13.3
Impact Factor
Theranostics 2026; 16(5):2284-2309. doi:10.7150/thno.126199 This issue Cite
Review
Role of central and peripheral serotonin in liver physiology and diseases
Université Côte D'Azur, INSERM, U1065, C3M, Nice, France.
Received 2025-10-2; Accepted 2025-10-31; Published 2026-1-1
Abstract
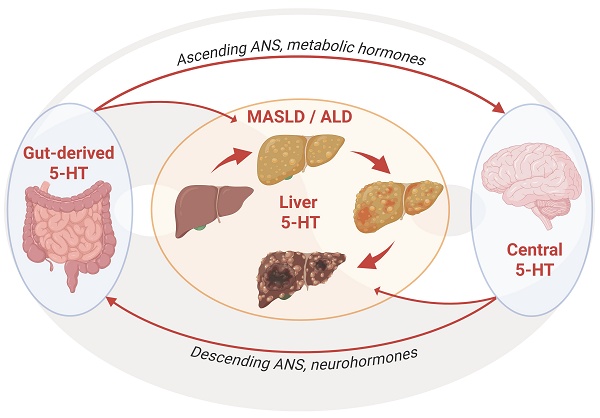
Steatotic liver diseases (SLD) associated with metabolic dysfunction (Metabolic dysfunction-Associated Steatotic Liver Disease: MASLD), chronic alcohol consumption (alcohol-associated liver disease: ALD), or both (MetALD) represent a major health issue worldwide. These chronic liver diseases are major drivers of fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality, with limited treatments currently available. Their progression is fueled by persistent disruptions in hepatic metabolism, inflammation, and tissue remodeling. The autonomic nervous system, notably its regulatory role in hepatic function, is receiving increasing attention as a key mediator of pathogenesis of liver disease. Serotonin (5-hydroxytryptamine, 5-HT) has emerged as a pivotal regulator of all these processes. Acting via combined central and peripheral pathways and a range of receptor subtypes, 5-HT modulates hepatic glucose and lipid metabolism, immune responses, fibrogenesis, and liver regenerative capacity. In this review, we explore the multifaceted role of 5-HT signaling in metabolic control, obesity, liver physiology, and chronic liver diseases. Our focus extends beyond the direct effects of 5-HT on liver cell populations to its interaction with the autonomic nervous system, metabolic hormones, and the gut-liver-brain axis. Lastly, we discuss how 5-HT's dual origin and pleiotropic effects may offer therapeutic avenues for MASLD, ALD, and HCC.
Introduction
Obesity and excessive alcohol intake are two major contributors to chronic liver diseases, notably metabolic dysfunction-associated steatotic liver disease (MASLD, previously NAFLD) and alcohol-associated liver disease (ALD) [1]. MASLD, which closely mirrors the rising prevalence of obesity, type 2 diabetes, and metabolic syndrome, encompasses a spectrum of hepatic conditions from steatosis to steatohepatitis (MASH), fibrosis, and cirrhosis, and may ultimately progress to hepatocellular carcinoma (HCC) [1]. Similarly, ALD evolves through stages of fatty liver, steatohepatitis (ASH), fibrosis, and cirrhosis, with a substantial risk of HCC in advanced disease stages, often compounded by episodes of acute-on-chronic liver injury [1]. Indeed, patients with underlying ASH or cirrhosis and recent excessive alcohol consumption can develop alcohol-associated hepatitis (AH). Although MASLD and ALD originate from distinct etiologies-metabolic versus toxic-their downstream pathological mechanisms, including lipid accumulation, mitochondrial dysfunction, inflammation, fibrogenesis, and carcinogenesis, share striking similarities (Figure 1).
In the context of the search for common regulatory mechanisms, 5-HT has gained considerable attention for its emerging role in liver physiology and disease [2,3]. While classically studied as a central neurotransmitter involved in mood, appetite, and behavior, over 90% of total body 5-HT is actually produced in the periphery, primarily by enterochromaffin cells of the gastrointestinal tract [4]. Once synthesized, gut 5-HT is released into the bloodstream where it is stored in platelets and acts on several organs [5], including the liver [6]. This dual origin, central and peripheral, adds a layer of complexity to 5-HT signaling, particularly in the context of MASLD and ALD.
Further complexity arises from the broad diversity of 5-HT receptors, with at least 14 subtypes grouped into seven families (5-HT1 to 5-HT7) [7]. These receptors are variably expressed across liver cell types, including hepatocytes, hepatic stellate cells (HSC), cholangiocytes, endothelial cells, and immune cells, and mediate a wide range of effects from lipid metabolism and cell proliferation to inflammation, fibrogenesis, and even tumor progression [2]. Growing evidence also implicates serotonergic signaling in the tumor microenvironment, supporting angiogenesis and cell proliferation in HCC [8]. In parallel, central 5-HT, by acting through neuroendocrine and autonomic pathways, indirectly influences liver function, particularly in the context of energy homeostasis and metabolic control [9,10]. This connection to the autonomic nervous system (ANS) is particularly important as the liver is densely innervated by both sympathetic and parasympathetic fibers [11]. The ANS governs a wide array of hepatic functions, such as glucose production, bile secretion, and immune surveillance through efferent signals from the central nervous system [11]. Central 5-HT modulates the activity of hypothalamic and brainstem nuclei that control autonomic output [12], thereby influencing liver physiology. Conversely, afferent vagal signaling from the liver to the brain contributes to central sensing of metabolic status [13], creating a bidirectional communication axis.
Altogether, 5-HT serves as a key integrator of gut, brain, and liver functions, acting both locally and systemically through an intricate network of serotonergic and autonomic pathways. Disentangling the respective contributions of peripheral versus central 5-HT, and their interaction with the ANS, are critical to understanding how serotonergic dysregulation contributes to the pathogenesis of MASLD and ALD. In this review, we aim to unravel the complex role of 5-HT in liver physiology and pathology, with a particular emphasis on its integration within the gut-liver-brain axis and its interconnection with other metabolic organs. We review the distinct sources of 5-HT, laying the necessary groundwork to differentiate the respective contributions of central versus peripheral 5-HT in the regulation of liver functions. This framework allows us to explore how disruptions in 5-HTergic signaling, from synthesis, degradation, receptor expression, to downstream pathways, can contribute to the onset and progression of chronic liver diseases, such as MASLD and ALD.
Progression of steatotic liver diseases. Steatotic liver diseases are associated with metabolic dysfunction (MASLD, blue) (Worldwide prevalence of 32.4%), chronic alcohol use (ALD, red) or both (MetALD), characterized by the progression from healthy liver to steatosis, steatohepatitis, and/or alcoholic hepatitis, that can both evolve in mild to severe fibrosis (cirrhosis) and ultimately lead to hepatocarcinoma (HCC). Blue percentages indicate estimated incidence of progression for MASLD, red for ALD. Double arrows show potential reversibility in the progression of the pathology. Created with BioRender.com.

I. Multiple sources of 5-HT
In mammals, the vast majority of 5-HT is produced in the gastrointestinal tract by enterochromaffin (EC) cells, in response to gut mechanical stimuli, nutrients (e.g., glucose, amino acids, short-chain fatty acids) or microbiota metabolites [4]. It has been evaluated that 90% of 5-HT is synthesized in EC cells, by the rate-limiting enzyme tryptophan hydroxylase 1 (TPH1) [14], which converts tryptophan into 5-hydroxytryptophan, further converted to 5-Hydroxytryptamine by the aromatic amino acid decarboxylase (AADC). Gut microbiota influences TPH1 activity and 5-HT production in the gut through multiple mechanisms [15]. While certain microbial species can upregulate the expression of TPH1 and enhance local 5-HT synthesis - particularly through metabolites such as short-chain fatty acids (SCFAs) including acetate, butyrate, and propionate - others can suppress TPH1 expression via the production of secondary bile acids or tryptamine derivatives, thereby reducing mucosal 5-HT synthesis [16-19]. Other microbial species can synthesize tryptophan (providing more substrate for TPH1-mediated 5-HT synthesis), or express the AADC, hence contributing to 5-HT production [20]. Through these actions, the microbiota plays a pivotal role in modulating 5-HT availability in the inflamed gut and/or in a context of obesity [21]. In turn, by modulating the growth of certain bacteria, or reducing tryptophan availability due to its synthesis, 5-HT may shape gut microbiota composition to stimulate inflammatory and immune responses [22]. Once secreted by EC cells, 5-HT is either acting locally in a para- or autocrine manner or it is released into the systemic circulation, as a hormone. In the bloodstream, most of the 5-HT is stored in blood platelets [23]. Upon platelet activation, 5-HT is released into various organs, including the liver, pancreas, and adipose tissue, thereby regulating their homeostasis or metabolic activity [24] through activation of multiple 5-HT receptors (Figure 2). Cells expressing TPH1 and capable of synthesizing 5-HT are also found in the liver (i.e., stellate cells and cholangiocytes) [25,26], pancreas (β-cells) [27], and adipose tissue (adipocytes) [28], suggesting an additional local source of 5-HT in those organs (Figure 3).
Another important source of 5-HT is the nervous system, where it is synthesized by the tryptophan hydroxylase 2 (TPH2) in lower relative quantities and serves as a neurotransmitter. 5-HT is present in the enteric neurons (ENS) of the peripheral nervous system (PNS), where it regulates intestinal motility and anxiety [29,30], and in the raphe neurons of the brainstem within the central nervous systems (CNS), where it regulates a wide range of physiological, psychological, and cognitive functions such as appetite, sleep, mood, fear, reward, learning, and memory [31].
From these different sources, 5-HT can further influence most of the metabolic functions controlled by the autonomous nervous system. 5-HT regulates glucose and lipid homeostasis, appetite and satiety, energy expenditure, and thermogenesis by modulating the sympathetic and parasympathetic activity in the liver, the pancreas or the adipose tissues [9]. For instance, 5-HT neurons from the rostral medullary raphe (rMR) indirectly project to the coeliac (CG) and superior mesenteric (sMG) ganglions that relay sympathetic innervation to the liver [32-34], pancreas [32,35], and adipose tissues [32,36]. There is also evidence showing that 5-HT immunoreactive axons are detectable within sympathetic nerves and could directly innervate sympathetic ganglions [37-43] to modulate sympathetic outflow [44,45]. In addition, the descending parasympathetic (efferent) transmission of the vagal nerve, which originates from the dorsal motor nucleus of the vagus (DMV) and innervates various organs, is also facilitated by 5-HT, an effect probably mediated by 5-HT3 receptors located on vagal nerve terminals [46,47]. The resulting stimulation of both descending sympathetic [48,49] and parasympathetic [50,51] pathways can modulate the activity of ENS 5-HT neurons, which would facilitate 5-HT release from EC cells [52] (Figure 3). In turn, circulating 5-HT may stimulate the sympathetic (spinal) and parasympathetic (vagal) sensory afferents, thereby forming a feedback loop within the brain-gut axis [53]. Furthermore, 5-HT neurons from the dorsal raphe nuclei (DRN) directly project to the nucleus of the tractus solitary (NTS) [54,55] in the medulla to further modulate the ascending parasympathetic (afferent) transmission of the vagal nerve coming from the liver [32,34], pancreas [32,35] and adipose tissues [56-59] (Figure 3). Interestingly, 5-HT producing neurons have been found within the sensory nodose ganglion (NG) of the vagus nerve [60,61] and the NTS [62], with a population of 5-HT immunoreactive neurons projecting from the nodose ganglion to the NTS [63,64], although it is unclear whether they release 5-HT in a physiologically-relevant manner [65]. This suggests that 5-HT innervation is found along both the sympathetic and parasympathetic systems to modulate the autonomic afferent and efferent signaling from and to various organs. Finally, some evidences have demonstrated sparse 5-HT innervation in liver, around the portal vein, portal artery, bile duct, and within hepatic lobules [66-68], suggesting that 5-HT regulates hepatic blood flow [69,70] but could also modulate hepatocellular functions.
Together, this highlights the intricated connections between central and peripheral 5-HT and supports the idea that a precise control of 5-HT levels is essential for liver physiology and related organs' metabolic functions. For example, traumatic brain injury and neurodegenerative diseases (Parkinson, Alzheimer) are associated with reduced peripheral 5-HT signaling [71-73], decreased lipogenesis, lipid accumulation in liver and adipose tissues, and alterations in the gut microbiota composition [71,74].
Expression of peripheral 5-HT receptor subtypes across metabolic and immune systems. The diagram illustrates the cell-specific expression of 5-HT receptors in key peripheral tissues involved in metabolic regulation: gut (enterocytes), liver (HC, hepatocytes; HSC, hepatic stellate cells; ChC, Cholangiocytes; KC Kupffer Cells), pancreas (α and β cells), white and brown adipose tissues, and in the immune system (B, B cells; T, T cells and Mϕ, macrophages. Multiple 5-HT receptor subtypes (e.g., 5-HT1A/1D/1F, 5-HT2A/2B, 5-HT3, 5-HT4, 5-HT7) are differentially expressed depending on the cell type, highlighting the widespread and pleiotropic roles of serotonin in peripheral physiology. Created with BioRender.com.

II. Central 5-HT Signaling and Liver Physiology
a. Regulation of liver physiology by central 5-HT
The main function of the liver is to regulate metabolism, detoxification, and energy homeostasis. Hepatic cytochrome P450 (CYP) monooxygenases play a key role in the metabolism of endogenous substances such as steroids, fatty acids, and neurotransmitters, as well as xenobiotics like drugs and toxins (for review see [75]). Beyond its metabolic and detoxifying functions, the liver is a major immune organ responding to exogenous antigens, metabolites, and molecular patterns. In obesity, sentinel cells such as liver-resident macrophages (Kupffer cells) and monocyte-derived macrophages sense the persistent accumulation of metabolites, antigens, and pattern molecules. This recognition shifts the liver from an immune-tolerant to an activated immune state, with reduced anti-inflammatory cytokines (TGF-β, IL-10) and increased pro-inflammatory cytokines (IL-1, IL-6, TNF-α). The resulting interplay between immune cells and hepatocytes sustains low-grade inflammation in MASH, while liver sinusoidal endothelial cells (LSECs), macrophages, innate lymphoid cells (ILCs), and neutrophils further contribute to disease progression and fibrosis (for review see [76]). A fine regulation of these hepatic functions must therefore be in place, in part through the nervous system, to coordinate appropriate responses.
The regulation of hepatic CYP enzymes is primarily controlled by endocrine signals, particularly growth hormone (GH), glucocorticoids (GR), thyroid hormones, and sexual hormones that act via nuclear receptors to modulate CYP gene expression [77]. The brain 5-HTergic system seems to play a significant role in the neuroendocrine regulation of hepatic CYP activity. Indeed, 5-HT projections from the DRN and median raphe nuclei (MRN) innervate the paraventricular (PVN) and arcuate (ARC) nuclei of the hypothalamus, key centers for hormone regulation [77,78]. Selective depletion of 5-HT innervation in the ARC or PVN by neurotoxic lesioning, which reduces 5-HT levels in these areas, produces opposite effects, with ARC-5-HT depletion decreasing, and PVN-5-HT depletion increasing CYP2C11 expression and function [79]. This data suggests that 5-HT exerts an opposite, region-specific effect on liver CYP enzymes, depending on the hypothalamic nucleus involved. Interestingly, depletion of central 5-HT in DRN and MRN by genetic ablation, neurotoxic lesioning or following a tryptophan-deficient diet, increases the liver expression and activity of CYP1A1/A2, CYP2C11, and CYP3A1 [80-82], suggesting that 5-HT acts predominantly as a negative regulator of these enzymes. In line with this, stimulation of brain 5-HT by chronic intracerebroventricular administration of its precursor 5-HTP decreases the expression and activity of liver CYP1A2, CYP2A2, CYP1B, CYP2C11, and CYP3A1/2 [78].
Central and peripheral sources of 5-HT in the regulation of metabolic functions. In the central nervous system, 5-HT is synthesized in the raphe nuclei (dorsal raphe, DR; median raphe, MR; rostral median raphe, rMR) by the tryptophan hydroxylase 2 (TPH2). 5-HTergic raphe (5-HT), noradrenergic locus coeruleus (LC), and subfornical organ (SFO) neurons are interconnected with the hypothalamic paraventricular nucleus (PVN) and the arcuate nucleus (ARC) to modulate autonomic sympathetic (rostral ventrolateral medulla, RVLM; Noradrenaline, NA) and parasympathetic preganglionic neurons (nucleus of the solitary tract, NTS; dorsal motor nucleus of the vagus, DMV; Acetylcholine, ACh) in the brainstem. RVLM and rMR neurons project through the spinal cord via the intermediolateral nucleus (IML) to the sympathetic coeliac (CG) and superior mesenteric ganglions (sMG), that innervate the different organs, including the liver, pancreas, adipose tissue, and gut. DMV neurons project to various parasympathetic ganglions, including hepatic ganglia (HG) via the descending vagal nerve. 5-HT can modulate the descending and ascending (afferents, sensory) sympathetic and parasympathetic branches via 5-HT receptors (5-HTR). In the gut, 5-HT is synthesized in EC) cells by the TPH1, which hydroxylates the tryptophan (TRP) coming from the lumen into 5-hydroxytryptophan (5-HTP), further converted to 5-HT. The gut is also innervated by the enteric nervous system (ENS) that expresses TPH2. Enterocytes express the serotonin transporter (SERT) so they can uptake and release 5-HT from and to the lumen or lamina propria. Once released, 5-HT can reach the blood where it is uptaken by the platelets that also express the SERT. Upon activation, platelets can release 5-HT to the organs. By influencing 5-HT synthesis and transport, the gut microbiota (MB) can modulate the levels of 5-HT produced by the gut. The pancreas expresses TPH1 and releases 5-HT together with insulin (Ins). Adipocytes also express TPH1 and synthetize 5-HT. Ins and 5-HT regulate lipolysis in the adipose tissues, which release lipid droplets that can accumulate in the liver. In the liver, only hepatic stellate cells (HSC) and cholangiocytes (ChC) produce 5-HT as they express the TPH1. Hepatocytes (HC) can store 5-HT as they express the SERT. Created with BioRender.com.

Central 5-HT modulation of hepatic cytochrome activity orchestrates the balance between liver regeneration and metabolic processes. Together with their interplay with the brainstem adrenergic locus coeruleus (LC), the 5-HTergic raphe nuclei (RN) regulate both the hypothalamic paraventricular nucleus (PVN) via 5-HT1A receptors to inhibit hepatic metabolism during liver regeneration, and the arcuate nucleus (ARC) via the 5-HT2C to stimulate hepatic metabolism in response to stress. 5-HT1A signaling in the PVN reduces, while 5-HT2C signaling in the ARC stimulates CYP1A1, CYP1A2, CYP2C11, CYP3A1, and CYP3A2 expression or activity. Created with BioRender.com.

