen SY, Han ZL, Han ZZ, Jin MT. et al. Bacterial cellulose-based hydrogel with antibacterial activity and vascularization for wound healing. Carbohydr Polym. 2023;308:120647
154. Tian M, Zhou LP, Fan C, Wang LR, Lin XF, Wen YQ. et al. Bimetal-organic framework/GOx-based hydrogel dressings with antibacterial and inflammatory modulation for wound healing. Acta Biomater. 2023;158:252-65
155. Guo YR, Ding S, Shang CS, Zhang CG, Li MG, Zhang QH. et al. Multifunctional PtCuTe nanosheets with strong ROS scavenging and ROS-independent antibacterial properties promote diabetic wound healing. Adv Mater. 2024;36:2306292
156. Wang RY, Xu SX, Zhang MM, Feng W, Wang CW, Qiu XF. et al. Multifunctional chitosan-based hydrogels loaded with iridium nanoenzymes for skin wound repair. Carbohydr Polym. 2024;342:122325
157. Wei G, Liu QY, Wang XY, Zhou ZJ, Zhao XZ, Zhou WQ. et al. A probiotic nanozyme hydrogel regulates vaginal microenvironment for Candida vaginitis therapy. Sci Adv. 2023;9:adg0949
158. Wong K-Y, Nie Z, Wong M-S, Wang Y, Liu J. Metal-drug coordination nanoparticles and hydrogels for enhanced delivery. Adv Mater. 2024;36:e2404053
159. Qiao LP, Liang YP, Chen JY, Huang Y, Alsareii SA, Alamri AM. et al. Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing. Bioact Mater. 2023;30:129-41
160. Wang F, Deng S, Song C, Fu X, Zhang N, Li Q. et al. Pd@Au nanoframe hydrogels for closed-loop wound therapy. ACS Nano. 2025;19:15069-80
161. Song C, Li J, Wang F, Peng Z, Shen Y, Huang C. et al. Multifunctional AuAgPt nanoframes for a stimuli-responsive electrochemiluminescence aptasensor. ACS Nano. 2025;19:26161-9
162. Xiang S, Li J, Wang F, Yang H, Jiang Y, Zhang P. et al. Novel ultralow-potential electrochemiluminescence aptasensor for the highly sensitive detection of zearalenone using a resonance energy transfer system. Anal Chem. 2023;95:15125-32
163. Li J, Yang H, Cai R, Tan W. Novel nucleic acid-assisted ion-responsive ECL biosensor based on hollow AuAg nanoboxes with excellent SPR and effective coreaction acceleration. Anal Chem. 2024;96:11076-82
164. Deng S, Wang F, Li Y, Li J, Zhan J, Shen Y. et al. Triple helix molecular switch cascade multiple signal amplification strategies for ultrasensitive chloramphenicol detection. Anal Chem. 2024;96:20312-7
165. Xiang S, Li JX, Shi ML, Yang HF, Cai R, Tan WH. A novel ECL aptasensor for ultra-highly sensitive detection of patulin based on terbium organic gels as Co-reaction accelerator in a 3, 4, 9, 10-peryle-netetracarboxylic acid/K2S2O8 system. Sensors and Actuators B-Chemical. 2023;394:134356
166. Wang F, Song C, Li Y, Yang H, Yang D, Chen K. et al. Fully wearable devices for real-time health monitoring and multimodal sensing in nanomedicine using multiplexed green biofuels. Nano Lett. 2025;25:9853-62
167. Xu J, Li Y, Wang F, Li W, Zhan J, Deng S. et al. Machine learning assisted-intelligent lactic acid monitoring in sweat supported by a perspiration-driven self-powered sensor. Nano Lett. 2025;25:2968-77
168. Park JS, Bang BW, Hong SJ, Lee E, Kwon KS, Kim HK. et al. Efficacy of a novel hemostatic adhesive powder in patients with refractory upper gastrointestinal bleeding: a pilot study. Endoscopy. 2019;51:458-62
Author contact
![]() Corresponding authors: Lianjun Ma (horsejlmedu.cn), Xin Wang (xinwangjluedu.cn), Ying-Wei Yang (ywyangedu.cn).
Corresponding authors: Lianjun Ma (horsejlmedu.cn), Xin Wang (xinwangjluedu.cn), Ying-Wei Yang (ywyangedu.cn).
 Global reach, higher impact
Global reach, higher impact