13.3
Impact Factor
Theranostics 2026; 16(5):2539-2558. doi:10.7150/thno.126053 This issue Cite
Review
Modulating CD8⁺ T cell immunity through exercise: mechanistic insights and implications for precision immunotherapy
Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou, 510280, Guangdong, China.
*Equal contributors.
Received 2025-9-29; Accepted 2025-11-25; Published 2026-1-1
Abstract
CD8⁺ T lymphocytes are pivotal effectors of adaptive immunity, executing cytotoxic mechanisms essential for pathogen clearance, tumor surveillance and tissue protection. Their activity is shaped by antigenic stimulation, cytokine networks and the metabolic and structural architecture of the tissue microenvironment. Physical exercise has emerged as a potent, non-pharmacological modulator of CD8⁺ T cell biology, capable of influencing recruitment, activation, differentiation and functional persistence. Acute exercise mobilizes effector and memory subsets, enhances trafficking to peripheral tissues and transiently alters activation thresholds, while sustained training remodels subset composition, preserves mitochondrial competence and attenuates immunosenescence. These adaptations are orchestrated through integrated neuroendocrine, vascular and metabolic pathways that recalibrate chemokine gradients, nutrient availability and energetic support. However, the magnitude and direction of these effects are highly context-dependent, varying with host physiology, disease state and microenvironmental constraints. This Review integrates mechanistic and translational evidence across physiological and pathological settings—including cancer, infectious, neurological and metabolic diseases—to clarify when and how exercise can be leveraged to reinforce cytotoxic immunity. We highlight key methodological and biological challenges, and propose biomarker-guided, microenvironment-informed and adaptively titrated exercise interventions as a framework for advancing exercise from an adjunctive measure to a modulatory, precision immunotherapy.
Keywords: CD8⁺ T cells, physical exercise, immunomodulation, T cell dynamics, immune metabolism
1. Introduction
CD8⁺ T lymphocytes are pivotal effectors of adaptive immunity, orchestrating cytotoxic mechanisms essential for the elimination of virally infected cells and the eradication of malignant cells arising through oncogenic transformation [1-3]. Upon recognition of antigenic peptides presented by MHC (Major histocompatibility complex) class I molecules, naïve CD8⁺ T cells undergo clonal expansion and acquire effector functions, including the secretion of perforin and granzymes to induce target-cell apoptosis, and the production of pro-inflammatory cytokines such as IFN-γ and TNF-α to amplify immune responses [4, 5]. Their functional integrity is sustained through a tightly regulated continuum of activation, proliferation, differentiation and memory formation, governed by co-stimulatory signals from antigen-presenting cells, cytokine-mediated survival and differentiation cues, chemokine-directed trafficking, and metabolic inputs from the tissue microenvironment [6, 7]. These processes generate heterogeneous subsets—including short-lived effector cells, central and effector memory populations, and tissue-resident memory T cells—that collectively maintain immune surveillance and mediate rapid protective responses [8, 9]. In both physiological and pathological contexts, the magnitude and quality of CD8⁺ T cell responses critically influence the outcome of infection control, tumor surveillance and chronic inflammatory processes [10, 11].
Physical exercise is increasingly recognized as a potent modulator of immune homeostasis, exerting effects that span systemic physiology and local tissue niches to influence multiple aspects of T cell biology [12, 13]. Acute bouts of exercise mobilize T cells from lymphoid reservoirs such as the spleen, bone marrow and peripheral lymph nodes into the circulation, primarily through catecholamine-driven β₂-adrenergic receptor signaling that promotes detachment from stromal niches and enhances trafficking to peripheral tissues [14, 15]. This mobilization preferentially enriches antigen-experienced effector and effector memory CD8⁺ T cells, transiently boosting cytotoxic surveillance [16, 17]. In contrast, chronic exercise training drives longer-term remodeling of the T cell compartment, expanding the naïve pool, increasing central and effector memory subsets with high proliferative potential, and reducing terminally differentiated or senescent-like populations [18]. These adaptations are associated with improved proliferative capacity, reduced expression of exhaustion markers such as PD-1, and enhanced cytokine production, reflecting an attenuation of immunosenescence [19].
Over the past decade, both preclinical and clinical studies have expanded our understanding of how exercise shapes CD8⁺ T cell dynamics in health and disease. In healthy individuals, regular moderate-intensity exercise has been linked to increased naïve and central memory CD8⁺ T cell frequencies, improved proliferative capacity, and reduced accumulation of senescent or exhausted phenotypes [20]. In oncology, exercise has shown the capacity to remodel the tumor microenvironment (TME), increasing vascular perfusion and, in some models, enhancing tumor infiltration by cytotoxic T cells, thereby augmenting the efficacy of immune checkpoint inhibitors [21, 22]. Conversely, other studies have reported negligible or even adverse effects, particularly in immunologically “cold” tumors or in conditions of excessive training stress [23, 24]. Such variability underscores the complexity of exercise-immune interactions and suggests that outcomes are contingent upon a multidimensional interplay of exercise parameters, host factors, and the underlying immune-metabolic context.
In this review, we present the first comprehensive synthesis of evidence linking physical exercise to CD8⁺ T cell biology, spanning mechanisms from systemic physiology to tissue-specific immunity. We first analyze how exercise shapes CD8⁺ T cell mobilization, metabolic fitness, and immunosenescence, integrating insights from human studies and preclinical models. The discussion then moves to pathological contexts, examining how exercise shapes CD8⁺ T cell responses in cancer, infectious diseases, neurological disorders and metabolic syndromes, and how the unique immune-metabolic features of each disease microenvironment govern both the magnitude and the direction of these effects. Finally, we critically examine the methodological, mechanistic and translational challenges that currently limit the field, and outline strategies to harness exercise as a precision immunomodulatory intervention optimizing CD8⁺ T cell-mediated immunity in both health and disease.
2. Determinants and therapeutic relevance of CD8⁺ T cell function in the immune microenvironment
2.1 T cell subsets and their functions within the immune microenvironment
T lymphocytes are a central component of adaptive immunity and can be broadly classified into CD4⁺ and CD8⁺ αβ T cells, γδ T cells, and specialized populations such as regulatory T cells (Tregs), each with distinct developmental mechanisms and effector functions [25, 26]. CD4⁺ T cells primarily act as orchestrators of immune responses by providing cytokine-mediated help to other immune cells, supporting antibody production, enhancing antigen-presenting cell function, and optimizing cytotoxic T lymphocyte and natural killer cell activity [27]. Through differentiation into subsets such as Th1, Th2, Th17, and T follicular helper cells, CD4⁺ T cells adapt their effector profiles to the nature of the antigenic challenge and the cytokine environment [28]. CD8⁺ T cells serve as the primary cytotoxic arm of the adaptive immune system, recognizing antigenic peptides presented on MHC class I molecules and inducing target-cell death through perforin- and granzyme-dependent cytolysis or death receptor signaling, while also releasing pro-inflammatory cytokines that amplify local immune activation [29]. γδ T cells occupy an interface between innate and adaptive immunity, capable of rapid responses to stress-induced ligands and metabolites, and contributing to tissue integrity and early immune surveillance [30, 31]. Tregs, characterized by the transcription factor FOXP3 (Forkhead box P3) and high CD25 expression, are critical for maintaining peripheral tolerance, controlling immune activation thresholds, and preventing immune-mediated pathology [32, 33].