Bidirectional 5-HTergic control of CYP function is likely mediated by 5-HT1A receptors in the PVN and 5-HT2C receptors in the ARC. Stimulation of 5-HT2C receptors (excitatory) in the ARC increases the activity and expression of CYP2C11, CYP3A1/23, and CYP3A2, and is associated with an increase in the pituitary growth hormone-releasing hormone (GHRH) and serum GH levels [83]. Conversely, stimulation of the 5-HT1A receptors (inhibitory) in the PVN suppresses GH secretion, leading to a reduction in the expression and activity of CYP2C11 and CYP3A1 in the liver [77]. This effect is attributed to 5-HT-mediated stimulation of somatostatin release in the hypothalamus, which inhibits GH secretion and downregulates GH-dependent CYP enzymes [78]. Together, these data suggest that 5-HT exerts an opposite and region-specific regulatory effect on liver CYP enzymes, depending on the hypothalamic nucleus involved.
Noradrenaline (NA) plays a complementary role in hepatic CYP regulation, with the locus coeruleus (LC, the major noradrenergic center) projecting to the PVN and ARC nuclei influencing GH and other pituitary hormones [84]. Lesioning of the LC, which reduces NAergic input, alters GH secretion and increases hepatic CYP2C11 expression [84]. These findings indicate that, like serotonin, norepinephrine modulates CYP activity through neuroendocrine mechanisms. Using a combination of anatomical tracing and “chemogenetics”, a recent report has identified a brain-to-liver neural circuit that inhibits liver regeneration following chronic stress. This pathway involves NA neurons in the locus coeruleus (LC) projecting to 5-HT neurons in the medullary raphe nucleus (mRN), which modulates sympathetic innervation to the liver. This circuit becomes hyperactive under chronic stress, leading to sustained NE release in the liver, which suppresses pro-inflammatory macrophage activation and inhibits hepatic regenerative processes [85]. These findings suggest that brain 5-HT signaling could mediate the inhibitory action of stress on hepatic regeneration. Interestingly, an upregulation of most CYP isozymes and hepatic metabolism is also observed following chronic exposure to psychological stress (for review see [86,87]), whereas CYP downregulation is observed during liver regeneration [88]. Taken together, this data suggests that central 5-HT (together with the interplay with central NE) could regulate the balance between the metabolic and the regenerative activity of the liver [89,90], by adapting liver function to physiological demands, stress, and injury [91,92]. Since transient hepatic lipid accumulation (steatosis) is shown to be essential for regeneration after hepatectomy, it supports the idea that central 5-HT signaling could modulate the regenerative process by controlling hepatic lipid metabolism [93] (Figure 4).
b. Role of central 5-HT in the regulation of metabolic hormones
Beyond its classical role in the brain, central 5-HT plays a pivotal role in systemic metabolic regulation, in part through its intricate interactions with a range of key metabolic hormones-including insulin, leptin, ghrelin, glucagon, glucagon-like peptide 1 (GLP-1), glucocorticoids, and thyroid hormones-which collectively influence liver function and energy homeostasis (Figure 5).
Insulin is the hormone secreted by pancreatic β-cells in response to high blood glucose levels. Once secreted by the pancreas in response to food intake, insulin stimulates the storage of glucose and lipid in the liver [94,95] and suppresses appetite by stimulating pro-opiomelanocortin neurons on the ARC. Insulin can further increase the firing activity of DRN 5-HT neurons by direct activation of insulin receptors located on 5-HT neurons [96]. In turn, chronic DRN neuron activation or chronic intranasal administration of 5-HT improves hepatic lipid metabolism, reduces liver steatosis, and ameliorates systemic (muscle) tolerance to glucose, and hepatic and systemic (muscle) sensitivity to insulin [93,97]. This therefore suggests a potential role of central 5-HT deficiency in insulin resistance and metabolic dysfunctions [98].
Modulatory role of central 5-HT on hypothalamic metabolic hormones, food intake and liver function. Within the hypothalamus, the arcuate nucleus (ARC) and the paraventricular nucleus (PVN) are interconnected to regulate food intake via the orexinergic pathway (light red) and satiety via the anorexinergic pathway (light blue). Pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) neurons release α-melanocyte-stimulating hormone (α-MSH), which activates melanocortin 4 receptors (MC4R) in the PVN to promote satiety and suppress food intake. In contrast, ARC orexinergic neurons release neuropeptide Y (NPY), agouti-related peptide (AgRP), and gamma-aminobutyric acid (GABA), which inhibit the anorexigenic effects of POMC neurons on the PVN, thereby suppressing satiety signals and promoting feeding. These effects are mediated through inhibitory GABA receptors (GABAR) and NPY receptors (Y1 and Y5) on POMC and PVN neurons. Food intake activates the DRN and MRN, leading to the release of 5-HT in the ARC and PVN. This serotonergic input inhibits feeding via activation of NPY-5-HT1B receptors and promotes satiety via stimulation of POMC-5-HT2C and PVN-5-HT2A,6 receptors. Conversely, in the absence of food, DRN/MRN activity is suppressed through 5-HT1A receptor signaling, leading to reduced 5-HT release in the ARC and PVN, thereby lifting the inhibition on feeding. Peripheral metabolic hormones also modulate these central circuits: insulin, leptin, and GLP-1 exert anorexigenic effects through their respective receptors (InsR, LepR, and GLP-1R) located on DRN/MRN, ARC, or PVN neurons. In contrast, ghrelin exerts orexigenic effects by stimulating its receptor (GHSR) on NPY neurons. This complex interplay between central serotonergic pathways, hypothalamic feeding circuits, and peripheral metabolic hormones enables fine-tuned regulation of feeding and satiety, while also contributing to the homeostatic control of liver function in response to nutritional states. Created with BioRender.com.

Leptin is a satiety hormone primarily produced by adipose tissues in response to increased lipid storage, which signals the hypothalamus to reduce appetite and to increase energy expenditure. Leptin can act directly or indirectly (by stimulating hypothalamic PVN [99]) on the liver to inhibit gluconeogenesis, stimulate glycogenolysis, increase insulin sensitivity, inhibit lipogenesis, reduce triglyceride secretion, and stimulate fatty acid oxidation [100-103]. By preventing lipid accumulation in the liver, leptin is considered an anti-steatotic hormone [100,101,104] although exerting a pro-fibrotic action [105]. Hence, leptin resistance may occur rapidly and be exacerbated in MASLD [100,106]. DRN 5-HT neurons projecting to the ARC express the leptin receptor and the resulting activation of DRN-ARC by leptin inhibits feeding behaviors [107]. Conversely, DR 5-HT depletion prevents the effect of leptin on the reduction of food intake [108].
Ghrelin is an appetite stimulating hormone released by the empty stomach that acts through its receptor, growth hormone secretagogue receptor (GHSR), on liver and PVN neurons to promote food intake, stimulate hepatic glucose production, and inhibit insulin signals [109]. Whether ghrelin stimulates [110,111] or inhibits [112,113] 5-HT neurons activity is not clearly established yet. However, it is likely that ghrelin and 5-HT have opposite effects on food intake. Hence, intra-PVN injections of 5-HT antagonize the orexinergic effect of ghrelin [114], probably via activation of 5-HT2C receptors on pro-opiomelanocortin (POMC) neurons [115]. Conversely, inhibition of MRN activity by the stimulation of 5-HT1A receptors potentiates the orexinergic effect of ghrelin injection into the PVN [116]. This suggests that 5-HT and ghrelin could exert an opposite action on liver metabolic functions.
Glucocorticoids (GC) are secreted by the adrenal cortex and delivered to the liver via the bloodstream. Through activation of the glucocorticoid receptor (GR), GC influence many important liver functions such as gluconeogenesis, glucose uptake and utilization [117], de novo lipogenesis, lipid export, fatty-acid oxidation [118], and insulin sensitivity [119]. Thus, it has been suggested that chronic alterations in GC/GR signaling could lead to MASLD [120]. The role of 5-HT as an activator of the hypothalamus-pituitary-adrenal axis (HPA) and the subsequent release of adrenal GC is well established [121,122]. As part of a negative feedback loop, GC reduces 5-HT release in the PVN [123]. This suggests that dysregulation of the 5-HT/GC loop could be associated with alterations in liver functions.
Glucagon is a hormone released by pancreatic α-cells in response to low blood glucose levels to mobilize energy. By acting directly on the liver, glucagon promotes hepatic glucose production through stimulation of glycogenolysis and gluconeogenesis, while inhibiting glycolysis [124]. It also reduces hepatic lipid accumulation and secretion, adiposity, and body weight [125-128]. Glucagon may therefore reduce liver steatosis, and glucagon receptor agonists are currently being developed as a therapeutic strategy for MASH/MASLD [129]. The release of glucagon by the pancreas seems to be controlled by glucose-sensing neurons in the hypothalamus and the brainstem through the autonomic nervous system (ANS) [130,131]. In turn, the glucagon released by the pancreas reaches the brain to regulate hypothalamus and brainstem functions to lower hepatic glucose production and decreases food intake [132-134]. Although the interconnection between glucagon and central 5-HT has not been established yet, it could occur via their respective signaling in the hypothalamus and/or the brainstem. Locally in the liver, both synergic and opposite cross-talks exist between glucagon and 5-HT receptors: 5-HT2B and 5-HT7 receptors potentiate glucagon's hyperglycemic action by promoting gluconeogenesis, whereas 5-HT1A and 5-HT2A receptors antagonize glucagon's hyperglycemic action by stimulating glycogenesis.
Glucagon-like peptide 1 (GLP-1) is an incretin hormone produced from the same precursor as glucagon (proglucagon) by the intestine and the nucleus of the solitary tract (NTS) in the brainstem, in response to glucose and lipid. Intestinal GLP1 can either be secreted to reach hypothalamus via the blood circulation [135], act locally to stimulate gut 5-HT release [136] or activate the enteric nervous system that projects to NTS preproglucagon neurons which releases GLP1 in the hypothalamus. This combined effect modulates the autonomic nervous system to stimulate insulin secretion, which lowers hepatic glucose production [137], enhances hepatic glycogen storage [138], negatively regulates food intake, and reduces body weight [139]. Therefore, GLP1 receptor agonists have been proposed in the treatment of MASLD [140-144]. NTS GLP1 neuron activity is modulated by central 5-HT via 5-HT1A and 5-HT2C receptors [145]. Interestingly, the hypophagic effect of 5-HT2C receptor agonists is mediated by NTS GLP1 neurons [146]. In turn, GLP1 acts in the DRN to modulate central 5-HT release and reduce appetite and body weight [147,148].
Insulin-like growth factor 1 (IGF-1), is a hormone produced by the liver, with a molecular structure similar to insulin, that plays important roles in the development and growth during childhood, and has, to a lesser extent, metabolic effects such as stimulation of glucose uptake and lipid metabolism, and reduction of blood glucose in adults. In the liver, IGF-1 likely reduces steatosis, fibrosis, and the overall severity of MASLD [149]. 5-HT and IGF-1 bidirectionally interact within the brain, particularly in regions such as the hypothalamus, including the ARC and PVN. On one hand, 5-HT positively regulates IGF-1 by stimulating hypothalamic GHRH, which promotes growth hormone (GH) secretion from the anterior pituitary, thereby enhancing hepatic IGF-1 synthesis [150]. On the other hand, IGF-1 influences 5-HT neurotransmission, by stimulating serotonergic input from the DRN to the hypothalamus, particularly in the PVN and ARC, potentially influencing appetite, energy balance, and stress responses [151].
T3 and T4 thyroid hormones (TH) release from the thyroid gland is controlled by the thyroid stimulating hormone (TSH) secreted by the pituitary, itself under the control of the thyrotropin-releasing hormone (TRH) produced by the PVN. T3 and T4 exert negative feedback on both TSH and TRH secretion. By acting directly on the liver and indirectly via the PVN, TH regulates lipid and glucose homeostasis, through cholesterol modulation and fatty acid synthesis, increased lipolysis, lipid droplets formation, free fatty acid uptake, glucose production, and reduced insulin sensitivity [152]. In turn, T3 suppresses both hypothalamic 5-HT activity and TSH secretion, indicating direct negative feedback at the hypothalamic level and suggesting that the 5-HT/thyroid interplay regulates liver physiology.
c. Role of the autonomic nervous system in the regulation of liver functions and diseases: potential modulation by 5-HT
The autonomic nervous system, comprising the sympathetic and parasympathetic branches, serves as a crucial interface between the brain and peripheral organs, orchestrating the regulation of systemic metabolism through dynamic neural control of energy balance, inflammation, and organ-specific functions.
The sympathetic nervous system (SNS) plays a pivotal role in hepatic lipid and glucose metabolism, especially under metabolic stress conditions. High-fat diet challenge, liver steatosis, aging, and metabolic syndrome are associated with altered liver sympathetic tone, leading to changes in intrahepatic noradrenaline (NA) signaling [153-165]. This adrenergic signaling upregulates fatty acid transporters (CD36) and lipogenic enzymes (DGAT1 and DGAT2) [166], which accelerates hepatic triglyceride accumulation and steatosis [153]. Notably, liver sympathetic denervation is shown to reverse obesity-induced hepatic steatosis, supporting the role of the SNS in lipid accumulation [166,167]. Beyond lipid metabolism, NA also exerts significant control over glucose handling in the liver. It stimulates gluconeogenic pathways through β2-adrenergic receptor-mediated activation of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase, while suppressing glycogenesis [168,169]. These effects promote hepatic glucose output, contributing to hyperglycemia and the development of liver insulin resistance, which in turn exacerbates de novo lipogenesis and steatosis [170,171]. In addition, the SNS negatively regulates liver regeneration by preventing hepatocyte priming and the entry into the cell cycle after partial hepatectomy [172].
In parallel, sympathetic input enhances the pro-inflammatory state by promoting Kupffer cell cytokine production, particularly tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), thereby pushing the progression from steatosis to steatohepatitis [173,174]. Furthermore, the SNS directly contributes to liver fibrogenesis. NA and neuropeptide Y (NPY) activate HSC through α- and β-adrenergic receptors, triggering PI3K/MAPK pathways that drive HSC proliferation, α-smooth muscle actin (α-SMA) expression, and extracellular matrix synthesis [175-177]. Clinically, elevated plasma NA and increased sympathetic tone are observed in cirrhotic patients, correlating with portal hypertension and fibrosis severity [178]. However, sympathetic integrity also appears essential for adequate liver repair and regeneration, with loss of hepatic sympathetic fibers being associated with metabolic dysfunction and impaired regenerative capacity [176,177,179,180].
Conversely, the parasympathetic nervous system (PNS), via the vagus nerve, plays a largely protective role in liver inflammation and metabolism [181,182]. Acetylcholine (ACh) released from vagal efferents binds to α7-nicotinic ACh receptors (α7nAChRs) on Kupffer cells and hepatocytes, suppressing NF-κB activation and downstream transcription of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [183-185]. This cholinergic anti-inflammatory effect has been shown to reduce steatohepatitis in multiple murine models [182]. Pharmacological enhancement of vagal tone using galantamine, an inhibitor of the ACh degradation enzyme (acetylcholine esterase, AChE), reduces hepatic inflammation, steatosis, and systemic insulin resistance [186,187]. Conversely, selective hepatic vagotomy results in exacerbation of steatohepatitis, with increased inflammatory cytokine expression, and a concomitant decrease in peroxisome proliferator-activated receptor alpha (PPARα) activity leading to the aggravation of liver steatosis [185]. As a result, the PNS enhances liver insulin sensitivity and suppresses hepatic glucose output, serving as a metabolic counter-regulator to the SNS [170,188].
There is also some evidence supporting a fibrogenic role of parasympathetic innervation. For instance, parasympathetic modulation in vitro appears pro-fibrogenic with ACh promoting HSC proliferation and collagen I transcription via muscarinic M2 receptors [189,190]. Hence, hepatic vagotomy reduces HSC proliferation [191] and fibrogenesis [192] in diet- and toxin-induced mouse models of liver fibrosis. Nevertheless, parasympathetic signals are implicated in liver regeneration, particularly through vagal efferents that modulate hepatocyte proliferation and liver progenitor activation [193,194].
Collectively, these findings highlight a vicious cycle wherein sympathetic overactivation promotes hepatic steatosis, insulin resistance, inflammation, and fibrosis, while parasympathetic activity mitigates these processes and supports regenerative outcomes. Disruption of this autonomic balance, promoting sympathetic tone and vagal stillness, may increase the risk of the onset and progression of chronic liver diseases [195,196]. Interestingly, serotonin (5-HT) appears to mirror and potentially amplify the pathological actions of the sympathetic nervous system on liver metabolism and inflammation. Similar to noradrenaline, 5-HT exacerbates insulin resistance by stimulating hepatic glucose output and impairing insulin signaling [197], enhances liver steatosis via 5-HT2A receptors on hepatocytes [198,199], and fibrogenesis (upregulation of α-SMA, TGF-β1, and extracellular matrix components) through activation of 5-HT2A and 5-HT2B receptors on hepatic stellate cells [200]. In addition, 5-HT has emerged as a key modulator of liver regeneration, particularly via platelet-derived 5-HT which primes hepatocytes for cell cycle entry and regeneration following partial hepatectomy [201]. 5-HT may also directly modulate autonomic nerve terminal activity within the liver since a large body of evidence demonstrates that 5-HT influences autonomic efferent activity by acting on various prejunctional 5-HT receptors, to either inhibit or facilitate autonomic neurotransmitter release, thereby affecting processes like heart rate, blood pressure, and gastrointestinal motility (for review see [202]). Although such mechanisms remain unexplored in the liver, the dense autonomic innervation and the presence of multiple 5-HT receptor subtypes in liver raise the hypothesis that 5-HT could shape its function not only via hepatocytes or immune targets but also by modulating the activity of sympathetic and parasympathetic fibers. This putative serotonergic-autonomic interface may constitute a critical amplification loop.