Within the immune microenvironment, the composition, spatial organization, and activation state of these T cell subsets are dynamically regulated by antigenic stimuli, stromal interactions, chemokine gradients, cytokine networks, and metabolic availability [34]. The balance among effector, helper, cytotoxic, and regulatory functions determines the overall immune set point, influencing whether the local environment supports protective immunity, tolerance, or chronic inflammation. Disruption of this balance can occur through altered recruitment patterns, changes in differentiation trajectories, competitive utilization of survival niches, or shifts in the metabolic landscape, ultimately reshaping the hierarchy of T cell subset dominance and modifying the immune response capacity [35, 36].
2.2 Determinants of CD8⁺ T cell trafficking, persistence and functional competence
The capacity of CD8⁺ T cells to provide effective immune surveillance is contingent upon a coordinated sequence of trafficking, infiltration, and sustained functional competence within target tissues [2]. Entry into affected sites is guided by precisely orchestrated chemokine-chemokine receptor interactions, such as those involving CXCR3- or CCR5-bearing T cells responding to gradients of inflammatory chemokines, and is facilitated by integrin-mediated adhesion to endothelial ligands including ICAM-1 (Intercellular adhesion molecule 1) and VCAM-1 (Vascular cell adhesion molecule 1). The efficiency of this process depends on the alignment of chemotactic cues with permissive vascular and stromal architecture, enabling transendothelial migration and tissue parenchyma access [37]. Infiltration can be compromised by structural alterations in vasculature, remodeling of extracellular matrix components, or the establishment of stromal configurations that physically limit lymphocyte entry and dispersal [38].
Once within the target tissue, CD8⁺ T cells must sustain effector activity in a microenvironment that can impose profound metabolic, structural, and immunological constraints. Local hypoxia and restricted nutrient availability limit glycolytic flux and mitochondrial oxidative phosphorylation, impairing the bioenergetic capacity required for proliferation and cytotoxic function [39]. Accumulation of metabolic by-products, such as lactate or adenosine, disrupts intracellular signaling pathways, dampens cytokine production, and destabilizes immunological synapse formation [40]. Persistent antigenic stimulation can induce a progressive decline in effector potential, manifested as a transcriptionally and epigenetically imprinted state of dysfunction, often accompanied by sustained expression of multiple inhibitory receptors [41]. Additional regulatory influences from soluble mediators and cellular constituents of the microenvironment modulate activation thresholds, survival signals, and differentiation trajectories, further shaping the balance between cytotoxicity and tolerance. The convergence of these structural, metabolic, and regulatory factors determines the degree to which CD8⁺ T cells can maintain their surveillance and clearance functions within challenging tissue environments [42].
2.3 Immunological and clinical relevance of enhancing CD8⁺ T cell infiltration and function
Restoring or augmenting CD8⁺ T cell function represents a pivotal objective in both fundamental immunology and translational medicine. The ability of these cells to recognize and eliminate aberrant or compromised targets directly influences the balance between immune protection and immune escape, thereby shaping long-term health outcomes. Enhancing their functional capacity encompasses not only increasing cytotoxic potential but also optimizing activation thresholds, proliferative durability, and resistance to dysfunction or exhaustion. At the immunological level, improved CD8⁺ T cell performance can recalibrate effector-to-regulatory cell ratios, reinforce immune surveillance, and maintain the structural and functional integrity of diverse tissues [43]. From a clinical perspective, strategies that fine-tune CD8⁺ T cell responses offer the prospect of sustained immune competence, greater adaptability to emerging antigens, and the capacity to modulate inflammation without compromising tolerance. Such approaches must be precisely calibrated to the immune-metabolic context, ensuring that cytotoxic activity is maximized in protective scenarios while avoiding collateral damage in settings where immune restraint is essential. The integration of mechanistic understanding with personalized intervention frameworks holds the potential to translate CD8⁺ T cell optimization into durable improvements in health span and disease resilience.
2.4 Linking CD8⁺ T cell biology to exercise immunomodulation
Given the pivotal role of CD8⁺ T cells in immune surveillance, pathogen eradication, and the orchestration of tissue-specific immune responses, strategies that enhance their trafficking, metabolic competence, and effector potential hold considerable translational promise [44]. Physical exercise emerges as a uniquely multifaceted, non-pharmacological intervention capable of simultaneously modulating systemic physiology and remodeling the immune microenvironment, thereby influencing multiple stages of the CD8⁺ T cell response continuum. Through integrated neuroendocrine activation, vascular remodeling, cytokine redistribution, and modulation of nutrient and oxygen availability, exercise can recalibrate chemokine gradients, adjust activation thresholds, and augment resistance to exhaustion-induced functional decline [45, 46]. These adaptations may, in turn, shape clonal expansion dynamics, memory formation, and cytotoxic output, with outcomes highly contingent upon the pre-existing immune-metabolic state of the host and the structural composition of the target tissue microenvironment. Dissecting how exercise-driven systemic signals converge with the molecular pathways underlying CD8⁺ T cell dysfunction—spanning altered antigen presentation, inhibitory receptor signaling, and metabolic stress—across distinct pathological settings will be critical for advancing precision exercise immunotherapy. Such an approach will require integrating high-resolution immune profiling with physiological monitoring to enable biomarker-guided prescription, optimizing cytotoxic T cell immunity while accounting for inter-individual heterogeneity in responsiveness (Figure 1).
3. Exercise-induced modulating of CD8⁺ T cell immunity under physiological conditions
Exercise functions as a dynamic physiological regulator that modulates CD8⁺ T cell immunity at multiple levels—from transient mobilization and activation to long-term metabolic adaptation and delayed senescence [47, 48]. Under physiological conditions, repeated physical activity progressively shapes the functional and metabolic states of cytotoxic T cells, maintaining immune stability while synchronizing immune responsiveness with systemic metabolic demands.