Recent findings also highlight the pivotal role of brain-derived neurotrophic factor (BDNF) and its receptor TrkB in the maintenance of hepatic autonomic innervation, overall liver homeostasis, and insulin sensitivity [203-205]. In humans and rodents, BDNF deficiency is associated with increased appetite and weight gain, higher glucose levels, and elevated risk of liver steatosis and steatohepatitis [206-209]. In models of metabolic stress, such as HFD-induced MASH, liver tissue undergoes selective sympathetic neuropathy, driven by TNF-α-induced axonal degeneration [171]. This neuropathic loss impairs noradrenergic tone and disrupts sympathetic regulation of hepatocyte function, contributing to metabolic imbalance and liver inflammation [171]. On the other hand, BDNF levels are elevated in fibrotic liver tissues and appear to participate in both neuronal and fibrogenic signaling [210]. Notably, by acting on its TrkB receptor, BDNF can activate hepatic stellate cells and the upregulation of pro-fibrogenic markers [210]. In the meantime, it also plays a protective neurotrophic role via TGF-β/SMAD signaling [211], which may be essential for preserving liver innervation [212]. Loss of neurotrophic support may therefore not only facilitate inflammation and fibrosis but also hinder the neuronal plasticity and reinnervation necessary for effective liver regeneration.
5-HTergic signaling and BDNF may also cooperate in regulating hepatic autonomic nerve plasticity and/or degeneration, as observed in the brain [213,214]. Therefore, the progressive loss of autonomic nerve fibers observed in chronic liver disease may not only reflect neurodegeneration but also a failure of trophic and regenerative support, with 5-HT-BDNF co-signaling representing a key modulator in this process.
III. Role of Peripheral Serotonin in Metabolic and Liver Diseases
a. Obesity
Obesity is the most prevalent metabolic disease worldwide, affecting over 1 billion children and adults, approximately 13% of the global population and its prevalence continues to rise [215]. It is a major driver of a wide range of metabolic disorders, including type 2 diabetes, cardiovascular disease, and MASLD. Peripheral 5-HT is an important pathogenic contributor to obesity and associated dysglycemia [216] and dyslipidemia [217]. Human obesity is characterized by increased production and release of gut-derived 5-HT, which is strongly linked to abnormal glycemic control, altered lipid levels, and higher body mass [218]. Similarly, increased 5-HT levels and TPH1 activity are consistently observed in animal models of obesity [28,219-224].
In mice fed a high-fat diet (HFD), the pharmacological blockade of 5-HT synthesis with TPH inhibitors, either parachlorophenylalanine (PCPA) or LP-533401, decreases body weight gain, improves glucose tolerance, and lowers adiposity [219,225]. These effects are likely mediated by peripheral TPH1 because opposite effects (weight and body fat gain) are observed when inhibiting TPH2 by intracerebroventricular injections of PCPA [226]. The link between 5-HT synthesis and obesity has been confirmed genetically. Mice depleted for TPH1 (Tph1-/-) are protected from HFD-induced obesity and related metabolic dysfunctions, showing less weight gain, lower adiposity, reduced insulin resistance, and liver steatosis compared to control mice [227]. This work suggests that 5-HT-mediated obesity and metabolic dysfunction could be linked to local 5-HT synthesis by adipose tissues (AT). In mice fed an obesogenic diet, TPH1 expression and tissue 5-HT levels are augmented in white (W) and brown (B) AT [219,227]. Selective ablation of TPH1 in adipose tissues reduces weight gain and improves glucose tolerance and insulin sensitivity after HFD [28,219,223]. Reduced adiposity in epididymal and inguinal WAT and increased energy expenditure by BAT are also observed, similarly to PCPA-treated mice [219]. This suggests that adipocyte-derived 5-HT promotes energy storage in WAT while inhibiting energy expenditure in BAT.
The effect of 5-HT on adipose tissues is likely mediated by various 5-HT receptors, including 5-HT3, 5-HT2A, and 5-HT2B receptors. Mice genetically depleted for 5-HT3 receptors show reduced body gain, improved glucose tolerance, and increased BAT activity upon HFD challenge, suggesting a potential role of 5-HT3 receptors in thermogenesis [219]. Increased mRNA expression of 5-HT2A and 5-HT2B receptors is observed in the visceral adipose tissue (VAT) of human patients and WAT of mice (leptin deficient ob/ob, HFD) suffering of obesity [228], suggesting that alterations in the expression of these receptors contribute to AT expansion. Indeed, mice with selective knockout of 5-HT2A receptors in adipose tissues have reduced de novo lipogenesis in WAT, improved glucose tolerance, and are resistant to HFD-induced obesity [228]. On the other hand, 5-HT2B receptors seem involved in fatty acids lipolysis and free fatty acid (FFA) release [197]. Pharmacological inhibition or selective deletion of 5-HT2B receptors in adipose tissues reduces HFD-induced AT lipolysis, lowers the subsequent release of FFA in the blood circulation, and improves insulin sensitivity and glucose tolerance [197,228]. In addition, 5-HT2B receptor activation leads to reduced energy expenditure in BAT by suppressing the uncoupling protein 1 (UCP1), which promotes energy storage and weight gain [229].
Together, these data suggest that elevated levels of peripheral 5-HT might facilitate the development of metabolic dysfunction and obesity. In line with this, patients treated with selective serotonin reuptake inhibitor (SSRI) antidepressants, medications that increase 5-HT availability by blocking its reuptake through the serotonin transporter (SERT), show reduced BAT thermogenesis [229], which could contribute to SSRI-induced weight gain and metabolic dysfunction, although some of these effects may occur independently of 5-HT signaling [230]. Similarly, mice genetically deleted for the SERT (Sert-/-) exhibit metabolic dysfunctions such as glucose intolerance, insulin resistance, and obesity [231-233]. Overall, these data support the involvement of 5-HT in the regulation of energy expenditure [234,235] and suggest that targeting peripheral 5-HT may constitute a therapeutic strategy for the treatment of obesity and associated metabolic dysfunctions.
List of hepatic cytochromes regulated by central 5-HT signaling, their function, and the links to liver physiology, metabolism dysregulation, and liver diseases.
| Name | Functions | Link with chronic liver diseases | Ref |
|---|---|---|---|
| CYP1A1 | -generation of ROS -cholesterol, arachidonic acid and glucose metabolism -immune response | -lipid deposition, cholesterol accumulation, fatty liver -steatohepatitis, neutrophil infiltration -proliferation and differentiation of HSC -fibrosis -HCC | [381-385] |
| CYP1A2 | -generation of ROS -cholesterol metabolism and lipid peroxidation | -metabolic dysfunction -neutrophil infiltration -cirrhosis -HCC | [383,386] |
| CYP1B | -steroid hormone -lipid, glucose, and vitamin D metabolism -fat synthesis | - insulin sensitivity | [387] |
| CYP2A | -steroid metabolism | -hepatitis -cirrhosis | [388] |
| CYP2C11 | -steroid hormone, vitamin D, antidepressants, and antipsychotics metabolism | - liver injury - alcohol-related liver disease - fibrosis | [389-391] |
| CYP2D6 | -immune functions -promotes 5-HT synthesis | -autoimmune liver diseases | [392] |
| CYP3A | -bile acid metabolism -inflammatory cytokine release | -fibrosis -cirrhosis | [393] |
b. Diabetes
Diabetes mellitus refers to a group of metabolic disorders characterized by impaired glucose homeostasis. Type I (insulin-dependent) and Type II (insulin-resistant) diabetes are among the most clinically significant metabolic diseases, with Type II accounting for most cases [236]. Accumulating evidence implicates both central and peripheral 5-HT signaling in the regulation of insulin signaling and glucose homeostasis, with dysregulations of 5-HT levels being associated with impaired insulin function and diabetes development [237].
Postprandial insulin secretion inhibits the breakdown of stored fat into FFA (lipolysis) and, instead, promotes the synthesis of fatty acids and their storage as triglycerides in fat cells (lipogenesis). Importantly, β-cells also express TPH1 (and TPH2) [238,239], produce 5-HT, and store 5-HT in the same vesicles as insulin [240]. Upon glucose stimulation, 5-HT is co-released with insulin and acts in an autocrine and paracrine manner to potentiate insulin secretion-primarily via 5-HT2B and 5-HT3 receptors on β-cells [27,241-243], and 5-HT1F receptors on α-cells to suppress glucagon [240]. In addition, 5-HT can directly promote insulin granule exocytosis via receptor-independent serotonylation [244], a post-translational modification mediated by transglutaminase 2 (TGM2), which covalently links 5-HT to target proteins such as Rab GTPases [245]. This could alter cytoskeletal dynamics and intracellular signaling and enhance cellular motility and proliferation.
It is well established that patients with diabetes, both type I and II, exhibit elevated levels of plasma 5-HT, which correlate with their increased blood glucose levels [246-250]. This elevation is accompanied by a decrease in platelet 5-HT storage [249] due to reduced basal uptake and enhanced spontaneous release of 5-HT [247,249]. Rodent models such as streptozotocin-induced β-cell depletion mirror these findings, with elevated plasma [251] and gut [252,253] 5-HT levels, increased density of enteric 5-HTergic neurons [254,255], and restoration of these changes following insulin treatment. In contrast, central 5-HT signaling is generally reduced in diabetic rodents, with decreased levels of 5-HT and TPH in the brainstem, lower density of 5-HT neurons, downregulated SERT, altered 5-HT1A and 5-HT2 receptor expression [256-258], and diminished 5-HT release in the hypothalamus and cortex [257,259]-most of which are also corrected by insulin therapy.
A key regulator of postprandial insulin secretion is GLP-1, an incretin hormone secreted by enteroendocrine L-cells in response to nutrient intake. GLP-1 acts on GLP-1 receptors (GLP-1R) expressed on pancreatic β-cells to enhance insulin release and suppress glucagon effects. GLP-1R is the target of widely used GLP-1 receptor agonists for the management of obesity, type 2 diabetes and, in the near future, MASLD [144]. GLP-1 agonists also increase TPH expression in β-cells and stimulate 5-HT synthesis and its co-release with insulin [239]. In the gut, GLP-1R is highly expressed in EC cells, where its activation robustly stimulates 5-HT secretion, establishing GLP-1 as an upstream regulator of 5-HT signaling [136]. Conversely, 5-HT itself, through stimulation of 5-HT4 receptors on enteric neurons and L-cells, enhances GLP-1 release, forming a bidirectional feedforward loop [260]. Moreover, stimulation of 5-HT4 receptors improves intestinal barrier integrity and glucose tolerance in models of Type 1 diabetes [261] and HFD-induced metabolic dysfunction [262], respectively, paralleling GLP-1's protective effects [263].
GLP-2, co-secreted with GLP-1, complements its effects by maintaining mucosal integrity, promoting intestinal growth, and enhancing nutrient absorption through actions on GLP-2R, which are expressed on EC 5-HT cells and 5-HT-sensitive enteric neurons [264,265], further linking GLP-2 activity to 5-HTergic modulation of gut motility and epithelial function. GLP-2 thereby indirectly influences 5-HT availability and function, potentially enhancing both GLP-1 and 5-HT secretion. Meanwhile, 5-HT4 receptors, expressed on epithelial and neuronal compartments, mediate mucosal repair, anti-inflammatory effects, and promote peristalsis, functions that both overlap with and potentiate GLP-2's reparative actions.
This network of signaling reveals a tightly integrated endocrine and paracrine system wherein GLP-1, GLP-2, and 5-HT mutually regulate each other's release and downstream effects through synergistic receptor pathways. Disruption of any component can impair insulin secretion, contributing to the pathophysiology of diabetes. Collectively, these findings highlight a complex and bidirectional relationship between 5-HT and insulin in the regulation of glycemic levels and the pathophysiology of diabetes.
c. Metabolic dysfunction-associated steatotic liver diseases
MASLD is the most common chronic liver disease worldwide, affecting 34-38% of adults in the global population. Associated with obesity, insulin resistance, and other features of metabolic syndrome, MASLD represents a growing public health challenge due to the potential progression of liver steatosis to steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma [266]. MASLD is characterized by dysregulations of nutrient metabolism, notably lipids and carbohydrates, resulting from a combination of excessive dietary intake and impaired hepatic metabolic functions [76,144,267-273].
The presence of nutrients in the stomach stimulates 5-HT synthesis by TPH1 within the gastrointestinal tract, leading to elevated platelets levels of 5-HT in the bloodstream. The liver, being the first organ to receive blood from the gut via the portal vein, is exposed to these increased platelets 5-HT concentrations, at levels high enough to exert systemic, hormone-like effects on liver functions. Patients with obesity have increased basal TPH1 activity in the gut, resulting in a two-fold increase in plasma levels of 5-HT potentially reaching the liver [218]. Consistently, in mice, genetic deletion of the serotonin transporter (SERT), which increases extracellular 5-HT levels, results in weight gain and liver steatosis [233].
Obesity associated with HFD induces liver steatosis and inflammation, increases platelet number and aggregation in the liver sinusoids [274], and increases TPH1 activity in the gut (duodenum, jejunum, and colon), which augments 5-HT release from the gut [222] and platelet-derived 5-HT to the hepatic portal vein [198]. This elevation in TPH1 activity, as well as the subsequent elevation in 5-HT reaching liver, is likely implicated in the pathogenesis of metabolic liver diseases, as pharmacological inhibition of TPH1 reduces weight gain and adiposity, improves insulin sensitivity, and ameliorates liver steatosis in mice fed a HFD [199,219,224,225,275,276]. Since several peripheral organs express TPH1 as mentioned above, the potential therapeutic effect of TPH1 inhibitors could thus result from combined effects on these organs.
Selective depletion of gut-derived 5-HT by genetic ablation of gut TPH1 (villin-cre: tph1fl/fl) improves HFD-induced liver steatosis and triglyceride accumulation, but does not affect other systemic energy metabolism features, such as body weight, plasma cholesterol and triglycerides, glucose tolerance, and adiposity, suggesting that gut-derived 5-HT is specifically involved in liver lipogenesis [198]. This effect is likely mediated by 5-HT2A receptors located on hepatocytes, as their selective ablation (5-HT2A cKO, Albumin-Cre: Htr2afl/fl) protects against HFD-induced steatosis independently of systemic energy homeostasis [198]. Not only is steatosis diminished, but the inflammatory and fibrogenic responses are abolished in 5-HT2A-cKO mice upon HFD challenge, suggesting that 5-HT2A antagonists could represent a treatment strategy for steatohepatitis and liver fibrosis [198,277,278]. Similarly, pharmacologic blockade of 5-HT4 receptors (GR113808) prevents weight gain, insulin resistance, hepatic steatosis and inflammation induced by HFD in mice [279].
On the other hand, hepatocytes express 5-HT2B receptors that do not seem to participate in liver steatosis development as no changes were observed in mice selectively ablated for 5-HT2B receptors in hepatocytes (Albumin-Cre: Htr2bfl/fl) [198]. Instead, hepatocytic 5-HT2B receptors likely regulate glucose homeostasis by promoting gluconeogenesis through the stimulation of the two rate-limiting gluconeogenic enzymes: fructose-1,6-bisphosphatase and the glucose-6-phosphatase, and by inhibiting glucose uptake [197]. In addition, 5-HT2B receptors expressed in HSC, exert an inhibitory effect on liver regeneration by stimulating TGFβ expression, which also stimulates fibrogenesis [200]. Interestingly, 5-HT2B receptor expression in HSC is upregulated in fibrotic liver, suggesting its important role in the fibrogenesis process [200,280]. In line with this, pharmacological inhibition or genetic deletion (Htr2b-/-) of 5-HT2B receptors reduces liver fibrosis, promotes hepatocyte growth, and improves liver function in a mouse model of liver fibrosis (carbon tetrachloride (CCl4)-induced liver fibrosis mouse models) [200]. Together, these data suggest that antagonizing 5-HT2A receptors in hepatocytes and/or 5-HT2B receptors in HSC could dampen the MASLD progression. A similar protection against HFD-induced hepatic steatosis was also observed in mice ablated for TPH1 or 5-HT2B in adipocytes (adiponectin-cre; Tph1fl/fl or Htr2bfl/fl) or mice genetically deleted for the 5-HT3a receptor (Htr3a-/-) [225,228].
5-HT could also exert its pathogenic effects indirectly, via 5-HT2A receptor mediated upregulation of its degradation enzyme [281-284], the monoamine oxidase (MAO), located on mitochondria. Being the most exposed organ to gut-derived 5-HT, the liver is also the primary site of 5-HT metabolism with a high expressing of MAO [285]. Higher 5-HT degradation may lead to overproduction of reactive oxygen species (ROS) and formation of free radicals, which could contribute to increased oxidative stress, causing lipid peroxidation, cellular damages, and liver inflammation [281-284]. By stimulating the phosphorylation of c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (p38 MAPK), signal transducer and activator of transcription 3 (STAT3) and NF-κB, the 5-HT degradation system can upregulate pro-apoptotic factors such as Bcl-2 Associated X-protein (Bax), cleaved-caspase 3 and cleaved-caspase 9, and downregulate anti-apoptotic factors like Bcl-2, which ultimately results in apoptosis, secretion of TNF-α and IL-1β, and the progression of liver diseases [281,282]. Blocking 5-HT2A receptors with the selective antagonist sarpogrelate has been shown to prevent MAO upregulation and reduce the resulting increase ROS byproducts, which further prevent the JNK/p38 MAPK/STAT3 cascade activation, and reduced inflammation and apoptosis, thereby preventing carbon tetrachloride (CCl4)-induced hepatotoxicity in vitro and in vivo [281,282].
The progression of liver fibrosis is closely linked to both portal fibrosis and angiogenesis, two interconnected processes that result from the activation of portal myofibroblasts. These cells originate from various sources, including resident portal fibroblasts, perivascular mesenchymal cells, and potentially cholangiocytes undergoing transdifferentiation. In response to chronic injury and inflammation, they accumulate within the portal regions, where they drive extracellular matrix deposition and contribute to the formation of fibrotic septa that eventually bridge neighboring portal tracts. In the meantime, activated portal myofibroblasts release pro-angiogenic signals such as VEGF, PDGF, and Angiopoietin-2, promoting the growth of new capillary networks within the expanding fibrotic tissue. This close relationship between fibrogenesis and angiogenesis creates a self-reinforcing cycle that not only sustains the progression of fibrosis but also alters hepatic microcirculation by diverting blood flow and exacerbating portal hypertension, which may further amplify the fibrogenic response [286]. 5-HT may not only modulate portal hypertension through the activity of 5-HT1A and 5-HT2A receptors [69,70,287,288], but could also regulate angiogenesis by modulating endothelial cells function through VEGF-dependent or -independent manners, involving various 5-HT receptors such as 5-HT1B, 5-HT2A, 5-HT4 or 5-HT7 [289-293].
Both alterations in blood and bile flow can also promote liver disease, notably through the development of cholestasis, a condition characterized by the intrahepatic accumulation of bile acids, which can induce hepatocellular injury, inflammation and fibrosis. In response to sustained biliary stress, the liver undergoes bile duct remodeling, characterized by ductular reaction, proliferation of cholangiocytes (the epithelial cells lining the bile duct), and periductal fibrosis, which further perpetuate portal inflammation and fibrosis. Cholangiocytes express both TPH1 and TPH2, enabling local production of 5-HT [25,26]. This locally produced 5-HT acts in an autocrine fashion to inhibit cholangiocyte proliferation via the activation of 5-HT1A receptors [26]. In rodent models of cholestasis induced by bile duct ligation (BDL), TPH1 & 2 expression is upregulated while MAO expression is downregulated, leading to increased intra-biliary 5-HT level [26]. Genetic ablation of TPH2 results in an ~80% reduction in biliary 5-HT, promoting excessive proliferation of cholangiocytes and hepatocytes, expansion of immature ductules, and increased recruitment of liver progenitor cells. This aberrant proliferative response promotes extensive ductular remodeling and exacerbates liver fibrosis [26]. 5-HT secreted by cholangiocytes also acts in a paracrine manner on HSC to stimulate their trans differentiation into myofibroblasts and augment TGF-β1 secretion. In turn, TGF-β1 suppresses TPH2 expression in cholangiocytes, relieving the inhibitory effect of 5-HT on ductular proliferation and reinforcing a feedforward loop that perpetuates both fibrogenesis and ductular expansion [26]. BDL also upregulates 5-HT2A, 2B, 2C receptors expression in both cholangiocytes and HSC [25]. Stimulation of these receptors by 5-HT further enhances bile duct mass, collagen deposition, proinflammatory cytokine production, and secretion of senescence-associated secretory phenotypes (SASPs), whereas their antagonism significantly reduces these responses and attenuates liver fibrosis [25].
Together, these findings highlight the complex role of 5-HT signaling in regulating hepatic metabolism, inflammation, cholangiocyte proliferation, biliary remodeling, and fibrogenesis. Through distinct receptor pathways and tissue-specific effects, 5-HT therefore acts as a pivotal modulator in the progression of MASLD (and cholestatic liver diseases).
d. Alcohol associated liver diseases
While the role of central 5-HT signaling in alcohol use disorders and associated mood dysregulations have been extensively investigated, showing that chronic alcohol consumption enhances brain 5-HT innervation and signaling, with 5-HT1A receptors playing a key role in binge-alcohol drinking behavior [294-301], little is known regarding the potential role of peripheral 5-HT signaling in ALD.
Nevertheless, elevated blood and urine levels of 5-HT have been reported in patients with severe alcohol-associated cirrhosis and in corresponding animal models [302-304], likely resulting from elevated expression of intestinal TPH1 [304] and reduced storage of 5-HT in blood platelets [305-307]. Notably, mice genetically deleted for intestinal TPH1 are protected against alcohol-induced liver steatosis, inflammation, and alterations in lipogenesis pathways, probably by preventing neutrophil mobilization into the liver [308] and nuclear translocation of Sterol Regulatory Element-Binding Protein 1 (SREBP1) [304]. Similarly, hepatocyte-specific knockout of 5-HT2A receptors confers protection against alcohol-associated steatosis, and SREBP1 activation, thereby attenuating liver endoplasmic reticulum stress and inflammation [304]. Together, these findings suggest that intestinal 5-HT, acting through 5-HT2A receptors, contributes, at least in part, to the early stages of ALD. However, further studies are warranted to clarify the respective role of intestinal and liver-secreted 5-HT in the progression towards more advanced stages of the liver disease.
e. Hepatocellular carcinoma
5-HT likely exerts a potent pro-tumorigenic factor in HCC, influencing both disease progression and prognosis through multiple intertwined mechanisms. Elevated systemic and intraplatelet levels of 5-HT have been consistently reported in patients with HCC compared to cirrhotic controls, with plasma 5-HT concentrations emerging as a potentially sensitive and non-invasive biomarker for early-stage HCC detection across various underlying etiologies [8,309,310]. Moreover, elevated levels of 5-HT storage in platelets have been associated with increased risk of tumor recurrence, early relapse, and poor overall survival, suggesting that platelet-derived 5-HT acts as both a systemic marker and a local driver of tumorigenesis [311-316]. Mechanistically, 5-HT promotes hepatic cancer cell proliferation, invasion, and metastasis primarily via receptor-mediated signaling, notably through the 5-HT2B receptor, which is highly upregulated in HCC tissues [317-319]. Activation of 5-HT2B by 5-HT enhances the expression and nuclear activity of YAP (Yes-associated protein), a central effector of the Hippo pathway, thereby driving tumor growth through downstream ERK and YAP phosphorylation [319]. Interestingly, high levels of both intraplatelet 5-HT and YAP are independently associated with poor clinical outcomes, reinforcing the functional significance of the 5-HT-YAP axis in HCC pathogenesis [315,316].
Beyond 5-HT2B, HCC tissues show a distinct remodeling of 5-HT receptor expression. Upregulation of 5-HT1B, 5-HT1D, and 5-HT7 receptors, and downregulation of 5-HT2A and 5-HT5 receptors have also been observed in tumors [317,318]. Antagonism at 5-HT7 receptors significantly reduces tumor size in vivo, suggesting a proliferative or survival role of this receptor in liver malignancy [318]. 5-HT1B and 5-HT1D, similarly overexpressed in HCC, have been involved in the regulation of epithelial-mesenchymal transition and cell invasiveness in pancreatic cancer, pointing to a potential role in HCC progression, yet their exact contribution remains unclear in liver tissue.
5-HT also exerts tumor-promoting effects independently of receptor activation, through serotonylation. In HCC, elevated TGM2 expression correlates with poor differentiation, high levels of the HCC marker alpha-fetoprotein (AFP), and advanced tumor stage, with pharmacological inhibition of serotonylation suppressing tumor growth [321]. These findings suggest that serotonylation may contribute to tumor aggressiveness in HCC.
Collectively, these studies depict 5-HT as a potential modulator of HCC, acting through platelet-derived paracrine mechanisms, receptor-specific signaling, and epigenetic modifications. This positions 5-HT not only as a biomarker for early detection and prognosis but also as a promising target for therapeutic intervention in hepatocellular carcinoma.
f. Cardiovascular diseases
Cardiovascular diseases (CVD) represent major comorbidities of metabolic dysfunction and chronic liver disorders such as MASLD and HCC, reflecting their convergence within a shared cardiometabolic continuum. Beyond its hepatic and metabolic roles, 5-HT functions as a potent vasoactive mediator that regulates vascular tone, endothelial function, platelet aggregation, and myocardial remodeling. More than 95 % of circulating 5-HT is synthesized peripherally by enterochromaffin cells and stored in platelets, from which it is released upon activation during thrombus formation or acute inflammation [322]. Physiologically, 5-HT contributes to vascular homeostasis through the balanced activation of vasoconstrictive 5-HT2A/2B and vasodilatory 5-HT1B/7 receptors [322,323]. Under metabolic stress, hyperlipidemia and insulin resistance disrupt this equilibrium, promoting endothelial dysfunction, oxidative stress, and thrombosis [237,324].
Clinical and experimental data demonstrate that platelet-derived 5-HT contributes to atherogenesis, vascular inflammation, and myocardial injury. Elevated plasma 5-HT and reduced platelet storage are reported in obesity, diabetes, and MASLD, correlating with vascular stiffness, carotid intima-media thickness, and coronary artery calcification [325,326]. During coronary artery disease, platelet activation within the ischemic microenvironment triggers massive 5-HT release, which acts as a chemoattractant and activator of neutrophils [308]. In TPH1⁻/⁻ mice or following chronic SSRI treatment, depletion of platelet-derived 5-HT markedly reduces neutrophil recruitment, degranulation, and myocardial injury [308,327,328]. Mechanistically, 5-HT signaling through 5-HT7 receptors on neutrophils increases intracellular calcium, promoting CD11b externalization, myeloperoxidase (MPO) release, and reactive oxygen species (ROS) production, which enhance neutrophil adhesion to platelets and injured endothelium, aggravating reperfusion injury [308,328]. Conversely, pharmacological depletion of platelet 5-HT or long-term SSRI administration mitigates this inflammatory cascade and confers protection against ischemia/reperfusion injury [329]. In humans, plasma 5-HT levels correlate positively with neutrophil CD11b and MPO expression in acute coronary syndrome, whereas SSRI therapy suppresses both markers [328]. Consistently, patients treated with SSRIs display a reduced risk of first myocardial infarction, consistent with decreased platelet 5-HT uptake and aggregation [330]. At the cardiac level, 5-HT2B receptor activation in cardiomyocytes and fibroblasts triggers ERK1/2- and TGF-β-dependent remodeling, leading to hypertrophy and diastolic dysfunction [331]. Notably, similar 5-HT2B-driven fibrogenic programs operate in hepatic stellate cells during fibrosis [200], suggesting shared serotonergic mechanisms underpinning cardiac and hepatic remodeling. Elevated circulating and hepatic 5-HT levels observed in cirrhosis and HCC correlate with systemic and portal hypertension [303,332-334], further linking hepatic 5-HT dysregulation to extrahepatic vascular pathology. Recent findings also implicate intestinal tryptophan (Trp) metabolism in the regulation of systemic 5-HT levels and cardiovascular risk. Hence, dietary Trp is catabolized via three main pathways: (1) the kynurenine (Kyn) pathway in intestinal epithelial cells through indoleamine 2,3-dioxygenase 1 (IDO1) or in the liver via Trp 2,3-dioxygenase (TDO); (2) the microbial indole pathway, which converts Trp into indole metabolites; and (3) the 5-HT synthesis pathway EC cells via the TPH1 [329]. In mice lacking intestinal IDO1 or deprived of dietary Trp, the Kyn pathway blockade redirects metabolism toward 5-HT synthesis, elevating gut-derived and circulating 5-HT, compromising barrier integrity, and promoting systemic inflammation and atherosclerotic plaque formation [329]. Pharmacological inhibition of TPH1 normalizes these effects by reducing gut inflammation and plaque burden, whereas exogenous 5-HT supplementation aggravates vascular lesions [329]. At the cellular level, these deleterious effects are orchestrated through endothelial 5-HT1B/2A/2B receptors, which regulate vascular tone and inflammatory signaling, and macrophage 5-HT2A/2B/7 receptors, which drive cytokine release, inflammasome activation, and leukocyte recruitment during atherogenesis and ischemic injury [292,335-338].
Collectively, these findings identify 5-HT as a unifying molecular link between metabolic, hepatic, and cardiovascular disorders. Dysregulated peripheral 5-HT not only promotes hepatic steatosis, fibrosis, and tumor progression but also drives vascular inflammation and myocardial remodeling, amplifying morbidity and mortality across the metabolic syndrome. Understanding 5-HT signaling within this integrated liver-gut-cardiovascular axis may thus reveal therapeutic opportunities capable of concurrently mitigating hepatic and cardiovascular complications.
IV. Conclusion and Clinical Considerations
Extensive evidence suggests that 5-HT exerts context-dependent effects on metabolic and liver health, mediated through both central and peripheral pathways. Elevated peripheral 5-HT levels are generally associated with deleterious outcomes, promoting hepatic steatosis, inflammation, fibrogenesis, and tumor progression, thereby contributing to the development of MASLD, ALD, and HCC. In contrast, physiological 5-HT signaling can exert beneficial actions by stimulating hepatocyte proliferation and liver regeneration, particularly following injury or hepatectomy, creating a therapeutic paradox. These opposing outcomes likely depend on the relative contribution of gut-derived vs locally synthesized 5-HT, each exerting organ- or tissue-specific effects according to the repertoire of 5-HT receptor subtypes expressed. At the central levels, 5-HT primarily modulates appetite, energy expenditure, and autonomic outflow, indirectly influencing liver metabolism and systemic homeostasis. Although 5-HT cannot cross the blood-brain barrier, evidence suggests that peripheral 5-HT levels may inversely influence central 5-HT synthesis, notably by modulating Trp availability.
This duality highlights the importance of integrating both central and peripheral 5-HT signaling when considering therapeutic interventions. Decreasing overall 5-HT levels using the non-selective inhibitor of both central and peripheral TPH (PCPA), was shown to reduce obesity and adiposity, as well as diet- and alcohol-induced hepatic steatosis and inflammation [198,219,224,225,304,339]. However, prolonged brain 5-HT depletion by PCPA is also known to produce anxiety [340], to impair motivation [341] and cognitive functions [342], and to increase aggressivity [343] and vulnerability to stress [344]. While reducing overall 5-HT levels may seem like a feasible approach to halting the progression of liver disease, it could also greatly affect emotions and mood. Resolving this contradiction is essential to devise new 5-HT-targeting therapeutics for the treatment of chronic liver disease. In line with this, novel TPH1 inhibitors that do not cross the blood brain barrier have been developed, revealing that inhibition of peripheral 5-HT synthesis is sufficient to protect against diet-induced obesity, reduce blood glucose levels, adiposity, and prevent diet-induced liver steatosis [199,275].
Conversely, this suggests that drugs elevating 5-HT levels, such as selective serotonin reuptake inhibitors (SSRIs) or MAO inhibitors (MAOI) antidepressants may aggravate liver steatosis and steatohepatitis. For instance, 5-HTergic medications, particularly SSRIs, have been consistently associated with adverse metabolic and hepatic effects [345-350]. Chronic exposure to SSRIs such as sertraline, fluoxetine, and citalopram induces hepatotoxicity by impairing the liver's ability to metabolize drugs and fatty acids [351,352]. In epidemiological studies, antidepressants with high affinity for the serotonin transporter have been linked to elevated serum LDL cholesterol levels [353], while clinical trials in anxiety or depressive disorders reveal divergent effects across molecules. For instance, fluoxetine was associated with reductions in body weight and lipid markers, whereas paroxetine, citalopram, and sertraline were linked to increased weight, waist circumference, and systemic glucose, LDL, and triglycerides [354]. Long-term use of SSRIs or tricyclics has further been correlated with an increased risk of diabetes mellitus [355], and several reports confirm that fluoxetine, sertraline, venlafaxine, and related agents unfavorably impact lipid profiles, with venlafaxine showing the strongest association with dyslipidemia [356,357]. Fluoxetine has particularly been implicated in hepatic lipid accumulation via a dual mechanism, upregulating the SREBP1c-ACC1-FAS lipogenic axis through p38 MAPK signaling while suppressing lipolysis via downregulation of CES1/3 [358]. This is compounded by fluoxetine-induced increase in hepatic 5-HT synthesis, which in turn promotes steatosis, an effect reversed by TPH inhibition [359]. Human and murine studies further confirm that fluoxetine elevates serum triglycerides and LDL and increases liver expression of lipogenic genes while suppressing adipogenic and β-oxidation markers [360,361]. Similar hepatotoxic effects, such as acute liver injury, cholestasis, vanishing bile duct syndrome, chronic fibrosis, Kupffer cell hyperplasia, glycogen depletion, and nuclear damage have been reported for sertraline [362-364]. Similarly, MAO isoforms A and B are critical players in the intersection between 5-HT and liver diseases. MAO-A is upregulated in MASH liver and mediates oxidative stress-induced hepatocellular damage [365]. Similar findings in patients with obesity show elevated MAO-A expression correlating with increased ROS and vascular dysfunctions [366], while MAO-B levels are markedly increased in fibrotic liver cells [367]. Blockade of MAO activity using inhibitors (e.g., clorgyline, pargyline) has shown beneficial effects on food intake and obesity markers in mice [368], suggesting that selective modulation of MAO may offer therapeutic avenues.
Beyond antidepressants, 5-HT receptor agonists such as triptans are also associated with hepatotoxicity. Sumatriptan and rizatriptan, 5-HT1D receptors agonists, have been shown to cause mitochondrial and lysosomal dysfunctions in hepatocytes, marked by lipid peroxidation and oxidative stress [369]. Yet not all serotonergic drugs are deleterious. Buspirone, a 5-HT1A receptor agonist, reduces oxidative stress and protects against CCl₄-induced liver fibrosis [370], and portal vein-targeted 5-HT1A modulation has shown promise in reducing portal hypertension [288]. Several 5-HT receptors such as 5-HT7, 5-HT2B and 5-HT1D have also been involved in the deleterious effects of 5-HT on HCC progression in vitro. For instance, 5-HT increases the proliferation of HCC cells by reducing β-catenin degradation and subsequently enhancing Wnt/β-catenin signaling pathway, a process attenuated by a 5-HT7 antagonist [318]. Pharmacological inhibition of 5-HT2B receptors also reduces the proliferation and invasiveness of HCC cells by attenuating the activation of mammalian target of rapamycin (mTOR) and YAP pathways [319]. Finally, genetic silencing of 5-HT1D receptors was shown to inhibit the proliferation, migration and invasion of HCC cells, by attenuation Pi3K/Akt-dependent epithelial-mesenchymal transition (EMT) [371]. Interestingly, emerging evidence suggests a paradoxical protective role of SSRI and tricyclic antidepressants in HCC. By inducing cancer cell autophagy, inhibiting tumor growth, and synergizing with anti-cancer agents, such as sorafenib, SSRIs likely decrease mortality in HCC patients [372-379]. Furthermore, by reducing adiposity, hepatic steatosis, hepatocyte ballooning and liver fibrosis induced by HFD- or choline-deficient HFD, 5-HT2A receptor antagonists have recently emerged as promising therapeutic candidates for MASH, MASLD and HCC [277,380].
Taken together, these observations highlight the complex and ambivalent role of 5-HT signaling in liver health. While some 5-HT targeting therapeutics show potential for modulating metabolic, inflammatory, or neoplastic processes within the liver, the unintended consequences of chronic 5-HTergic modulation-particularly through SSRIs and MAOI-raise significant safety concerns. These include disruptions in lipid and glucose homeostasis, exacerbation of steatosis, hepatocellular injury, and fibrosis. The liver's central role in drug metabolism renders it especially vulnerable to sustained 5-HT input, whether via enhanced serotonin levels, altered degradation pathways, or receptor overstimulation.
These findings highlight the temporal and receptor-specific duality of 5-HT signaling in chronic liver disease. Although 5-HT contributes to fibrogenesis and cirrhosis development, it may also participate in limiting tumor aggressiveness or supporting hepatic regeneration under specific conditions. Therapeutic strategies aiming to modulate serotonergic pathways in chronic liver disease must therefore consider both the disease stage and the cellular targets involved in 5-HT signaling. As such, repositioning serotonergic drugs for the treatment of chronic liver diseases demands a highly cautious, mechanism-guided approach. It will be essential to discriminate between receptor-specific effects, distinguish central versus peripheral actions, and identify patient profiles most likely to benefit from such interventions.