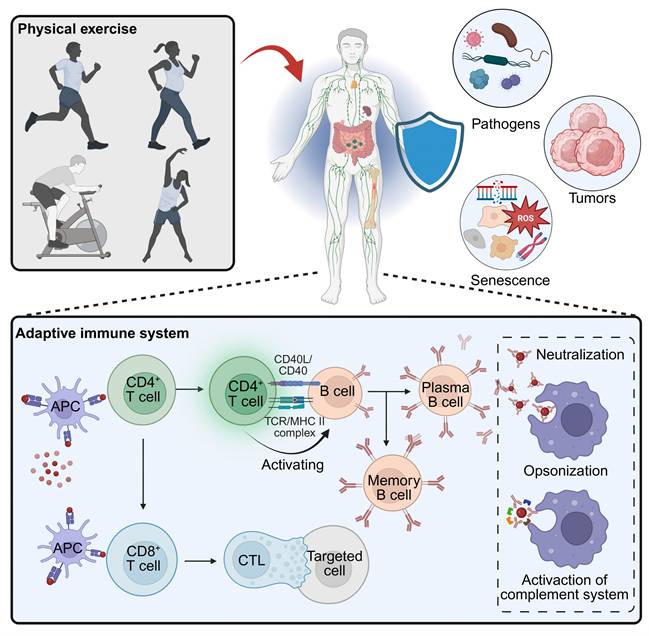
Exercise remodels adaptive immunity to enhance host defense. Physical activity elicits systemic cues that strengthen surveillance against pathogens, tumors, and senescent cells. Within the adaptive compartment, exercise optimizes APC-T-cell priming, amplifies CD4⁺ T-cell help for B-cell responses (neutralization, opsonization, complement), and improves CD8⁺ T cell trafficking, persistence, and cytolytic function in target tissues. Created in https://BioRender.com.
3.1 Acute modulating of CD8⁺ T cell mobilization and activation
Acute aerobic exercise modulates rapid redeployment and transient activation of CD8⁺ T cells, allowing the immune system to temporarily enhance cytotoxic surveillance. Transitional memory (TM) CD8⁺ T cells rise immediately and one hour post-exercise, though the increase is smaller than that of effector memory (EM) and EMRA subsets. The preferential mobilization of CD57⁺ CD8⁺ T cells—particularly within EM and EMRA compartments—identifies CD57 as a hallmark of exercise-responsive cytotoxic populations. Likewise, maximal exercise mobilizes peripheral CD8⁺ T cells, especially central memory (KLRG1⁺/CD57⁻) and senescent (KLRG1⁺/CD57⁺) subsets, increasing both frequency and absolute concentration in circulation [49]. Beyond redistribution, exercise transiently remodulates functional potential of circulating CD8⁺ T cells. Supramaximal high-intensity interval exercise induces a robust rise in CD8⁺ T cell numbers and strengthens their cytotoxic profile. Despite reduced glycolytic capacity post-exercise, these cells maintain viability and expansion potential, exhibit elevated IFN-γ release, and show enhanced nonspecific cytotoxic activity—evidence of short-term enhancement in immune surveillance [50]. A single bout of moderate-to-vigorous aerobic exercise also increases activated CD69⁺ CD8⁺ T cells in young adults, indicating an acute yet self-limited activation phase. While nutrient-sensing pathways in PBMCs remain stable, mitochondrial oxidative capacity increases at the tissue level, reflecting elevated systemic bioenergetic demand [51]. Collectively, these observations demonstrate that acute exercise modulates metabolically competent CD8⁺ T cells for transient activation, strengthening immune vigilance without eliciting chronic inflammatory signaling.
3.2 Sustained exercise remodulates metabolic fitness of CD8⁺ T cells
Beyond immediate mobilization, habitual exercise modulates long-term metabolic adaptation within the CD8⁺ T cell compartment, shaping their energetic reliance and functional resilience. Regular physical activity enhances mitochondrial dependence while reducing glucose utilization, particularly in effector memory subsets at rest. Upon activation, CD8⁺ T cells from physically active individuals demonstrate greater metabolic flexibility and reduced inflammatory output, such as lower IL-6 expression, compared with less active counterparts [52]. These features reflect a sustained remodulating of cytotoxic T cell metabolism that favors oxidative efficiency and limits inflammatory exhaustion. Aerobic fitness correlates positively with mitochondrial mass in naïve CD8⁺ T cells, reflecting enhanced metabolic capacity and energy efficiency [18]. Individuals with higher cardiorespiratory fitness display greater mitochondrial respiration and a more pronounced shift toward oxidative phosphorylation, suggesting that exercise-induced metabolic modulating maintains mitochondrial integrity and supports prolonged functional potential [53]. Collectively, these data indicate that repeated physiological challenges through exercise act as a metabolic conditioning signal, reinforcing mitochondrial health, sustaining effector competence, and preserving the balanced activation potential of the CD8⁺ T cell pool.
3.3 Exercise-mediated delay of CD8⁺ T cell immunosenescence
In addition to optimizing metabolism, exercise actively modulates the aging trajectory of CD8⁺ T cells, shaping their longevity, differentiation balance, and regenerative potential. Sustained physical activity preserves immune competence in older adults by maintaining a balanced CD4⁺/CD8⁺ ratio and supporting a higher proportion of naïve and central memory T cells [54]. This remodeling of subset composition reflects a modulatory preservation of immune diversity rather than a passive delay of decline. Active individuals display fewer terminally differentiated effector CD8⁺ T cells, even in CMV-seropositive populations, suggesting that exercise mitigates the cumulative effects of chronic antigenic load on the cytotoxic T cell compartment. Enhanced aerobic performance metrics such as VO₂max and 6-minute walk distance correlate with increased CD4⁺CD45RA⁺ and naïve CD8⁺ T cells, reinforcing the link between systemic fitness and adaptive immune preservation [55]. Moreover, moderate aerobic fitness is associated with the maintenance of telomere length in CD28⁺ CD8⁺ T cells, indicating that exercise modulates molecular longevity within the cytotoxic compartment. Individuals with moderate VO₂max exhibit a higher proportion of less differentiated CD8⁺ T cells and longer telomeres, whereas low fitness correlates with accumulation of terminally differentiated effector populations [56]. These findings together suggest that physiological exercise recalibrates CD8⁺ T cell differentiation and proliferative capacity through sustained epigenetic and metabolic conditioning, thereby delaying senescence-associated remodeling. Animal studies further substantiate these rejuvenating modulating effects. In aged rats, water-based endurance, resistance, and combined exercise regimens all reduce the proportion of aged and memory CD8⁺ T cells, with combined training most effectively restoring thymic structure and limiting memory CD4⁺ T cell accumulation [57]. In humans, low-dose combined resistance and endurance training for six weeks in elderly individuals increases the CD4⁺/CD8⁺ ratio and decreases systemic inflammatory mediators including IL-6, IL-8, IL-10, and VEGF, demonstrating that even modest physical training reestablishes immune homeostasis through functional remodulating of T cell balance [58]. Similarly, functional training modalities such as plank or Pilates exercise induce significant remodeling of immune cell composition. Plank exercise enhances CD8⁺ T cell abundance by approximately 28 % after 12 weeks, accompanied by improved respiratory capacity and overall physical performance. Aerobic and low-impact Pilates modulates further decrease immunosuppressive regulatory T cells—including IL-10-producing Tr1 and CD4⁺CD127⁻/loCD25⁺ Tregs—while increasing naïve cytotoxic CD8⁺ T cells [59, 60] (Figure 2) (Table 1). Collectively, these adaptations demonstrate that consistent exercise imposes a long-term modulating effect on CD8⁺ T cell immunity, rejuvenating the cytotoxic compartment, maintaining proliferative potential, and sustaining immune balance throughout physiological aging.
3.4 Integrative mechanisms underlying exercise-induced modulating of CD8⁺ T cell immunity
The capacity of exercise to remodulate CD8⁺ T cell immunity likely arises from the coordinated influence of metabolic, signaling, and epigenetic mechanisms that act across different temporal scales. At the metabolic level, repeated bouts of exercise may enhance mitochondrial biogenesis and oxidative phosphorylation while limiting excessive glycolytic flux [61, 62]. Activation of the AMPK-SIRT1-PGC-1α axis might promote mitochondrial turnover, preserve redox balance, and sustain bioenergetic efficiency, thereby preventing the metabolic exhaustion that often accompanies chronic stimulation [63]. Exercise-induced fluctuations in NAD⁺ availability and autophagic activity could maintain organelle integrity and ensure adaptive energy supply, establishing a metabolic environment that favors effector persistence and flexible recall responses. At the signaling level, transient endocrine and cytokine oscillations might recalibrate activation thresholds within the CD8⁺ T cell compartment. Periodic surges of catecholamines, IL-7, and IL-15 may transiently engage the PI3K-AKT-mTOR and STAT5 pathways, supporting controlled proliferation and survival while avoiding sustained activation [64, 65].
Exercise shapes CD8⁺ T cell mobilization, metabolic fitness, and immunosenescence. Different exercise modalities, including acute, chronic aerobic, supramaximal high-intensity interval, water-based, and low-intensity training, modulate CD8⁺ T cell dynamics. Exercise mobilizes naïve, effector, memory, and senescent CD8⁺ T cell subsets, enhances mitochondrial adaptation and cytotoxic mediator release (perforin, granzyme B, IFN-γ, TNF-α), and inhibits the transition of naïve to senescent cells. These effects collectively improve immune surveillance, sustain functional capacity, and delay aging-related decline in T cell immunity. Created in https://BioRender.com.
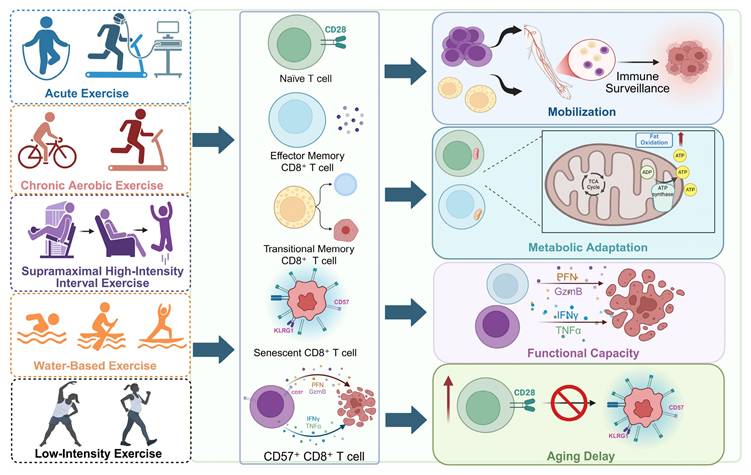
The impact of different exercise states on the physiological functions of T cells.
| Functional impact | Intensity | Outcomes | Mechanisms | Ref. |
|---|---|---|---|---|
| Mobilization | Acute exercise | Lateral-differentiated T cell subsets mobilization | Lateral T cell subsets have more β2-adrenergic receptors | [49] |
| Metabolism | HIIT | Increasing CD8+ T cell cytotoxicity decreasing glycolytic capacity | Increasing G-protein-coupled receptor activity | [50] |
| Mobilization | Moderate-to-vigorous exercise | Increasing CD69+/CD8+ T cells rising mitochondrial oxidative capacity | Upregulating glucose transporters | [51] |
| Metabolism | / | Increasing CD8+ T cell response inhibiting T cell inflammatory response | Enhancing mitochondrial dependency Reducing glucose dependency | [52] |
| Metabolism | Chronic exercise | Increasing mitochondrial mass and elevating VO2 | / | [18] |
| Metabolism | Acute endurance exercise | Rising inflammation index shifting to OXPHOS | / | [53] |
| Immunosenescence | / | Increasing proportion of naive T lymphocytes | IL-15 elevation to promote naive T cell survival | [55] |
| Immunosenescence | Moderate exercise | Increasing the proportion of CD4⁺ T cells | Enhancing telomerase activity or increasing expression of telomere-stabilizing proteins | [56] |
| Immunosenescence | Water-based training | Enhancing proliferation and activity of CD4⁺ and CD8⁺ T cells | Increasing antioxidant enzyme production and reducing oxidative stress | [57] |
| Immunosenescence | Low-dose exercise | Increasing CD4⁺/CD8⁺ T cell ratio reducing inflammation | Promoting thymic output of CD4⁺ T cells reducing the need for VEGF. | [58] |
| Mobilization | Plank exercise | Increasing CD4⁺ T cells and CD8⁺ T cells | Increasing rate of blood circulation | [59] |
| Immunosenescence | Moderate exercise | Increasing proportion of naive cytotoxic T cells | / | [60] |
Such rhythmic modulation appears to reinforce transcriptional modulates associated with stress adaptation and immune balance, potentially acting as a physiological checkpoint that prevents continuous TCR and mTOR engagement, both of which are linked to terminal differentiation and functional decline. At the chromatin level, recurrent metabolic and signaling perturbations might progressively reshape the epigenetic landscape of CD8⁺ T cells. Exercise-driven histone acetylation and DNA methylation changes—mediated in part by NAD⁺-dependent deacetylases such as SIRT1—may stabilize gene-regulatory networks that favor mitochondrial maintenance, DNA repair, and stem-like memory phenotypes. These epigenetic imprints could translate transient metabolic information into durable transcriptional states, preserving effector competence while minimizing inflammatory burden [66, 67]. Collectively, these mechanisms suggest that exercise acts as a physiological modulating signal that aligns CD8⁺ T cell immunity with systemic energy metabolism. Through integrated modulation of metabolic flux, signaling thresholds, and chromatin accessibility, regular physical activity might sustain a self-renewing, stress-resilient cytotoxic T cell pool capable of maintaining immune homeostasis.
4. Exercise-induced modulation of CD8⁺ T cell responses in cancer
Cancer represents the most comprehensively studied setting in which exercise modulates CD8⁺ T cell immunity, with mechanistic insights spanning metabolic reprogramming, cytokine signaling, and tumor-immune interactions. Accordingly, this section synthesizes the most detailed evidence available to date on how exercise shapes CD8⁺ T cell responses in malignant disease.