Acknowledgements
We thank Dr Vanessa Lanoue for her valuable editing work on the manuscript.
Funding
This work was supported by grants from INSERM (France), charities (AFEF to PG and CL; SNFGE-FARE), the National Research Agency (#ANR-19-CE14-0044-01, #ANR-21-CE14-0015-03, #ANR-22-CE14-0027-01, #ANR-23-CE14-0048-03, #ANR-24-CE18-2311-02 and #ANR-25-CE17-4347-04 for PG and "Investments for the Future "IDEX UCAJedi (#ANR-15-IDEX-01). This work was also realized through the Adipo-Cible Research Study Group, supported by the French government through the France 2030 investment plan managed by the National Research Agency (ANR), as part of the Initiative of Excellence of Université Côte d'Azur under reference number ANR-15-IDEX-01. AB was supported by Université Nice Côte d'Azur (ATER 406759, 2024-2025).
Author contributions
A.B. and P.G conceptualized the review topic and designed the structure of the manuscript. A.B. and C.L. performed the literature search and drafted the manuscript. A.B. prepared the figures and tables. C.L and P.G provided critical revisions and additional references. All authors read and approved the final version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hagström H, Hegmar H, Moreno C. Interactions between the metabolic syndrome and alcohol consumption increases the risk of liver disease. United Eur Gastroenterol J. 2024;12:168-76
2. Mao B, Liu S, Zhu S. et al. The janus face of serotonin: Regenerative promoter and chronic liver disease aggravator. Heliyon. 2024;10:e30703
3. Papadimas GK, Tzirogiannis KN, Mykoniatis MG, Grypioti AD, Manta GA, Panoutsopoulos GI. The emerging role of serotonin in liver regeneration. Swiss Med Wkly. 2012;142:w13548
4. Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698-709
5. Rieder M, Gauchel N, Bode C, Duerschmied D. Serotonin: a platelet hormone modulating cardiovascular disease. J Thromb Thrombolysis. 2021;52:42-7
6. Nocito A, Georgiev P, Dahm F. et al. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatol Baltim Md. 2007;45:369-76
7. Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533-54
8. Elshayeb EI, Mohamed Abdel RK, Nada FE, Mohamed AH, Ehab Ahmed AE. Serum Serotonin as a novel marker for hepatocellular carcinoma. Adv Res Gastroenterol Hepatol. 2016;1:555571
9. Moon JH, Oh CM, Kim H. Serotonin in the regulation of systemic energy metabolism. J Diabetes Investig. 2022;13:1639-45
10. Park J, Jeong W, Yun C, Kim H, Oh CM. Serotonergic regulation of hepatic energy metabolism. Endocrinol Metab. 2021;36:1151-60
11. Jensen KJ, Alpini G, Glaser S. Hepatic nervous system and neurobiology of the liver. Compr Physiol. 2013;3:655-65
12. Conde K, Fang S, Xu Y. Unraveling the serotonin saga: from discovery to weight regulation and beyond - a comprehensive scientific review. Cell Biosci. 2023;13:143
13. Brito CF, Fonseca RC, Rodrigues-Ribeiro L. et al. Vagus nerve mediated liver-brain-axis is a major regulator of the metabolic landscape in the liver. Int J Mol Sci. 2025;26:2166
14. Alcaino C. Mechanosensitive release of 5-HT from specialized intestinal epithelial cells. Nat Rev Gastroenterol Hepatol. 2023;20:4
15. Hata T, Asano Y, Yoshihara K. et al. Regulation of gut luminal serotonin by commensal microbiota in mice. PLOS ONE. 2017;12:e0180745
16. Yano JM, Yu K, Donaldson GP. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264-76
17. Reigstad CS, Salmonson CE, Rainey JF III. et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395-403
18. Agus A, Planchais J, Sokol H. Gut Microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716-24
19. Fung TC, Vuong HE, Luna CDG. et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol. 2019;4:2064-73
20. Li G, Dong S, Liu C, Yang J, Rensen PCN, Wang Y. Serotonin signaling to regulate energy metabolism: a gut microbiota perspective. Life Metab. 2025;4:loae039
21. Hu G, Zhu Y, Ding S, Zheng L. Role of gut microbiota in the 5-hydroxytryptamine signal transduction mechanism. Metab Transl Med. 2023 1
22. Kwon YH, Wang H, Denou E. et al. Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cell Mol Gastroenterol Hepatol. 2019;7:709-28
23. Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci Basic Clin. 2010;153:47-57
24. El-Merahbi R, Löffler M, Mayer A, Sumara G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015;589:1728-34
25. Kyritsi K, Chen L, O'Brien A. et al. Modulation of the tryptophan hydroxylase 1/monoamine oxidase-A/5-hydroxytryptamine/5-hydroxytryptamine receptor 2A/2B/2C axis regulates biliary proliferation and liver fibrosis during cholestasis. Hepatol Baltim Md. 2020;71:990-1008
26. Omenetti A, Yang L, Gainetdinov RR. et al. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am J Physiol-Gastrointest Liver Physiol. 2011;300:G303-15
27. Kim K, Oh CM, Ohara-Imaizumi M. et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology. 2015;156:444-52
28. Shong KE, Oh CM, Namkung J, Park S, Kim H. Serotonin regulates de novo lipogenesis in adipose tissues through serotonin receptor 2A. Endocrinol Metab. 2020;35:470-9
29. Zhang H, Leitner DR, Hasegawa Y, Waldor MK. Peripheral serotonergic neurons regulate gut motility and anxiety-like behavior. Curr Biol. 2024;34:R133-4
30. Li Z, Chalazonitis A, Huang Y. et al. Essential roles of Enteric Neuronal Serotonin in Gastrointestinal Motility and the Development/Survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998-9009
31. Pourhamzeh M, Moravej FG, Arabi M. et al. The roles of serotonin in neuropsychiatric disorders. Cell Mol Neurobiol. 2021;42:1671-92
32. Sun M, Wan Y, Shi M, Meng ZX, Zeng W. Neural innervation in adipose tissue, gut, pancreas, and liver. Life Metab. 2023;2:load022
33. Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol. 2004;280A:808-20
34. Lautt WW. Afferent and efferent neural roles in liver function. Prog Neurobiol. 1983;21:323-48
35. Lkhagvasuren B, Mee-Inta O, Zhao ZW, Hiramoto T, Boldbaatar D, Kuo YM. Pancreas-brain crosstalk. Front Neuroanat. 2021;15:691777
36. Jiang H, Ding X, Cao Y, Wang H, Zeng W. Dense Intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 2017;26:686-692.e3
37. Smirnov VM, Lychkova AE. Synergism of sympathetic and parasympathetic systems in the regulation of gastric motility. Bull Exp Biol Med. 2002;134:12-4
38. Bacon SJ, Smith AD. Preganglionic sympathetic neurones innervating the rat adrenal medulla: immunocytochemical evidence of synaptic input from nerve terminals containing substance P, GABA or 5-hydroxytryptamine. J Auton Nerv Syst. 1988;24:97-122
39. Hwang YK, Oh JS. Interaction of the vagus nerve and serotonin in the gut-brain axis. Int J Mol Sci. 2025;26:1160
40. Sah DW, Matsumoto SG. Evidence for serotonin synthesis, uptake, and release in dissociated rat sympathetic neurons in culture. J Neurosci Off J Soc Neurosci. 1987;7:391-9
41. Norevall LI, Matsson L, Forsgren S. 5-Hydroxytryptamine immunoreactivity is detectable in sympathetic nerve fibres in rat oral tissues. Histochem J. 1996;28:485-93
42. Karhulaa T, Soinila S, Lakomy M, Majewski M, Kaleczyk J, Häppölä O. 5-Hydroxytryptamine-immunoreactive nerve fibers in the rat and porcine prevertebral sympathetic ganglia: effect of precursor loading and relation to catecholaminergic neurons. Neurosci Lett. 1995;194:85-8
43. Karhula T, Panula P, Steinbusch H, Häppölä O. Immunohistochemical localization of 5-hydroxytryptamine, histamine and histidine decarboxylase in the rat major pelvic and coeliac-superior mesenteric ganglion. J Auton Nerv Syst. 1990;31:91-9
44. Kiraly M, Ma RC, Dun NJ. Serotonin mediates a slow excitatory potential in mammalian celiac ganglia. Brain Res. 1983;275:378-83
45. Wallis DI, Dun NJ. A comparison of fast and slow depolarizations evoked by 5-HT in guinea-pig coeliac ganglion cells in vitro. Br J Pharmacol. 1988;93:110-20
46. Yamano M, Ito H, Kamato T, Miyata K. Characteristics of inhibitory effects of serotonin (5-HT)3-receptor antagonists, YM060 and YM114 (KAE-393), on the von Bezold-Jarisch reflex induced by 2-Methyl-5-HT, veratridine and electrical stimulation of vagus nerves in anesthetized rats. Jpn J Pharmacol. 1995;69:351-6
47. Yoshioka M, Ikeda T, Togashi H, Saito Y, Saito H. Effect of 5-hydroxytryptamine on gastric motility and efferent gastric vagus nerve activity in rats. Res Commun Chem Pathol Pharmacol. 1990;70:3-10
48. Larsson I, Dahlström A, Pettersson G, Larsson PA, Kewenter J, Ahlman H. The effects of adrenergic antagonists on the serotonin levels of feline enterochromaffin cells after splanchnic nerve stimulation. J Neural Transm. 1980;47:89-98
49. Bellono NW, Bayrer JR, Leitch DB. et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170:185-198.e16
50. Ahlman H, Dahlström A. Vagal mechanisms controlling serotonin release from the gastrointestinal tract and pyloric motor function. J Auton Nerv Syst. 1983;9:119-40
51. Spencer NJ, Kyloh MA, Travis L, Hibberd TJ. Identification of vagal afferent nerve endings in the mouse colon and their spatial relationship with enterochromaffin cells. Cell Tissue Res. 2024;396:313-27
52. Dowling LR, Strazzari MR, Keely S, Kaiko GE. Enteric nervous system and intestinal epithelial regulation of the gut-brain axis. J Allergy Clin Immunol. 2022;150:513-22
53. Jiang C, Zhan Q, Zeng C. The 5-HT-related gut-brain axis in obesity. Life Sci. 2024;358:123171
54. Maley B, Elde R. The ultrastructural localization of serotonin immunoreactivity within the nucleus of the solitary tract of the cat. J Neurosci. 1982;2:1499-506
55. Schaffar N, Kessler JP, Bosler O, Jean A. Central serotonergic projections to the nucleus tractus solitarii: Evidence from a double labeling study in the rat. Neuroscience. 1988;26:951-8
56. Kreier F, Fliers E, Voshol PJ. et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat — functional implications. J Clin Invest. 2002;110:1243-50
57. Blaszkiewicz M, Willows JW, Johnson CP, Townsend KL. The Importance of peripheral nerves in adipose tissue for the regulation of energy balance. Biology. 2019;8:10
58. Wang Y, Ye L. The afferent function of adipose innervation. Diabetes. 2024;73:348-54
59. Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014;35:473-93
60. Fueri C, Faudon M, Hery M, Hery F. Release of serotonin from perikarya in cat nodose ganglia. Brain Res. 1984;304:173-7
61. Gaudin-Chazal G, Portalier P, Barrit MC, Puizillout JJ. Serotonin-like immunoreactivity in paraffin-sections of the nodose ganglia of the cat. Neurosci Lett. 1982;33:169-72
62. Calza` L, Giardino L, Grimaldi R, Rigoli M, Steinbusch HW, Tiengo M. Presence of 5-HT-positive neurons in the medial nuclei of the solitary tract. Brain Res. 1985;347:135-9
63. Nosjean A, Compoint C, Buisseret-Delmas C. et al. Serotonergic projections from the nodose ganglia to the nucleus tractus solitarius: an immunohistochemical and double labeling study in the rat. Neurosci Lett. 1990;114:22-6
64. Gaudin-Chazal G, Puizillout JJ. Quantitative autoradiographic studies of 5-HT-accumulating neurones in the nodose ganglia of the cat after perikaryal or terminal uptake. Brain Res. 1983;270:239-49
65. Browning KN. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front Neurosci. 2015;9:413
66. McCuskey RS. Anatomy of efferent hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280A:821-6
67. El-Salhy M, Stenling R, Grimelius L. Peptidergic innervation and endocrine cells in the human liver. Scand J Gastroenterol. 1993;28:809-15
68. Stoyanova II. Relevance of mast cells and hepatic lobule innervation to liver injury. Romanian J Gastroenterol. 2004;13:203-9
69. Mastai R, Giroux L, Semret M, Huet PM. Ritanserin decreases portal pressure in conscious and unrestrained cirrhotic rats. Gastroenterology. 1990;98:141-5
70. Vorobioff J, Garcia-Tsao G, Groszmann R. et al. Long-term hemodynamic effects of ketanserin, a 5-hydroxytryptamine blocker, in portal hypertensive patients. Hepatology. 1989;9:88-91
71. Mercado NM, Zhang G, Ying Z, Gómez-Pinilla F. Traumatic brain injury alters the gut-derived serotonergic system and associated peripheral organs. Biochim Biophys Acta BBA - Mol Basis Dis. 2022;1868:166491
72. Wang JY, Chen JY, Lian YZ. et al. Reduced platelet 5-HT content is associated with rest tremor in Parkinson's disease. Parkinsonism Relat Disord. 2023;108:105314
73. Kumar AM, Sevush S, Kumar M, Ruiz J, Eisdorfer C. Peripheral serotonin in Alzheimer's disease. Neuropsychobiology. 1995;32:9-12
74. Sancho-Alonso M, Sarriés-Serrano U, Miquel-Rio L. et al. New insights into the effects of serotonin on Parkinson's disease and depression through its role in the gastrointestinal tract. Span J Psychiatry Ment Health. 2025;18:216-27
75. Jiang YJ, Cao YM, Cao YB, Yan TH, Jia CL, He P. A Review: Cytochrome P450 in alcoholic and non-alcoholic fatty liver disease. Diabetes Metab Syndr Obes. 2024 Volume 17: 1511-21
76. Luci C, Bourinet M, Leclère PS, Anty R, Gual P. Chronic inflammation in non-alcoholic steatohepatitis: Molecular mechanisms and therapeutic strategies. Front Endocrinol. 2020;11:597648
77. Bromek E, Rysz M, Haduch A, Wójcikowski J, Daniel WA. Activation of 5-HT1A receptors in the hypothalamic paraventricular nuclei negatively regulates cytochrome P450 expression and activity in rat liver. Drug Metab Dispos. 2018;46:786-93
78. Rysz M, Bromek E, Daniel WA. Activation of brain serotonergic system by repeated intracerebral administration of 5-hydroxytryptophan (5-HTP) decreases the expression and activity of liver cytochrome P450. Biochem Pharmacol. 2016;99:113-22
79. Rysz M, Bromek E, Haduch A, Liskova B, Wójcikowski J, Daniel WA. The reverse role of the hypothalamic paraventricular (PVN) and arcuate (ARC) nuclei in the central serotonergic regulation of the liver cytochrome P450 isoform CYP2C11. Biochem Pharmacol. 2016;112:82-9
80. Kot M, Pilc A, Daniel WA. Simultaneous alterations of brain and plasma serotonin concentrations and liver cytochrome P450 in rats fed on a tryptophan-free diet. Pharmacol Res. 2012;66:292-9
81. Rysz M, Bromek E, Haduch A, Sadakierska-Chudy A, Daniel WA. Damage to the brain serotonergic system increases the expression of liver cytochrome P450. Drug Metab Dispos. 2015;43:1345-52
82. Haduch A, Bromek E, Kuban W. et al. The effect of brain serotonin deficit (TPH2-KO) on the expression and activity of liver cytochrome P450 enzymes in aging male Dark Agouti rats. Pharmacol Rep. 2023;75:1522-32
83. Bromek E, Rysz M, Haduch A, Daniel WA. Stimulation of 5-HT2C serotonin receptor subtype in the hypothalamic arcuate nuclei (ARC) increases the cytochrome P450 activity in the liver. Pharmacol Rep. 2019;71:1210-2
84. Kot M, Sadakierska-Chudy A, Haduch A. et al. The role of the dorsal noradrenergic pathway of the brain (locus coeruleus) in the regulation of liver cytochrome P450 activity. Eur J Pharmacol. 2015;751:34-41
85. Zhou Y, Lin X, Jiao Y. et al. A brain-to-liver signal mediates the inhibition of liver regeneration under chronic stress in mice. Nat Commun. 2024;15:10361
86. Konstandi M, Johnson EO, Lang MA. Consequences of psychophysiological stress on cytochrome P450-catalyzed drug metabolism. Neurosci Biobehav Rev. 2014;45:149-67
87. Konstandi M, Johnson EO, Lang MA. Stress as a potential regulatory factor in the outcome of pharmacotherapy. Front Neurosci. 2022;16:737716
88. Thasler WE, Dayoub R, Mühlbauer M. et al. Repression of cytochrome P450 activity in human hepatocytes in vitro by a novel hepatotrophic factor, Augmenter of Liver Regeneration. J Pharmacol Exp Ther. 2006;316:822-9
89. Chembazhi UV, Bangru S, Hernaez M, Kalsotra A. Cellular plasticity balances the metabolic and proliferation dynamics of a regenerating liver. Genome Res. 2021;31:576-91
90. Wang X, Menezes CJ, Jia Y. et al. Metabolic inflexibility promotes mitochondrial health during liver regeneration. Science. 2024;384:eadj4301
91. Solhi R, Lotfinia M, Gramignoli R, Najimi M, Vosough M. Metabolic hallmarks of liver regeneration. Trends Endocrinol Metab. 2021;32:731-45
92. Caldez MJ, Van Hul N, Koh HWL. et al. Metabolic remodeling during liver regeneration. Dev Cell. 2018;47:425-438.e5
93. Xiu J, Han R, Liu Z. et al. Hijacking dorsal raphe to improve metabolism and depression-like behaviors via BDNF gene transfer in mice. Diabetes. 2021;70:1780-93
94. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13:572-87
95. Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol. 2011;46:200-15
96. Martin H, Bullich S, Martinat M. et al. Insulin modulates emotional behavior through a serotonin-dependent mechanism. Mol Psychiatry. 2024;29:1610-9
97. Derkach KV, Bondareva VM, Chistyakova OV, Berstein LM, Shpakov AO. The effect of long-term intranasal serotonin treatment on metabolic parameters and hormonal signaling in rats with high-fat diet/low-dose streptozotocin-induced type 2 diabetes. Int J Endocrinol. 2015;2015:245459