4.1 Exercise modulates CD8⁺ T cell immunity in precancerous conditions
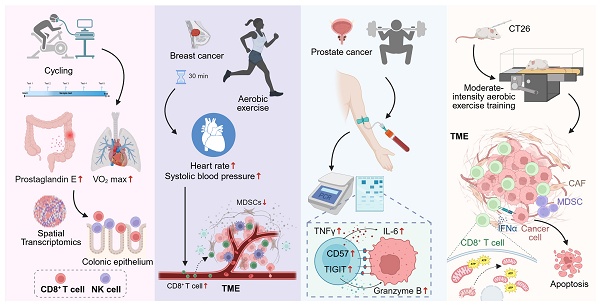
Physical activity is increasingly recognized as a physiological modulating cue that reshapes immune homeostasis and mitigates early tumorigenic risk, particularly in individuals with precancerous conditions [68-70]. In Lynch syndrome—a hereditary disorder predisposing to colorectal cancer—aerobic exercise not only enhances cardiorespiratory fitness but also modulates CD8⁺ T cell immunity within the intestinal mucosa. A 12-month structured cycling regimen promotes sustained enrichment of cytotoxic CD8⁺ T cells in the colonic epithelium, accompanied by reduced prostaglandin E levels and diminished systemic and local inflammation. Transcriptomic and spatial analyses further reveal that regular exercise reconfigures the mucosal immune landscape toward an antitumor, CD8⁺ T cell-dominant phenotype, coupled with increased NK cell infiltration and activation signatures. These findings suggest that exercise acts not merely as an immunomodulator but as a physiological modulating signal that enhances cytotoxic readiness and tissue-resident immunity, thereby reducing malignant progression risk in individuals with Lynch syndrome [71] (Figure 3A).
Exercise modulates CD8⁺ T cell responses across precancerous and cancer contexts. (A) In individuals with Lynch syndrome, long-term structured cycling enhances cardiorespiratory fitness, reduces colonic prostaglandin E levels, and promotes CD8⁺ T cell and NK cell infiltration into the colonic epithelium, as revealed by spatial transcriptomics. (B) In newly diagnosed breast cancer, a single session of moderate-intensity aerobic exercise transiently mobilizes circulating CD8⁺ T cells and NK cells, while reducing myeloid-derived suppressor cells, correlating with exercise-induced hemodynamic changes. (C) In prostate cancer, acute high-intensity exercise mobilizes CD8⁺ T cells with increased expression of CD57, TIGIT, and granzyme B, alongside transient inflammatory cytokine responses. (D) In mouse models, moderate-intensity aerobic training enhances CD8⁺ T cell infiltration and function within TME, preserves mitochondrial fitness, increases IFN-γ and ATP production, and promotes cancer cell apoptosis, thereby reducing tumor burden. Created in https://BioRender.com.
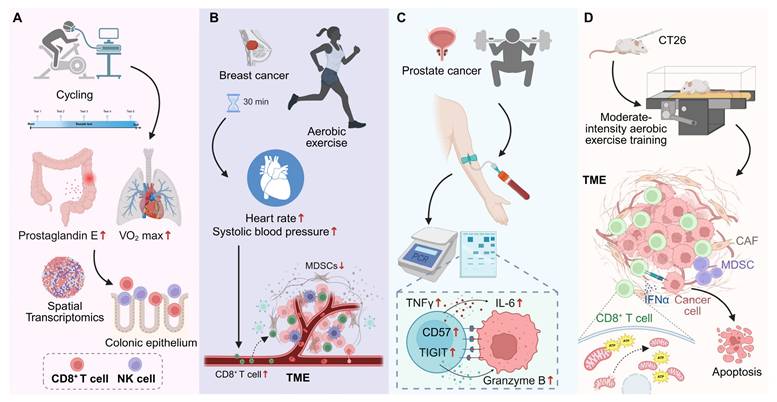
4.2 Exercise-driven remodulating of CD8⁺ T cell trafficking and tumor infiltration
Recent findings highlight exercise as a physiological remodulating cue that shapes CD8⁺ T cell trafficking and infiltration within the TME. Rather than passively enhancing immune cell recruitment, exercise actively remodulates the migratory and homing behavior of cytotoxic T cells, facilitating their entry into tumor tissue and strengthening immune surveillance [72, 73]. Epidemiological and translational studies show that habitual physical activity before cancer diagnosis selectively increases CD8⁺ T cell densities at the invasive front and tumor core in colorectal cancer, independent of age, sex, or stage—an effect not observed for total CD3⁺ T cells, underscoring the selective modulating of cytotoxic subsets [74]. In breast cancer, even brief bouts of moderate-intensity exercise trigger a transient yet directed mobilization of CD8⁺ T cells and NK cells, accompanied by a reduction in immunosuppressive myeloid-derived suppressor cells. This pattern suggests exercise-induced recalibration of the peripheral immune pool toward cytotoxic dominance, modulated by tumor hormone receptor status [75]. Similarly, a 10-minute acute exercise session increases circulating CD8⁺ T cells by approximately 34%, with mobilization tightly correlated to hemodynamic parameters such as systolic pressure and heart rate, implying a mechanistic link between vascular stress and T cell trafficking modulating [16] (Figure 3B). In lymphoma and early-stage prostate cancer, high-intensity exercise induces transient mobilization of CD8⁺ T cells expressing CD57, TIGIT, and Granzyme B, reflecting a shift toward effector modulating. In animal models, moderate-intensity training reduces tumor burden through enhanced CD8⁺ T cell frequency and improved CD4⁺/CD8⁺ balance, with negative correlations between cytotoxic T cell abundance and tumor mass [76]. Collectively, these findings demonstrate that exercise exerts a dose- and intensity-dependent modulating effect on CD8⁺ T cell trafficking, promoting effective infiltration and immune orchestration within the TME.
4.3 Exercise-mediated restoration of CD8⁺ T cell functionality in cancer
Exercise functions as a remoduting signal that restores functional integrity to metabolically and epigenetically exhausted CD8⁺ T cells within tumors [77]. In multiple myeloma, a six-month structured training program significantly reduces exhaustion marker expression (PD-1, TIGIT, TIM-3, LAG-3), reshaping the transcriptional and metabolic modulating of CD8⁺ T cells toward a rejuvenated cytotoxic state [78]. Mechanistically, exercise-induced myokines and metabolites remodel central carbon metabolism in both mice and humans, augmenting mitochondrial respiration and effector cytokine production. Adoptive transfer of CD8⁺ T cells from exercised mice confers superior antitumor efficacy in untrained hosts, providing direct evidence that exercise-driven metabolic remodulating enhances cytotoxic potential and memory formation [79]. Moreover, moderate aerobic exercise improves CD8⁺ tumor-infiltrating lymphocyte (TIL) function by preserving mitochondrial integrity and oxidative capacity. Enhanced IFN-γ release and ATP generation correspond with reduced mitochondrial loss and hypoxia within tumors, indicating that exercise modulates mitochondrial fitness and effector persistence in situ [80] (Figure 3D). Collectively, these findings illustrate that structured physical activity not only alleviates CD8⁺ T cell exhaustion but reinstates their functional modulating, transforming exercise into a non-pharmacological intervention capable of restoring antitumor immunity.