98. Horáček J, Kuzmiaková M, Höschl C, Andĕl M, Bahbonh R. The relationship between central serotonergic activity and insulin sensitivity in healthy volunteers. Psychoneuroendocrinology. 1999;24:785-97
99. Powis JE, Bains JS, Ferguson AV. Leptin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol-Regul Integr Comp Physiol. 1998;274:R1468-72
100. Martínez-Uña M, López-Mancheño Y, Diéguez C, Fernández-Rojo MA, Novelle MG. Unraveling the role of leptin in liver function and its relationship with liver diseases. Int J Mol Sci. 2020;21:9368
101. Hackl MT, Fürnsinn C, Schuh CM. et al. Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis. Nat Commun. 2019;10:2717
102. Metz M, Beghini M, Wolf P. et al. Leptin increases hepatic triglyceride export via a vagal mechanism in humans. Cell Metab. 2022;34:1719-1731.e5
103. Huang W, Dedousis N, Bandi A, Lopaschuk GD, O'Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147:1480-7
104. Huynh FK, Neumann UH, Wang Y, Rodrigues B, Kieffer TJ, Covey SD. A role for hepatic leptin signaling in lipid metabolism via altered very low density lipoprotein composition and liver lipase activity in mice. Hepatol Baltim Md. 2013;57:543-54
105. Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206-13
106. Cernea S, Roiban AL, Both E, Huţanu A. Serum leptin and leptin resistance correlations with NAFLD in patients with type 2 diabetes. Diabetes Metab Res Rev. 2018;34:e3050
107. Maxwell ND, Smiley CE, Sadek AT. et al. Leptin activation of dorsal raphe neurons inhibits feeding behavior. Diabetes. 2024;73:1821-31
108. Sadek AT, Cowan HB, Jimenez KM. et al. Serotonin as a regulator of leptin-mediated food intake control within a novel neuronal circuit between the hypothalamus and raphe nuclei. J Endocr Soc. 2021;5:A55-6
109. Lin Y, Liang Z, He L. et al. Gut ghrelin regulates hepatic glucose production and insulin signaling via a gut-brain-liver pathway. Cell Commun Signal. 2019;17:8
110. Ogaya M, Kim J, Sasaki K. Ghrelin postsynaptically depolarizes dorsal raphe neurons in rats in vitro. Peptides. 2011;32:1606-16
111. Hansson C, Alvarez-Crespo M, Taube M. et al. Influence of ghrelin on the central serotonergic signaling system in mice. Neuropharmacology. 2014;79:498-505
112. Ghersi MS, Casas SM, Escudero C. et al. Ghrelin inhibited serotonin release from hippocampal slices. Peptides. 2011;32:2367-71
113. Reich N, Hölscher C. Beyond appetite: Acylated ghrelin as a learning, memory and fear behavior-modulating hormone. Neurosci Biobehav Rev. 2022;143:104952
114. Currie PJ, John CS, Nicholson ML, Chapman CD, Loera KE. Hypothalamic paraventricular 5-hydroxytryptamine inhibits the effects of ghrelin on eating and energy substrate utilization. Pharmacol Biochem Behav. 2010;97:152-5
115. Schellekens H, De Francesco PN, Kandil D. et al. Ghrelin's Orexigenic effect is modulated via a serotonin 2C receptor interaction. ACS Chem Neurosci. 2015;6:1186-97
116. Wauson SE, Sarkodie K, Schuette LM, Currie PJ. Midbrain raphe 5-HT1A receptor activation alters the effects of ghrelin on appetite and performance in the elevated plus maze. J Psychopharmacol (Oxf). 2015;29:836-44
117. Præstholm SM, Correia CM, Goitea VE. et al. Impaired glucocorticoid receptor expression in liver disrupts feeding-induced gene expression, glucose uptake, and glycogen storage. Cell Rep. 2021;37:109938
118. Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60:1500-10
119. Clyburn C, Browning KN. Glutamatergic plasticity within neurocircuits of the dorsal vagal complex and the regulation of gastric functions. Am J Physiol-Gastrointest Liver Physiol. 2021;320:G880-7
120. Polyzos SA, Targher G. Role of glucocorticoids in metabolic dysfunction-associated steatotic liver disease. Curr Obes Rep. 2024;13:242-55
121. Osei-Owusu P, James A, Crane J, Scrogin KE. 5-Hydroxytryptamine 1A receptors in the paraventricular nucleus of the hypothalamus mediate oxytocin and adrenocorticotropin hormone release and some behavioral components of the serotonin syndrome. J Pharmacol Exp Ther. 2005;313:1324-30
122. Heisler LK, Pronchuk N, Nonogaki K. et al. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci. 2007;27:6956-64
123. Habash T, Eskay RL, Kuenzel WJ, Castonguay TW. Interactions of glucocorticoids, NPY and hypothalamic serotonin. Nutr Neurosci. 2000;3:183-92
124. Edgerton DS, Kraft G, Smith M, Farmer B, Williams P, Cherrington AD. A physiologic increase in brain glucagon action alters the hepatic gluconeogenic/glycogenolytic ratio but not glucagon's overall effect on glucose production. Am J Physiol Endocrinol Metab. 2023;324:E199-208
125. Al-Massadi O, Fernø J, Diéguez C, Nogueiras R, Quiñones M. Glucagon control on food intake and energy balance. Int J Mol Sci. 2019;20:3905
126. Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and lipid metabolism. Front Physiol. 2019;10:413
127. Wang H, Zhao M, Sud N. et al. Glucagon regulates hepatic lipid metabolism via cAMP and Insig-2 signaling: implication for the pathogenesis of hypertriglyceridemia and hepatic steatosis. Sci Rep. 2016;6:32246
128. Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab. 2011;13:118-25
129. Kajani S, Laker RC, Ratkova E, Will S, Rhodes CJ. Hepatic glucagon action: beyond glucose mobilization. Physiol Rev. 2024;104:1021-60
130. Thorens B. Neuronal glucose sensing mechanisms and circuits in the control of insulin and glucagon secretion. Physiol Rev. 2024;104:1461-86
131. Thorens B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes Metab. 2011;13(Suppl 1):82-8
132. Mighiu PI, Yue JTY, Filippi BM. et al. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat Med. 2013;19:766-72
133. Edgerton DS, Cherrington AD. Glucagon's yin and yang effects on hepatic glucose production. Nat Med. 2013;19:674-5
134. LaPierre MP, Abraham MA, Yue JTY, Filippi BM, Lam TKT. Glucagon signalling in the dorsal vagal complex is sufficient and necessary for high-protein feeding to regulate glucose homeostasis in vivo. EMBO Rep. 2015;16:1299-307
135. Cabou C, Burcelin R. GLP-1, the gut-brain, and brain-periphery axes. Rev Diabet Stud RDS. 2011;8:418-31
136. Lund ML, Egerod KL, Engelstoft MS. et al. Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol Metab. 2018;11:70-83
137. Yang M, Wang J, Wu S. et al. Duodenal GLP-1 signaling regulates hepatic glucose production through a PKC-δ-dependent neurocircuitry. Cell Death Dis. 2017;8:e2609-e2609
138. Knauf C, Cani PD, Perrin C. et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115:3554-63
139. Holt MK, Richards JE, Cook DR. et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68:21-33
140. Havranek B, Loh R, Torre B, Redfield R, Halegoua-DeMarzio D. Glucagon-like peptide-1 receptor agonists improve metabolic dysfunction-associated steatotic liver disease outcomes. Sci Rep. 2025;15:4947
141. Nevola R, Epifani R, Imbriani S. et al. GLP-1 Receptor agonists in non-alcoholic fatty liver disease: Current evidence and future perspectives. Int J Mol Sci. 2023;24:1703
142. Njei B, Al-Ajlouni Y, Lemos SY. et al. Efficacy and safety of GLP-1 receptor agonists in patients with metabolic dysfunction-associated steatotic liver disease: A systematic review and meta-analysis of randomized controlled trials. Cureus. 2024;16:e71366
143. Abushamat LA, Shah PA, Eckel RH, Harrison SA, Barb D. The emerging role of glucagon-like peptide-1 receptor agonists for the treatment of metabolic dysfunction-associated steatohepatitis. Clin Gastroenterol Hepatol. 2024;22:1565-74
144. Farrugia M, Pini E, Tran A, Chevalier N, Anti R, Gual P. Incretins and MASLD: at the crossroads of endocrine and hepatic disorders. Curr Obes Rep. 2025;14:56
145. Holt MK, Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Serotonergic modulation of the activity of GLP-1 producing neurons in the nucleus of the solitary tract in mouse. Mol Metab. 2017;6:909-21
146. Wagner S, Brierley DI, Leeson-Payne A. et al. Obesity medication lorcaserin activates brainstem GLP-1 neurons to reduce food intake and augments GLP-1 receptor agonist induced appetite suppression. Mol Metab. 2023;68:101665
147. Anderberg RH, Richard JE, Eerola K. et al. Glucagon-like peptide-1 and its analogues act in the dorsal raphe and modulate central serotonin to reduce appetite and body weight. Diabetes. 2017;66:1062-73
148. Nonogaki K. The Regulatory Role of the central and peripheral serotonin network on feeding signals in metabolic diseases. Int J Mol Sci. 2022;23:1600
149. Kineman RD, del Rio-Moreno M, Waxman DJ. Liver-specific actions of GH and IGF1 that protect against MASLD. Nat Rev Endocrinol. 2025;21:105-17
150. Musumeci G, Trovato F, Avola R, Imbesi R, Castrogiovanni P. Serotonin/growth hormone/insulin-like growth factors axis on pre- and post-natal development: A contemporary review. OA Anat. 2013;1:12
151. Hoshaw BA, Hill TI, Crowley JJ. et al. Antidepressant-like behavioral effects of IGF-I produced by enhanced serotonin transmission. Eur J Pharmacol. 2008;594:109-16
152. Marino L, Kim A, Ni B, Celi FS. Thyroid hormone action and liver disease, a complex interplay. Hepatol Baltim Md. 2025;81:651-69
153. Ghosh PM, Shu ZJ, Zhu B. et al. Role of β-adrenergic receptors in regulation of hepatic fat accumulation during aging. J Endocrinol. 2012;213:251-61
154. Lelou E, Corlu A, Nesseler N. et al. The role of catecholamines in pathophysiological Liver Processes. Cells. 2022;11:1021
155. Yi C-X, La Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta BBA - Mol Basis Dis. 2010;1802:416-31
156. Bruinstroop E, Fliers E, Kalsbeek A. Hypothalamic control of hepatic lipid metabolism via the autonomic nervous system. Best Pract Res Clin Endocrinol Metab. 2014;28:673-84
157. Edinburgh RM, Frampton J. Liver sympathetic nerve activity and steatosis. J Physiol. 2020;598:11-2
158. Guarino D, Nannipieri M, Iervasi G, Taddei S, Bruno RM. The role of the autonomic nervous system in the pathophysiology of obesity. Front Physiol. 2017;8:665
159. Brumer RP, Corrêa-Velloso JC, Thomas SJ, Sandiford OA, Thomas AP, Bartlett PJ. Short-term high-fat diet feeding of mice suppresses catecholamine-stimulated Ca2+ signalling in hepatocytes and intact liver. J Physiol. 2023;601:1383-405
160. Püschel GP. Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280A:854-67
161. Carnagarin R, Tan K, Adams L. et al. Metabolic dysfunction-Associated Fatty Liver Disease (MAFLD)—A condition associated with heightened sympathetic activation. Int J Mol Sci. 2021;22:4241
162. Shi Y, Shu ZJ, Wang H. et al. Altered expression of hepatic β-adrenergic receptors in aging rats: implications for age-related metabolic dysfunction in liver. Am J Physiol Regul Integr Comp Physiol. 2018;314:R574-83
163. Shi Y, Pizzini J, Wang H. et al. β2-Adrenergic receptor agonist induced hepatic steatosis in mice: modeling nonalcoholic fatty liver disease in hyperadrenergic states. Am J Physiol-Endocrinol Metab. 2021;321:E90-104
164. Katz MS, Dax EM, Gregerman RI. Beta adrenergic regulation of rat liver glycogenolysis during aging. Exp Gerontol. 1993;28:329-40
165. Fraeyman N, Van de Velde E, Van Ermen A. et al. Effect of maturation and aging on β-adrenergic signal transduction in rat kidney and liver. Biochem Pharmacol. 2000;60:1787-95
166. Hurr C, Simonyan H, Morgan DA, Rahmouni K, Young CN. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J Physiol. 2019;597:4565-80
167. Dimitriadis K, Iliakis P, Vakka A. et al. Effects of Sympathetic denervation in metabolism regulation: A novel approach for the treatment of MASLD? Cardiol Rev. 2025 online ahead of print
168. Erraji-Benchekroun L, Couton D, Postic C. et al. Overexpression of β2-adrenergic receptors in mouse liver alters the expression of gluconeogenic and glycolytic enzymes. Am J Physiol-Endocrinol Metab. 2005;288:E715-22
169. Morgan HJN, Delfino HBP, Schavinski AZ. et al. Hepatic noradrenergic innervation acts via CREB/CRTC2 to activate gluconeogenesis during cold. Metabolism. 2024;157:155940
170. Hur MH, Song W, Cheon D-H. et al. Chemogenetic stimulation of the parasympathetic nervous system lowers hepatic lipid accumulation and inflammation in a nonalcoholic steatohepatitis mouse model. Life Sci. 2023;321:121533
171. Liu K, Yang L, Wang G. et al. Metabolic stress drives sympathetic neuropathy within the liver. Cell Metab. 2021;33:666-675.e4
172. Oben JA, Roskams T, Yang S. et al. Sympathetic nervous system inhibition increases hepatic progenitors and reduces liver injury. Hepatol Baltim Md. 2003;38:664-73
173. Henriksen JH, Møller S, Ring-Larsen H, Christensen NJ. The sympathetic nervous system in liver disease. J Hepatol. 1998;29:328-41
174. Mizuno K, Ueno Y. Autonomic nervous system and the liver. Hepatol Res. 2017;47:160-5
175. Sigala B, McKee C, Soeda J. et al. Sympathetic nervous system catecholamines and neuropeptide Y neurotransmitters are upregulated in human NAFLD and modulate the fibrogenic function of hepatic stellate cells. PloS One. 2013;8:e72928
176. Oben JA, Roskams T, Yang S. et al. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut. 2004;53:438-45
177. Xue J, Jiang T, Humaerhan J. et al. Impact of liver sympathetic nervous system on liver fibrosis and regeneration after bile duct ligation in rats. J Mol Neurosci. 2024;74:4
178. Henriksen JH, Ring-Larsen H, Christensen NJ. Sympathetic nervous activity in cirrhosis: A survey of plasma catecholamine studies. J Hepatol. 1985;1:55-65
179. Adori C, Daraio T, Kuiper R. et al. Disorganization and degeneration of liver sympathetic innervations in nonalcoholic fatty liver disease revealed by 3D imaging. Sci Adv. 2021;7:eabg5733
180. Adori M, Bhat S, Gramignoli R. et al. Hepatic innervations and nonalcoholic fatty liver disease. Semin Liver Dis. 2023;43:149-62
181. Gao X, van der Veen JN, Zhu L. et al. Vagus nerve contributes to the development of steatohepatitis and obesity in phosphatidylethanolamine N-methyltransferase deficient mice. J Hepatol. 2015;62:913-20
182. Hwang J, Okada J, Liu L, Pessin JE, Schwartz GJ, Jo YH. The development of hepatic steatosis depends on the presence of liver-innervating parasympathetic cholinergic neurons in mice fed a high-fat diet. PLoS Biol. 2024;22:e3002865
183. Borovikova LV, Ivanova S, Zhang M. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458-62
184. Li Y, Xu Z, Yu Y. et al. The vagus nerve attenuates fulminant hepatitis by activating the Src kinase in Kuppfer cells. Scand J Immunol. 2014;79:105-12
185. Nishio T, Taura K, Iwaisako K. et al. Hepatic vagus nerve regulates Kupffer cell activation via α7 nicotinic acetylcholine receptor in nonalcoholic steatohepatitis. J Gastroenterol. 2017;52:965-76
186. Consolim-Colombo FM, Sangaleti CT, Costa FO. et al. Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI Insight. 2017;2:e93340
187. Satapathy SK, Ochani M, Dancho M. et al. Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Mol Med. 2011;17:599-606
188. Bruinstroop E, Fliers E, Kalsbeek A. Restoring the autonomic balance to reduce liver steatosis. J Physiol. 2019;597:4683-4
189. Morgan ML, Sigala B, Soeda J. et al. Acetylcholine induces fibrogenic effects via M2/M3 acetylcholine receptors in non-alcoholic steatohepatitis and in primary human hepatic stellate cells. J Gastroenterol Hepatol. 2016;31:475-83
190. Oben JA, Yang S, Lin H, Ono M, Diehl AM. Acetylcholine promotes the proliferation and collagen gene expression of myofibroblastic hepatic stellate cells. Biochem Biophys Res Commun. 2003;300:172-7
191. Bockx I, Vander Elst I, Roskams T, Cassiman D. The hepatic vagus nerve stimulates hepatic stellate cell proliferation in rat acute hepatitis via muscarinic receptor type 2. Liver Int. 2010;30:693-702
192. Lam H-B, Yeh C-H, Cheng K-C, Hsu C-T, Cheng J-T. Effect of cholinergic denervation on hepatic fibrosis induced by carbon tetrachloride in rats. Neurosci Lett. 2008;438:90-5
193. Izumi T, Imai J, Yamamoto J. et al. Vagus-macrophage-hepatocyte link promotes post-injury liver regeneration and whole-body survival through hepatic FoxM1 activation. Nat Commun. 2018;9:5300
194. Cassiman D, Libbrecht L, Sinelli N, Desmet V, Denef C, Roskams T. The vagal nerve stimulates activation of the hepatic progenitor cell compartment via muscarinic acetylcholine receptor type 3. Am J Pathol. 2002;161:521-30
195. Jung I, Lee DY, Lee MY. et al. Autonomic imbalance increases the risk for non-alcoholic fatty liver disease. Front Endocrinol. 2021;12:752944
196. Mravec B, Szantova M. The role of the nervous system in liver diseases. Hepatol Res. 2024;54:970-80
197. Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012;16:588-600
198. Choi W, Namkung J, Hwang I. et al. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat Commun. 2018;9:4824