4.4 Exercise-induced remodulating of the TME and CD8⁺ T cell dynamics
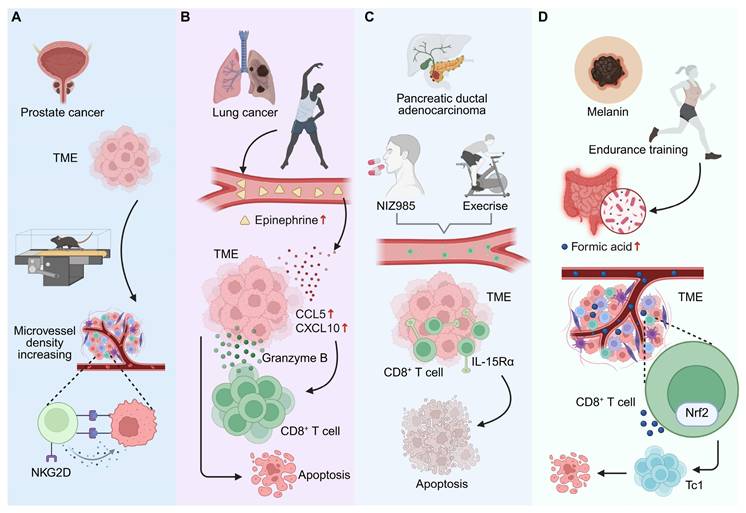
The TME critically governs immune responsiveness and tumor progression [81]. Exercise functions as a systemic remodulating cue that remodels both vascular and immune niches within the TME, thereby reprogramming CD8⁺ T cell trafficking, persistence, and effector engagement. Aerobic exercise restructures the melanoma microenvironment through ERK5-dependent vascular normalization, enhancing perfusion and facilitating CD8⁺ T cell infiltration. In YUMMER tumors, this vascular-immune remodeling suppresses tumor growth, increases cytotoxic T cell accumulation, and induces phenotypic shifts in tumor-associated macrophages, including elevated MHC II expression [82]. Similarly, voluntary running enhances antitumor immunity by increasing CD8⁺ T cell infiltration and NKG2D expression within tumors. Exercise generates a microvascular network characterized by higher density and improved regularity, which optimizes immune cell delivery and oxygenation. After 28 days of continuous exercise, tumors exhibit a marked rise in CD8⁺ T cell abundance, correlating with improved vascular function and reduced tumor mass [83] (Figure 4A). Notably, exercise-induced extracellular vesicles (EVs) extend this remodulating effect beyond systemic circulation. In triple-negative breast cancer, exercise-derived EVs transform the TME into a more immunologically active state by promoting CD8⁺ T cell infiltration and activation. Pre- and post-implantation administration of these EVs suppresses tumor growth in both EO771 and 4T1 models, particularly converting immune-excluded 4T1 tumors into T cell-responsive, inflamed phenotypes [84]. These findings underscore that exercise-induced systemic cues—including EVs and vascular remodeling—modulate a permissive microenvironment for CD8⁺ T cell immunity, thereby transforming cold tumors into immunologically “hot” states and potentiating responsiveness to immunotherapy.
4.5 Exercise-induced neuroimmune remodulating enhances CD8⁺ T cell-mediated antitumor immunity
Accumulating evidence suggests that exercise engages neuroimmune cross-talk to remodulate the activation and trafficking of CD8⁺ T cells within tumors [85, 86]. Moderate-intensity exercise elevates systemic epinephrine, which in turn upregulates chemokines CCL5 and CXCL10 in the TME, driving chemotactic recruitment and intratumoral accumulation of CD8⁺ T cells. This neuroendocrine-immune coupling results in significant tumor growth inhibition, an effect that can be recapitulated by exogenous epinephrine, confirming the β-adrenergic-dependent modulating of cytotoxic T cell migration and activation [87] (Figure 4B). Mechanistically, activation of β₂-adrenergic receptors during exercise mobilizes effector lymphocytes through cAMP-dependent signaling cascades that enhance TCR sensitivity and cytotoxic potential. Pharmacological modulation of this axis—specifically β₁-receptor blockade combined with PDE4 inhibition—further amplifies CD8⁺ T cell recruitment and effector activity [88]. These observations position neuroimmune signaling as a dynamic modulating interface through which exercise fine-tunes the magnitude, localization, and efficacy of CD8⁺ T cell responses against tumors.
4.6 Exercise enhances tumor immunotherapy efficacy through CD8⁺ T cell modulating
Exercise amplifies the efficacy of cancer immunotherapy by remodulating CD8⁺ T cell metabolism, infiltration, and effector activation [14, 89]. Through this integrative physiological conditioning, exercise enhances tumor immune surveillance and potentiates the action of immune checkpoint inhibitors [90]. In pancreatic ductal adenocarcinoma (PDAC), exercise stimulates IL-15/IL-15Rα signaling, driving expansion and recruitment of CD8⁺ T cells into tumors and strengthening cytotoxic responses. Pharmacologic activation of this pathway using the IL-15 superagonist NIZ985 mimics these effects, sensitizing tumors to PD-1 blockade and augmenting chemotherapy efficacy [89] (Figure 4C). In colorectal cancer, acute exercise preceding immuno-chemotherapy accelerates early CD8⁺ T cell infiltration, producing a transient enhancement of immune efficacy that significantly reduces tumor volume during combination anti-PD-1 therapy [91]. In breast cancer, exercise remodulates the tumor vasculature and immune milieu through the CXCL9/CXCL11-CXCR3 signaling axis, improving vessel perfusion, reducing hypoxia, and enabling sustained CD8⁺ T cell infiltration. This vascular-immune coordination renders previously resistant tumors responsive to PD-1 and CTLA-4 blockade [20]. Beyond direct immune activation, exercise-induced metabolic remodulating via the gut microbiota contributes to the enhancement of CD8⁺ T cell-mediated immunity. In melanoma, exercise increases microbial production of formate, a metabolite that promotes Tc1 differentiation and cytotoxic function through Nrf2 activation. Elevated formate levels correlate with heightened antigen-specific CD8⁺ T cell activity and improved checkpoint inhibitor efficacy in vivo [92] (Figure 4D). Collectively, these findings demonstrate that exercise not only complements immunotherapy but modulates a metabolically and transcriptionally resilient CD8⁺ T cell response, thereby extending the therapeutic window for effective tumor control.
5. Exercise-induced modulation of CD8⁺ T cell responses in non-malignant diseases
While cancer provides the most extensively studied context for exercise-induced modulation of CD8⁺ T cell immunity, emerging evidence suggests that similar principles also extend to a range of non-malignant diseases. However, the depth and maturity of mechanistic insights vary considerably across these conditions. In this chapter, we summarize current knowledge on how exercise influences CD8⁺ T cell responses in infectious, neurological, and metabolic diseases, highlighting both shared immunological themes and areas where evidence remains preliminary. This broader perspective helps clarify the generalizability and limitations of exercise-induced CD8⁺ T cell modulation beyond cancer.
Exercise-mediated modulation of tumor microenvironment and CD8⁺ T cell-related antitumor immunity. (A) In prostate cancer, exercise increases microvessel density in TME, promoting NKG2D-related immune responses and potentially enhancing immune cell delivery to tumors. (B) In lung cancer, moderate-intensity exercise raises systemic epinephrine levels, which upregulate chemokines CCL5 and CXCL10 in the TME, recruiting CD8⁺ T cells and leading to tumor cell apoptosis. (C) In PDAC, exercise enhances CD8⁺ T cell mobilization and intratumoral accumulation via IL-15/IL-15Rα signaling. Pharmacologic activation with NIZ985 mimics these effects, enhancing chemotherapy and immunotherapy efficacy. (D) In melanoma, endurance training increases formic acid production in the gut microbiota. Formic acid activates Nrf2 in CD8⁺ T cells, promoting Tc1 differentiation and enhancing antitumor immune responses. Created in https://BioRender.com.
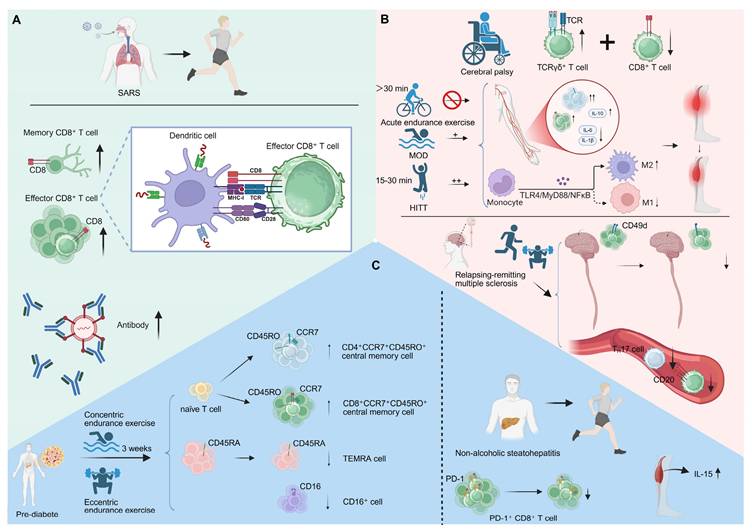
Exercise-induced T cell mobilization and immune modulation in various conditions. (A) Acute exercise mobilizes SARS-CoV-2-specific CD8⁺ T cells, increasing their circulation and enhancing immune surveillance. (B) In cerebral palsy, sufficient exercise intensity is needed for CD8⁺ T cell activation. HIIT enhances CD8⁺ T cell infiltration and function post-stroke and modulates macrophage activation. In relapsing-remitting multiple sclerosis, a 10-week multimodal exercise program reduces CD49d expression on CD8⁺ T cells and decreases proinflammatory Th17 cell levels. (C) In pre-diabetes, three weeks of concentric or eccentric endurance exercise reverses T cell senescence, increasing naïve and central memory T cells. In NASH, exercise reduces the accumulation of PD-1⁺ CD8⁺ T cells in the liver and elevates muscle - derived IL-15, mitigating chronic liver injury. Created in https://BioRender.com.
5.1 Exercise-induced CD8+ T cell mobilization and immune modulation in infection
By recalibrating the interplay between activation, memory formation, and exhaustion, exercise shapes CD8⁺ T cell functionality to sustain effective defense without excessive inflammation. This dynamic immunoregulation is reflected across distinct viral settings, where exercise intensity, duration, and context collectively influence the modulating of cytotoxic T cell responses. In influenza infection, an eight-week treadmill exercise program enhances resistance to influenza while dampening long-term CD8⁺ T cell memory formation in the lungs. Despite reduced granzyme B⁺ CD8⁺ T cell and antibody responses, exercised mice retain protection upon reinfection, indicating that moderate exercise recalibrates antiviral immunity by limiting inflammation yet preserving effective CD8⁺ T cell defense [93]. Similarly, an eight-week endurance regimen augments antiviral responses by increasing CD8⁺ T cell infiltration into the lungs and reducing TNF-α production in influenza-specific cytotoxic cells. In obese hosts, exercise restores delayed immune activation, whereas in lean counterparts it promotes early viral clearance with restrained inflammation [94]. These findings demonstrate that exercise intensity and metabolic context cooperatively modulate CD8⁺ T cell functionality to balance protection and tissue preservation during viral infection.
Emerging evidence in SARS-CoV-2 infection further supports this immune modulating paradigm. Acute exercise mobilizes a broad spectrum of SARS-CoV-2-specific CD8⁺ T cells, enhancing immune surveillance in individuals with natural immunity to the virus [95]. Exercise induces a 2.5-fold increase in circulating functional CD8⁺ T cells, which recognize various viral peptides, including those from the spike, nucleocapsid, and membrane proteins. This response is further accompanied by a transient rise in SARS-CoV-2 neutralizing antibodies. Notably, exercise predominantly mobilizes MHC I-restricted CD8⁺ T cells, maintaining broad TCR-β diversity [96] (Figure 5A). Additionally, exercise rehabilitation restored exercise tolerance in post-hospitalised COVID-19 patients and enhanced immune homeostasis, as evidenced by increased naïve and memory CD8⁺ T cell subsets. Structured physical activity may thus remodulate CD8⁺ T cell dynamics toward a rejuvenated phenotype, suggesting that targeted exercise regimens can exert immunorestorative effects in post-COVID syndrome [97].
In persistent viral infections such as cytomegalovirus (CMV), regular combined exercise training rejuvenates CD8⁺ T cell immunity in CMV-seropositive older adults and enhances influenza vaccine responsiveness. These programs reduce CMV-specific immunoglobulins, restore naïve CD8⁺ T cell pools, and shift the effector-to-naïve balance, accompanied by elevated IL-10 and an anti-inflammatory milieu [98]. In parallel, prolonged endurance training reverses infection-driven immunosenescence. A six-week strength-endurance regimen selectively reduces senescent T lymphocytes and expands naïve CD8⁺ populations in CMV-seropositive older women, reinforcing that sustained physical activity remodulates CD8⁺ T cell homeostasis and counteracts the age- and infection-related decline in immune flexibility [99]. At the acute level, exercise intensity governs T cell redeployment, with vigorous exertion eliciting stronger mobilization and type I cytokine expression in CD8⁺ T cells. CMV infection further skews this response toward highly differentiated phenotypes, although its amplifying effect is independent of intensity [100]. Together, these findings suggest that both acute and chronic exercise dynamically orchestrate CD8⁺ T cell trafficking, differentiation, and cytokine modulating, integrating metabolic stress and viral history to fine-tune immune activation.