199. Pagire SH, Pagire HS, Park K-Y. et al. Identification of new non-BBB permeable tryptophan hydroxylase inhibitors for treating obesity and fatty liver disease. Mol Basel Switz. 2022;27:3417
200. Ebrahimkhani MR, Oakley F, Murphy LB. et al. Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nat Med. 2011;17:1668-73
201. Lesurtel M, Graf R, Aleil B. et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104-7
202. González-Hernández A, Marichal-Cancino BA, MaassenVanDenBrink A, Villalón CM. Serotonergic modulation of neurovascular transmission: A focus on prejunctional 5-HT receptors/mechanisms. Biomedicines. 2023;11:1864
203. Nakagawa T, Tsuchida A, Itakura Y. et al. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436-44
204. Jo Y-H, Chua SC. The brain-liver connection between BDNF and glucose control. Diabetes. 2013;62:1367-8
205. Genzer Y, Chapnik N, Froy O. Effect of brain-derived neurotrophic factor (BDNF) on hepatocyte metabolism. Int J Biochem Cell Biol. 2017;88:69-74
206. Gray J, Yeo GSH, Cox JJ. et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55:3366-71
207. Ichimura-Shimizu M, Kojima M, Suzuki S. et al. Brain-derived neurotrophic factor knock-out mice develop non-alcoholic steatohepatitis. J Pathol. 2023;261:465-76
208. Fox EA, Byerly MS. A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R994-1004
209. Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci Off J Soc Neurosci. 2007;27:14265-74
210. Sun T, Liu X, Yang G. et al. Neurotrophic factors stimulate the activation of hepatic stellate cells in liver fibrosis. Biochem Biophys Res Commun. 2022;630:167-74
211. Song Y, Wei J, Li R. et al. Tyrosine kinase receptor B attenuates liver fibrosis by inhibiting TGF-β/SMAD signaling. Hepatol Baltim Md. 2023;78:1433-47
212. Causing CG, Gloster A, Aloyz R. et al. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257-67
213. Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589-94
214. Popova NK, Naumenko VS. Neuronal and behavioral plasticity: the role of serotonin and BDNF systems tandem. Expert Opin Ther Targets. 2019;23:227-39
215. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet Lond Engl. 2024;403:1027-50
216. Martin AM, Yabut JM, Choo JM. et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc Natl Acad Sci. 2019;116:19802-4
217. Tatayeva R, Tussupova A, Koygeldinova S, Serkali S, Suleimenova A, Askar B. The level of serotonin and the parameters of lipid metabolism are dependent on the mental status of patients with suicide attempts. Psychiatry Int. 2024;5:773-92
218. Young RL, Lumsden AL, Martin AM. et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018;42:1880-9
219. Oh C-M, Namkung J, Go Y. et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun. 2015;6:6794
220. Kim H-J, Kim JH, Noh S. et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10:722-31
221. Bertrand RL, Senadheera S, Markus I. et al. A Western diet increases serotonin availability in rat small intestine. Endocrinology. 2011;152:36-47
222. Sun W, Guo Y, Zhang S. et al. Fecal Microbiota transplantation can alleviate gastrointestinal transit in rats with high-fat diet-induced obesity via regulation of serotonin biosynthesis. BioMed Res Int. 2018;2018:8308671
223. Saponara E, Chen R, Reding T. et al. Single or combined ablation of peripheral serotonin and p21 limit adipose tissue expansion and metabolic alterations in early adulthood in mice fed a normocaloric diet. PLOS ONE. 2021;16:e0255687
224. Wu Y, Ma J, Chen J. et al. Ablation of CD44 attenuates adipogenesis in white Adipocytes via the tryptophan 5-hydroxylase 2/5-hydroxytryptamine axis to protect mice from high-fat diet-induced obesity. Am J Pathol. 2025;195:247-64
225. Namkung J, Shong KE, Kim H, Oh CM, Park S, Kim H. Inhibition of serotonin synthesis induces negative hepatic lipid balance. Diabetes Metab J. 2018;42:233-43
226. Breisch ST, Zemlan FP, Hoebel BG. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science. 1976;192:382-5
227. Crane JD, Palanivel R, Mottillo EP. et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015;21:166-72
228. Choi WG, Choi W, Oh TJ. et al. Inhibiting serotonin signaling through HTR2B in visceral adipose tissue improves obesity-related insulin resistance. J Clin Invest. 2021;131:e145331
229. Suchacki KJ, Ramage LE, Kwok TC. et al. The serotonin transporter sustains human brown adipose tissue thermogenesis. Nat Metab. 2023;5:1319-36
230. Rami M, Guillamat-Prats R, Rinne P. et al. Chronic intake of the selective serotonin reuptake inhibitor fluoxetine enhances atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:1007-19
231. Homberg JR, la Fleur SE, Cuppen E. Serotonin transporter deficiency increases abdominal fat in female, but not male rats. Obes Silver Spring Md. 2010;18:137-45
232. Zha W, Ho HTB, Hu T, Hebert MF, Wang J. Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance. Sci Rep. 2017;7:1137
233. Rosa LF, Haasis E, Knauss A, Guseva D, Bischoff SC. Serotonin reuptake transporter deficiency promotes liver steatosis and impairs intestinal barrier function in obese mice fed a Western-style diet. Neurogastroenterol Motil. 2023;35:e14611
234. Kesić M, Baković P, Farkaš V. et al. Constitutive serotonin tone as a modulator of brown adipose tissue thermogenesis: A rat study. Life. 2023;13:1436
235. Baković P, Kesić M, Kolarić D, Štefulj J, Čičin-Šain L. Metabolic and molecular response to high-fat diet differs between rats with constitutionally high and low serotonin tone. Int J Mol Sci. 2023;24:2169
236. Lu X, Xie Q, Pan X. et al. Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal Transduct Target Ther. 2024;9:1-25
237. Cai Y, Li X, Zhou H, Zhou J. The serotonergic system dysfunction in diabetes mellitus. Front Cell Neurosci. 2022;16:899069
238. Ohta Y, Kosaka Y, Kishimoto N. et al. Convergence of the insulin and serotonin programs in the pancreatic β-cell. Diabetes. 2011;60:3208-16
239. Ebou MH, Singh-Estivalet A, Launay JM. et al. Glucocorticoids inhibit basal and hormone-induced serotonin synthesis in pancreatic beta cells. PLOS ONE. 2016;11:e0149343
240. Almaça J, Molina J, Menegaz D. et al. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Rep. 2016;17:3281-91
241. Bennet H, Mollet IG, Balhuizen A. et al. Serotonin (5-HT) receptor 2b activation augments glucose-stimulated insulin secretion in human and mouse islets of Langerhans. Diabetologia. 2016;59:744-54
242. Kim H, Toyofuku Y, Lynn FC. et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804-8
243. Ohara-Imaizumi M, Kim H, Yoshida M. et al. Serotonin regulates glucose-stimulated insulin secretion from pancreatic β cells during pregnancy. Proc Natl Acad Sci U S A. 2013;110:19420-5
244. Paulmann N, Grohmann M, Voigt JP. et al. Intracellular serotonin modulates insulin secretion from pancreatic β-cells by protein serotonylation. PLOS Biol. 2009;7:e1000229
245. Walther DJ, Peter JU, Winter S. et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet α-granule release. Cell. 2003;115:851-62
246. Takada A, Shimizu F, Takao T, Masuda J. Measurement of tryptophan metabolites in healthy old men and patients of type 2 diabetes mellitus (T2DM). Food Nutr Sci. 2018;9:1206-20
247. Hara K, Hirowatari Y, Shimura Y, Takahashi H. Serotonin levels in platelet-poor plasma and whole blood in people with type 2 diabetes with chronic kidney disease. Diabetes Res Clin Pract. 2011;94:167-71
248. Prince Sandeep V, Vinodchandran M A. et al. Serum tryptophan, insulin, and serotonin in type 2 diabetic patients: A pilot study. Biomedicine. 2022;42:1162-5
249. Pietraszek MH, Takada Y, Takada A. et al. Blood serotonergic mechanisms in type 2 (non-insulin-dependent) diabetes mellitus. Thromb Res. 1992;66:765-74
250. Manjarrez-Gutiérrez G, Herrera-Márquez R, Neri-Gómez T, Hernández-Rodríguez J. Serotonin levels in plasma and platelets of adolescents with type 1 diabetes. Glob Adv Res J Med Med Sci. 2014;3:267-74
251. Asuaje Pfeifer M, Liebmann M, Beuerle T, Grupe K, Scherneck S. Role of serotonin (5-HT) in GDM prediction considering islet and liver interplay in prediabetic mice during gestation. Int J Mol Sci. 2022;23:6434
252. Martin FJ, Mi´guez JM, Aldegunde M, Atienza G. Effect of streptozotocin-induced diabetes mellitus on serotonin measures of peripheral tissues in rats. Life Sci. 1994;56:51-9
253. Takahara H, Fujimura M, Taniguchi S, Hayashi N, Nakamura T, Fujimiya M. Changes in serotonin levels and 5-HT receptor activity in duodenum of streptozotocin-diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G798-808
254. Bulc M, Palus K, Całka J, Kosacka J, Nowicki M. Streptozotocin-induced diabetes causes changes in serotonin-positive neurons in the small intestine in pig model. Int J Mol Sci. 2022;23:4564
255. Mezei D, Bódi N, Szalai Z, Márton Z, Balázs J, Bagyánszki M. Immediate insulin treatment prevents diabetes-induced gut region-specific increase in the number of myenteric serotonergic neurons. Appl Sci. 2021;11:5949
256. Manjarrez-Gutiérrez G, Mondragón-Herrera JA, Hernández-Rodríguez J. Diabetes mellitus causes changes in the activity and expression of brain tryptophan-5-hydroxylases and in the number of serotonergic neurons, which do not return to normal with insulin treatment. Gac Med Mex. 2022;158:182-9
257. Sandrini M, Vitale G, Vergoni AV, Ottani A, Bertolini A. Streptozotocin-induced diabetes provokes changes in serotonin concentration and on 5-HT1A and 5-HT2 receptors in the rat brain. Life Sci. 1997;60:1393-7
258. Abraham PM, Anju TR, Jayanarayanan S, Paulose CS. Serotonergic receptor upregulation in cerebral cortex and down regulation in brainstem of streptozotocin induced diabetic rats: antagonism by pyridoxine and insulin. Neurosci Lett. 2010;483:23-7
259. Ohtani N, Ohta M, Sugano T. Microdialysis study of modification of hypothalamic neurotransmitters in streptozotocin-diabetic rats. J Neurochem. 1997;69:1622-8
260. Ripken D, van der Wielen N, Wortelboer HM, Meijerink J, Witkamp RF, Hendriks HFJ. Nutrient-induced glucagon like peptide-1 release is modulated by serotonin. J Nutr Biochem. 2016;32:142-50
261. Hu S, Kou Y, Liu X, Rong W, Han H, Zhang G. Activation of the 5-hydroxytryptamine 4 receptor ameliorates tight junction barrier dysfunction in the colon of type 1 diabetic mice. Acta Biochim Biophys Sin. 2023;55:1874-83
262. Vanslette AM, Toft PB, Lund ML, Moritz T, Arora T. Serotonin receptor 4 agonism prevents high fat diet induced reduction in GLP-1 in mice. Eur J Pharmacol. 2023;960:176181
263. Wang JX, Chang SY, Jin ZY. et al. Lactobacillus reuteri-enriched eicosatrienoic acid regulates glucose homeostasis by promoting GLP-1 secretion to protect intestinal barrier integrity. J Agric Food Chem. 2025;73:393-408
264. Guan X, Karpen HE, Stephens J. et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150-64
265. Koopmann MC, Nelson DW, Murali SG. et al. Exogenous glucagon-like peptide-2 (GLP-2) augments GLP-2 receptor mRNA and maintains proglucagon mRNA levels in resected rats. J Parenter Enter Nutr. 2008;32:254-65
266. Younossi ZM, Kalligeros M, Henry L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. 2025;31:S32-50
267. Bertola A, Bonnafous S, Anty R. et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PloS One. 2010;5:e13577
268. Patouraux S, Rousseau D, Bonnafous S. et al. CD44 is a key player in non-alcoholic steatohepatitis. J Hepatol. 2017;67:328-38
269. Schneck A-S, Anty R, Patouraux S. et al. Roux-En Y gastric bypass results in long-term remission of hepatocyte apoptosis and hepatic histological features of non-alcoholic steatohepatitis. Front Physiol. 2016;7:344
270. Soysouvanh F, Rousseau D, Bonnafous S. et al. Osteopontin-driven T-cell accumulation and function in adipose tissue and liver promoted insulin resistance and MAFLD. Obesity (Silver Spring). 2023;31:2568-82
271. Bourinet M, Anty R, Gual P, Luci C. Roles of innate lymphoid cells in metabolic and alcohol-associated liver diseases. JHEP Rep. 2023;6:100962
272. Farrugia MA, Le Garf S, Chierici A. et al. therapeutic physical exercise programs in the context of NASH cirrhosis and liver transplantation: A systematic review. Metabolites. 2023;13:330
273. Le Garf S, Nègre V, Anty R, Gual P. Metabolic fatty liver disease in children: A growing public health problem. Biomedicines. 2021;9:1915
274. Malehmir M, Pfister D, Gallage S. et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25:641-55
275. Bae EJ, Choi WG, Pagire HS. et al. Peripheral selective oxadiazolylphenyl alanine derivatives as tryptophan hydroxylase 1 inhibitors for obesity and fatty liver disease. J Med Chem. 2021;64:1037-53
276. Ming X, Chung ACK, Mao D. et al. Pancreatic sirtuin 3 deficiency promotes hepatic steatosis by enhancing 5-hydroxytryptamine synthesis in mice with diet-induced obesity. Diabetes. 2021;70:119-31
277. Pagire HS, Pagire SH, Jeong B. et al. Discovery of a peripheral 5HT2A antagonist as a clinical candidate for metabolic dysfunction-associated steatohepatitis. Nat Commun. 2024;15:645
278. Redenšek Trampuž S, van Riet S, Nordling Å, Ingelman-Sundberg M. Mechanisms of 5-HT receptor antagonists in the regulation of fibrosis in a 3D human liver spheroid model. Sci Rep. 2024;14:1396
279. Kim MH, Kim SJ, Park WJ, Lee DH, Kim KK. GR113808, a serotonin receptor 4 antagonist, prevents high-fat-diet-induced obesity, fatty liver formation, and insulin resistance in C57BL/6J mice. BMC Pharmacol Toxicol. 2024;25:76
280. Ruddell RG, Oakley F, Hussain Z. et al. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol. 2006;169:861-76
281. Zhang Y, Wang Y, Zhang K. et al. Profile of 5-HT2A receptor involved in signaling cascades associated to intracellular inflammation and apoptosis in hepatocytes and its role in carbon tetrachloride-induced hepatotoxicity. Cell Signal. 2023;105:110612
282. Zhang YX, Li C, Liang XR. et al. Role of 5-HT degradation in acute liver injury induced by carbon tetrachloride. Eur J Pharmacol. 2021;908:174355
283. Zhang Y, Liang X, Guan J. et al. Carbon tetrachloride induced mitochondrial division, respiratory chain damage, abnormal intracellular [H+] and apoptosis are due to the activation of 5-HT degradation system in hepatocytes. Toxicol Appl Pharmacol. 2022;439:115929
284. Fu J. Activation of the 5-hydroxytryptamine degradation system in cells and organ injury. J Cell Signal. 2023;4:125-7
285. Jonnakuty C, Gragnoli C. What do we know about serotonin? J Cell Physiol. 2008;217:301-6
286. Lemoinne S, Thabut D, Housset C. Portal myofibroblasts connect angiogenesis and fibrosis in liver. Cell Tissue Res. 2016;365:583-9
287. Kaumann AJ, Morgan JS, Groszmann RJ. ICI 169,369 selectively blocks 5-hydroxytryptamine2 receptors and lowers portal pressure in portal hypertensive rats. Gastroenterology. 1988;95:1601-6
288. Zhu CP, Liu SQ, Wang KQ. et al. Targeting 5-Hydroxytryptamine Receptor 1A in the Portal Vein to Decrease Portal Hypertension. Gastroenterology. 2024;167:993-1007
289. Furrer K, Rickenbacher A, Tian Y. et al. Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc Natl Acad Sci. 2011;108:2945-50
290. Banskota S, Gautam J, Regmi SC. et al. BJ-1108, a 6-amino-2,4,5-trimethylpyridin-3-ol analog, inhibits serotonin-induced angiogenesis and tumor growth through PI3K/NOX pathway. PLOS ONE. 2016;11:e0148133
291. Profirovic J, Strekalova E, Urao N. et al. A novel regulator of angiogenesis in endothelial cells: 5-hydroxytriptamine 4 receptor. Angiogenesis. 2013;16:15-28
292. Iwabayashi M, Taniyama Y, Sanada F. et al. Role of serotonin in angiogenesis: Induction of angiogenesis by sarpogrelate via endothelial 5-HT1B/Akt/eNOS pathway in diabetic mice. Atherosclerosis. 2012;220:337-42
293. Machida T, Iizuka K, Hirafuji M. 5-hydroxytryptamine and its receptors in systemic vascular walls. Biol Pharm Bull. 2013;36:1416-9
294. Belmer A, Patkar OL, Pitman KM, Bartlett SE. Serotonergic neuroplasticity in alcohol addiction. Brain Plast Amst Neth. 2016;1:177-206
295. Belmer A, Patkar OL, Lanoue V, Bartlett SE. 5-HT1A receptor-dependent modulation of emotional and neurogenic deficits elicited by prolonged consumption of alcohol. Sci Rep. 2018;8:2099
296. Belmer A, Depoortere R, Beecher K, Newman-Tancredi A, Bartlett SE. Neural serotonergic circuits for controlling long-term voluntary alcohol consumption in mice. Mol Psychiatry. 2022;27:4599-610
297. Zaniewska M, Mosienko V, Bader M, Alenina N. Tph2 gene expression defines ethanol drinking behavior in mice. Cells. 2022;11:874
298. Marcinkiewcz CA. Serotonergic systems in the pathophysiology of ethanol dependence: Relevance to clinical alcoholism. ACS Chem Neurosci. 2015;6:1026-39
299. Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology. 2015;40:590-600
300. Underwood MD, Mann JJ, Arango V. Morphometry of dorsal raphe nucleus serotonergic neurons in alcoholism. Alcohol Clin Exp Res. 2007;31:837-45
301. Marcinkiewcz CA, Lowery-Gionta EG, Kash TL. Serotonin's complex role in alcoholism: Implications for treatment and future research. Alcohol Clin Exp Res. 2016;40:1192-201
302. Xu R, Vatsalya V, He L. et al. Altered urinary tryptophan metabolites in alcohol-associated liver disease. Alcohol Clin Exp Res. 2023;47:1665-76
303. Beaudry P, Hadengue A, Callebert J. et al. Blood and plasma 5-hydroxytryptamine levels in patients with cirrhosis. Hepatology. 1994;20:800-3
304. Hwang I, Nam JE, Choi W. et al. Serotonin regulates lipogenesis and endoplasmic reticulum stress in alcoholic liver disease. Diabetes Metab J. 2025;49:798-811
305. Borcsiczky D, Szalay F, Tekes K, Tarcali J, Magyar K, de Châtel R. Platelet serotonin (5-HT) content is decreased in patients with alcoholic liver cirrhosis, but elevated in Gilbert's syndrome. J Hepatol. 1996;25:781-2
306. Bailly D, Vignau J, Lauth B. et al. Platelet serotonin decrease in alcoholic patients. Acta Psychiatr Scand. 1990;81:68-72
307. Pivac N, Mück-Seler D, Mustapić M, Nenadić-Sviglin K, Kozarić-Kovacić D. Platelet serotonin concentration in alcoholic subjects. Life Sci. 2004;76:521-31
308. Duerschmied D, Suidan GL, Demers M. et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood. 2013;121:1008-15
309. Abd El Moety HA, Maharem DA, Gomaa SH. Serotonin: is it a marker for the diagnosis of hepatocellular carcinoma in cirrhotic patients? Alex J Med. 2013;49:369-78
310. Mamdouh F, Abdel Alem S, Abdo M. et al. Serum serotonin as a potential diagnostic marker for hepatocellular carcinoma. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. 2019;39:780-5
311. Padickakudy R, Pereyra D, Offensperger F. et al. Bivalent role of intra-platelet serotonin in liver regeneration and tumor recurrence in humans. J Hepatol. 2017;67:1243-52
312. Aryal B, Shimizu T, Kadono J. et al. Post-resection exhaustion of intra-platelet serotonin: Also an indicator of early hepatocellular carcinoma recurrence? J Cancer. 2017;8:3984-91
313. Aryal B, Yamakuchi M, Hashiguchi T, Imoto Y. Intra-platelet serotonin as a biomarker in HCC recurrence: When Time Matters. J Cancer. 2019;10:2384-5
314. Yang Q, Liu S, Deng C, Shu B, Zhang J, Zhai M. Preoperative serum and intra-platelet serotonin in prognosis: Useful or useless? J Cancer. 2018;9:3713-4
315. Shu B, Zhai M, Miao X. et al. Serotonin and YAP/VGLL4 balance correlated with progression and poor prognosis of hepatocellular carcinoma. Sci Rep. 2018;8:9739
316. Liu S, Zhai M, Xiao W. et al. Intra-platelet serotonin and YAP contributed to poor prognosis of hepatocellular carcinoma. Life Sci. 2021;270:119140
317. Soll C, Riener M-O, Oberkofler CE. et al. Expression of serotonin receptors in human hepatocellular cancer. Clin Cancer Res. 2012;18:5902-10
318. Fatima S, Shi X, Lin Z. et al. 5-Hydroxytryptamine promotes hepatocellular carcinoma proliferation by influencing b-catenin. Mol Oncol. 2016;10:195-212
319. Liu S, Miao R, Zhai M. et al. Effects and related mechanisms of serotonin on malignant biological behavior of hepatocellular carcinoma via regulation of Yap. Oncotarget. 2017;8:47412-24
320. Gurbuz N, Ashour AA, Alpay SN, Ozpolat B. Down-regulation of 5-HT1B and 5-HT1D receptors inhibits proliferation, clonogenicity and invasion of human pancreatic cancer cells. PloS One. 2014;9:e105245
321. Dong R, Wang T, Dong W. et al. TGM2-mediated histone serotonylation promotes HCC progression via MYC signalling pathway. J Hepatol. 2025;83:105-118
322. Ni W, Watts SW. 5-hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT). Clin Exp Pharmacol Physiol. 2006;33:575-83
323. Watts SW. Oh, the places you'll go!. My many colored serotonin (apologies to Dr. Seuss). Am J Physiol - Heart Circ Physiol. 2016;311:H1225-33
324. Ma Y, Liang X, Li C. et al. 5-HT 2A receptor and 5-HT degradation play a crucial role in atherosclerosis by modulating macrophage foam cell formation, vascular endothelial cell inflammation, and hepatic steatosis. J Atheroscler Thromb. 2022;29:322-36
325. Oh CM, Park S, Kim H. Serotonin as a New Therapeutic Target for Diabetes Mellitus and Obesity. Diabetes Metab J. 2016;40:89-98
326. Sadykova D, Nigmatullina R, Salakhova K. et al. Membrane transporter of serotonin and hypercholesterolemia in children. Int J Mol Sci. 2024;25:767
327. Cloutier N, Allaeys I, Marcoux G. et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc Natl Acad Sci U S A. 2018;115:E1550-9
328. Mauler M, Herr N, Schoenichen C. et al. Platelet serotonin aggravates myocardial ischemia/reperfusion injury via neutrophil degranulation. Circulation. 2019;139:918-31
329. Chajadine M, Laurans L, Radecke T. et al. Harnessing intestinal tryptophan catabolism to relieve atherosclerosis in mice. Nat Commun. 2024;15:6390
330. Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation. 2001;104:1894-8
331. Jaffré F, Callebert J, Sarre A. et al. Involvement of the serotonin 5-HT2B receptor in cardiac hypertrophy linked to sympathetic stimulation: control of interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha cytokine production by ventricular fibroblasts. Circulation. 2004;110:969-74
332. Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol. 2008;48:666-75
333. Watts SW, Davis RP. 5-Hydroxtryptamine Receptors in Systemic Hypertension: an arterial focus. Cardiovasc Ther. 2011;29:54-67
334. Brenner B, Harney JT, Ahmed BA. et al. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. 2007;102:206-15
335. Jiang M, Ding H, Huang Y. et al. Endothelial serotonin receptor 1B acts as a mechanosensor to drive atherosclerosis. Circ Res. 2025;136:887-901
336. Liu Y, Wang Z, Fang L. et al. Deficiency of 5-HT2B receptors alleviates atherosclerosis by regulating macrophage phenotype through inhibiting interferon signalling. Br J Pharmacol. 2025;182:4894-910
337. Flanagan TW, Sebastian MN, Battaglia DM, Foster TP, Maillet EL, Nichols CD. Activation of 5-HT2 receptors reduces inflammation in vascular tissue and cholesterol levels in high-fat diet-fed apolipoprotein E knockout mice. Sci Rep. 2019;9:13444
338. Brasil D, Temsah RM, Kumar K, Kumamoto H, Takeda N, Dhalla NS. Blockade of 5-HT(2A) receptors by sarpogrelate protects the heart against myocardial infarction in rats. J Cardiovasc Pharmacol Ther. 2002;7:53-9
339. Wang L, Fan X, Han J. et al. Gut-derived serotonin contributes to the progression of non-alcoholic steatohepatitis via the liver HTR2A/PPARγ2 pathway. Front Pharmacol. 2020;11:553
340. Trujillo V, Valentim-Lima E, Mencalha R. et al. Neonatal serotonin depletion induces hyperactivity and anxiolytic-like sex-dependent effects in adult rats. Mol Neurobiol. 2021;58:1036-51
341. Hori Y, Mimura K, Nagai Y. et al. Reduced serotonergic transmission alters sensitivity to cost and reward via 5-HT1A and 5-HT1B receptors in monkeys. PLOS Biol. 2024;22:e3002445
342. Wallace A, Pehrson AL, Sánchez C, Morilak DA. Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. Int J Neuropsychopharmacol. 2014;17:1695-706
343. Studer E, Näslund J, Andersson E, Nilsson S, Westberg L, Eriksson E. Serotonin depletion-induced maladaptive aggression requires the presence of androgens. PLOS ONE. 2015;10:e0126462
344. Sachs BD, Ni JR, Caron MG. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc Natl Acad Sci. 2015;112:2557-62
345. Khalil SM, MacKenzie KR, Maletic-Savatic M, Li F. Metabolic bioactivation of antidepressants: advance and underlying hepatotoxicity. Drug Metab Rev. 2024;56:97-126
346. Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: A Review for clinicians. Am J Psychiatry. 2014;171:404-15
347. Park SH, Ishino R. Liver injury associated with antidepressants. Curr Drug Saf. 2013;8:207-23
348. Billioti de Gage S, Collin C, Le-Tri T. et al. Antidepressants and hepatotoxicity: A cohort study among 5 million individuals registered in the French national health insurance database. CNS Drugs. 2018;32:673-84
349. Carvajal García-Pando A, García del Pozo J, Sánchez AS, Velasco MA, Rueda de Castro AM, Lucena MI. Hepatotoxicity associated with the new antidepressants. J Clin Psychiatry. 2002;63:135-7
350. Huang CY, You YS, Lai JM, Lin CL, Hsu HY, Hsieh YW. The association between antidepressant use and drug-induced liver injury: A nationwide, population-based case-control study in Taiwan. Drugs - Real World Outcomes. 2024;11:513-20
351. Almansour MI, Jarrar YB, Jarrar BM. In vivo investigation on the chronic hepatotoxicity induced by sertraline. Environ Toxicol Pharmacol. 2018;61:107-15
352. Chen S, Xuan J, Wan L. et al. Sertraline, an antidepressant, induces apoptosis in hepatic cells through the mitogen-activated protein kinase pathway. Toxicol Sci. 2014;137:404-15
353. Noordam R, Aarts N, de Keyser CE, Hofman A, Stricker BH, Visser LE. Antidepressants with a high serotonin reuptake transporter affinity and serum lipid levels in a population-based study in older adults. J Psychopharmacol (Oxf). 2015;29:1112-8
354. Beyazyüz M, Albayrak Y, Eğilmez OB, Albayrak N, Beyazyüz E. Relationship between SSRIs and metabolic syndrome abnormalities in patients with generalized anxiety disorder: A Prospective Study. Psychiatry Investig. 2013;10:148-54
355. Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166:591-8
356. Richards-Belle A, Austin-Zimmerman I, Wang B. et al. Associations of antidepressants and antipsychotics with lipid parameters: Do CYP2C19/CYP2D6 genes play a role? A UK population-based study. J Psychopharmacol (Oxf). 2023;37:396-407
357. Cao J, Chen Z, Wang Y. et al. Overweight and glucose/lipid metabolism abnormality associated with SSRIs: a pharmacovigilance study based on the FDA adverse event reporting system. Front Pharmacol. 2025;15:1517546
358. Feng XM, Xiong J, Qin H. et al. Fluoxetine induces hepatic lipid accumulation via both promotion of the SREBP1c-related lipogenesis and reduction of lipolysis in primary mouse hepatocytes. CNS Neurosci Ther. 2012;18:974-80
359. Ayyash A, Holloway AC. Fluoxetine-induced hepatic lipid accumulation is linked to elevated serotonin production. Can J Physiol Pharmacol. 2021;99:983-8
360. Pan S, Tan Y, Yao S. et al. Fluoxetine induces lipid metabolism abnormalities by acting on the liver in patients and mice with depression. Acta Pharmacol Sin. 2018;39:1463-72
361. Bozdag D, van Voorthuizen J, Korpel N, Lentz S, Gurer-Orhan H, Kamstra JH. Dysregulation of adipogenesis and disrupted lipid metabolism by the antidepressants citalopram and sertraline. Toxicol Appl Pharmacol. 2024;486:116937
362. Renemane L, Rancans E. Sertraline induced acute hepatocellular liver injury in patient with major depressive disorder: a case report. Front Psychiatry. 2024;15:1456455
363. Conrad MA, Cui J, Lin HC. Sertraline-associated cholestasis and ductopenia consistent with vanishing bile duct syndrome. J Pediatr. 2016;169:313-315.e1
364. Fartoux-Heymann L, Hézode C, Zafrani ES, Dhumeaux D, Mallat A. Acute fatal hepatitis related to sertraline. J Hepatol. 2001;35:683-4
365. Nocito A, Dahm F, Jochum W. et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology. 2007;133:608-18
366. Sturza A, Olariu S, Ionică M. et al. Monoamine oxidase is a source of oxidative stress in obese patients with chronic inflammation 1. Can J Physiol Pharmacol. 2019;97:844-9
367. Sun M, Huang Y, Sun X. et al. Evaluation of monoamine oxidase B fluctuation in liver fibrosis cell and mice models via a specificity fluorescent probe. Sens Actuators B Chem. 2024;417:136111
368. Feldman JM. Effect of the monoamine oxidase inhibitors clorgyline and pargyline on the hyperphagia of obese mice. Behav Brain Res. 1988;29:147-58
369. Khalili Fard J, Hamzeiy H, Sattari M, Eghbal MA. Protective roles of N-acetyl cysteine and/or taurine against sumatriptan-induced hepatotoxicity. Adv Pharm Bull. 2016;6:627-37
370. Abdel-Salam OME, Shaffie NM, Mohammed NA, Youness ER, Morsy SMY, Sleem AA. The 5-HT1A agonist buspirone decreases liver oxidative stress and exerts protective effect against CCl4- toxicity. J Exp Clin Toxicol. 2017;1:13-26
371. Zuo X, Chen Z, Cai J. et al. 5-Hydroxytryptamine receptor 1D aggravates hepatocellular carcinoma progression through FoxO6 in AKT-dependent and independent manners. Hepatology. 2019;69:2031-47
372. Chen VC, Lin CF, Hsieh YH. et al. Hepatocellular carcinoma and antidepressants: a nationwide population-based study. Oncotarget. 2016;8:30464-70
373. Zhang H, Xu H, Tang Q, Bi F. The selective serotonin reuptake inhibitors enhance the cytotoxicity of sorafenib in hepatocellular carcinoma cells. Anticancer Drugs. 2021;32:793-801
374. Bhagavathula AS, Woolf B, Rahmani J, Vidyasagar K, Tesfaye W. Selective serotonin reuptake inhibitor use and the risk of hepatocellular carcinoma: a systematic review and dose-response analysis of cohort studies with one million participants. Eur J Clin Pharmacol. 2022;78:547-55
375. Huang YH, Yeh CT. Anticancer Effects of antidepressants in hepatocellular carcinoma cells. Anticancer Res. 2023;43:1201-6
376. Dong F, He K, Zhang S. et al. SSRI antidepressant citalopram reverses the Warburg effect to inhibit hepatocellular carcinoma by directly targeting GLUT1. Cell Rep. 2024;43:114818
377. Chang CM, Hsieh MS, Yang TC. et al. Selective serotonin reuptake inhibitors and the risk of hepatocellular carcinoma in hepatitis B virus-infected patients. Cancer Manag Res. 2017;9:709-20
378. Chen LJ, Hsu TC, Chan HL. et al. Protective effect of escitalopram on hepatocellular carcinoma by inducing autophagy. Int J Mol Sci. 2022;23:9247
379. Huang KL, Chen YL, Stewart R, Chen VC. Antidepressant use and mortality among patients with hepatocellular carcinoma. JAMA Netw Open. 2023;6:e2332579
380. Tay RE, Ho CM, Ang NDZ. et al. Serotonin receptor 5-HT2A as a potential target for HCC immunotherapy. J Immunother Cancer. 2025;13:e011088
381. Zhang HF, Lin XH, Yang H. et al. Regulation of the activity and expression of aryl hydrocarbon receptor by ethanol in mouse hepatic stellate cells. Alcohol Clin Exp Res. 2012;36:1873-81
382. Divanovic S, Dalli J, Jorge-Nebert LF. et al. Contributions of the three CYP1 monooxygenases to pro-inflammatory and inflammation-resolution lipid mediator pathways. J Immunol. 2013;191:3347-57
383. Lu J, Shang X, Yao B. et al. The role of CYP1A1/2 in cholesterol ester accumulation provides a new perspective for the treatment of hypercholesterolemia. Acta Pharm Sin B. 2023;13:648-61
384. He Q, Li JK, Li F. et al. Mechanism of action of gypenosides on type 2 diabetes and non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2015;21:2058-66
385. Zhang Y, Fu Q, Wu T. et al. 5-Methoxyflavone ameliorates non-alcoholic fatty liver disease through targeting the cytochrome P450 1A1. Free Radic Biol Med. 2023;195:178-91
386. Chen H, Shen ZY, Xu W. et al. Expression of P450 and nuclear receptors in normal and end-stage Chinese livers. World J Gastroenterol. 2014;20:8681-90
387. Li F, Zhu W, Gonzalez FJ. Potential role of CYP1B1 in the development and treatment of metabolic diseases. Pharmacol Ther. 2017;178:18-30
388. Kirby GM, Batist G, Alpert L, Lamoureux E, Cameron RG, Alaoui-Jamali MA. Overexpression of cytochrome P-450 isoforms involved in aflatoxin B1 bioactivation in human liver with cirrhosis and hepatitis. Toxicol Pathol. 1996;24:458-67
389. Shen Y, Liu J, Yao B. et al. Non-alcoholic fatty liver disease changes the expression and activity of hepatic drug-metabolizing enzymes and transporters in rats. Toxicol Lett. 2024;396:36-47
390. Haduch A, Wójcikowski J, Daniel WA. Effect of neuroleptics on cytochrome P450 2C11 (CYP2C11) in rat liver. Pharmacol Rep PR. 2011;63:1491-9
391. Wójcikowski J, Haduch A, Daniel WA. Effect of antidepressant drugs on cytochrome P450 2C11 (CYP2C11) in rat liver. Pharmacol Rep PR. 2013;65:1247-55
392. Yu AM, Idle JR, Byrd LG, Krausz KW, Küpfer A, Gonzalez FJ. Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics. 2003;13:173-81
393. Qin X, Zhang Y, Lu J, Huang S, Liu Z, Wang X. CYP3A deficiency alters bile acid homeostasis and leads to changes in hepatic susceptibility in rats. Toxicol Appl Pharmacol. 2021;429:115703
Author contact
![]() Corresponding authors: Arnauld Belmer Ph.D., and Philippe Gual, Ph.D., Inserm UMR1065/C3M, Bâtiment Universitaire ARCHIMED, Team "Chronic liver diseases associated with obesity and alcohol", 151 route Saint-Antoine de Ginestière, BP 2 3194, 06204 Nice Cedex 03, France, arnauld.belmerfr and philippe.gualfr.
Corresponding authors: Arnauld Belmer Ph.D., and Philippe Gual, Ph.D., Inserm UMR1065/C3M, Bâtiment Universitaire ARCHIMED, Team "Chronic liver diseases associated with obesity and alcohol", 151 route Saint-Antoine de Ginestière, BP 2 3194, 06204 Nice Cedex 03, France, arnauld.belmerfr and philippe.gualfr.
 Global reach, higher impact
Global reach, higher impact