In chronic active infections such as HIV, exercise exerts distinct immunomodulatory effects. A 12-week non-linear resistance training program enhanced immune competence in individuals living with HIV, elevating circulating CD4⁺ and CD8⁺ T cell counts while suppressing pro-inflammatory cytokines and increasing IL-10. This coordinated shift toward an anti-inflammatory and immunoregulatory profile indicates that structured resistance exercise can restore T cell homeostasis and counteract chronic immune activation in HIV infection [101]. Notably, exhaustive exercise transiently mobilized mature and naïve T lymphocytes, yet HIV-infected individuals exhibited blunted CD4⁺ T cell recruitment and exaggerated CD8⁺ responses. This dysregulated T cell redistribution underscores impaired immune adaptability despite effective viral suppression, revealing that physical stress exposes persistent defects in T cell mobilization and homeostatic control in treated HIV infection [102].
5.2 CD8⁺ T cell-based immune modulation in neurological disorders induced by exercise
Exercise profoundly influences neuroimmune interactions, reshaping CD8⁺ T cell behavior across a spectrum of neurological conditions. Adults with cerebral palsy exhibit elevated baseline levels of TCRγδ⁺ T cells, indicative of chronic low-grade inflammation, and display a blunted CD8⁺ T cell mobilization following acute endurance exercise. The absence of post-exercise cytotoxic activation, despite comparable perceived exertion, likely reflects insufficient physiological intensity. These findings suggest that adequate exercise intensity is essential to modulate functional CD8⁺ T cell activation in individuals with cerebral palsy, underscoring the need for tailored immunometabolic interventions [103]. Similarly, high-intensity interval training (HIIT) enhances CD8⁺ T cell infiltration and effector activity after stroke, outperforming moderate-intensity aerobic training. HIIT elevates the CD4⁺/CD8⁺ ratio and circulating IL-10 while suppressing proinflammatory cytokines (IL-1β, IL-6) and rebalancing macrophage polarization through the TLR4/MyD88/NFκB axis. These adaptations highlight that exercise intensity dynamically modulates T cell-macrophage crosstalk to restrain post-stroke inflammation and muscle atrophy [104]. Moreover, a 10-week multimodal exercise program in individuals with relapsing-remitting multiple sclerosis remodulates CD8⁺ T cell immunity by reducing CD49d expression and limiting CNS homing. Exercise also lowers CD8⁺CD20⁺ and Th17 frequencies, collectively promoting a less encephalitogenic immune state. Such remodeling exemplifies exercise-driven modulating of CD8⁺ T cell trafficking and phenotype, ultimately dampening neuroinflammation and disease activity [105] (Figure 5B). A four-month moderate aerobic exercise program improved sleep and mood while recalibrating immune balance in patients with chronic insomnia. Exercise lowered circulating CD4⁺ and CD8⁺ T cell counts together with reduced cortisol levels, indicating a coordinated neuroendocrine-immune adjustment that restores physiological and immune equilibrium [106].
5.3 Metabolic disorders and exercise-induced CD8⁺ T cell modulation
Exercise has emerged as a potent regulator of immune homeostasis, capable of remodulating CD8⁺ T cell-mediated metabolic-immune interactions and attenuating the progression of metabolic disorders such as pre-diabetes and non-alcoholic steatohepatitis (NASH). [107, 108]. In pre-diabetic individuals, three weeks of concentric or eccentric endurance exercise remodulates T cell immunity by reversing hallmarks of cellular senescence and reshaping the CD8⁺ T cell compartment. Exercise increases the proportion of naïve and central memory CD4⁺ and CD8⁺ T cells—particularly CD4⁺CCR7⁺CD45RO⁺ and CD8⁺CCR7⁺CD45RO⁺ subsets—while reducing terminally differentiated effector memory (TEMRA) and CD16⁺ cells. This coordinated remodeling suggests that exercise promotes the renewal and mobilization of naïve T cells while facilitating the clearance of senescent populations, thereby rejuvenating immune competence in pre-diabetes [109]. Notably, a single bout of vigorous exercise selectively mobilizes differentiated CD8⁺ EMRA T cells; however, this response is markedly attenuated in individuals with type 1 diabetes. Reduced expression of adhesion and activation markers indicates defective trafficking and activation potential of these effector cells [110]. Similarly, acute maximal exercise lowers circulating CD4⁺ T cells and the CD4⁺/CD8⁺ ratio while increasing NK-cell activity, yet fails to enhance CD8⁺ T cell recruitment in type 1 diabetes [111]. These findings suggest that the capacity of exercise to modulate transient immune surveillance through CD8⁺ T cell redeployment is impaired in type 1 diabetes.
In a similar vein, exercise attenuates the progression of NASH by remodulating intrahepatic immune networks, particularly through reducing PD-1⁺ CD8⁺ T cell accumulation linked to liver inflammation and fibrosis. The decline in exhausted CD8⁺ T cells parallels improved hepatic architecture and suppressed proinflammatory cytokine production. Concurrently, elevated muscle-derived IL-15 orchestrates systemic immunometabolic communication. Collectively, these adaptations highlight exercise-driven remodulating of CD8⁺ T cell phenotypes as a pivotal mechanism mitigating chronic immune-mediated liver injury in NASH [112] (Figure 5C). Notably, an eight-week wheel-running exercise program restored bone integrity in ovariectomized mice by activating CD8⁺ T cells and elevating IFN-γ production. The resulting IFN-γ-dependent suppression of NF-κB and MAPK signaling inhibited osteoclastogenesis, linking exercise-induced CD8⁺ T cell activation to immune-mediated protection against bone loss [113] (Table 2).
6. Clinical evidence on exercise-mediated modulation of CD8⁺ T cell dynamics in aging, metabolic and cancer contexts
Clinical evidence increasingly indicates that exercise modulates immune function, particularly through the regulation of CD8⁺ T cell dynamics, metabolic fitness, and immune responsiveness. Structured training programs enhance immune surveillance and confer benefits across aging, metabolic and cancer contexts. A 12-week combined strength and endurance training remodulates CD8⁺ T cell immunity in older adults by constraining excessive differentiation and preserving a less-senescent phenotype. This intervention redirects kynurenine metabolism toward kynurenic-acid synthesis, mitigating inflammatory and neurotoxic stress. Collectively, these coordinated metabolic and phenotypic shifts indicate that structured exercise recalibrates immunometabolic circuits to sustain functional CD8⁺ T cell competence during aging [114]. Both cardiorespiratory and resistance exercise interventions acutely remodel circulating CD8⁺ T cell pools (CD45RA⁺CD62L⁻ and CD57⁺CD45RA⁺CD62L⁻) in older adults. Resistance exercise expands cytotoxic populations in sedentary individuals, whereas endurance modalities preferentially enhance responsiveness in trained participants. These modality- and fitness-dependent effects illustrate how exercise parameters can be tuned to remodulate transient CD8⁺ T cell redeployment and optimize cytotoxic readiness [115].
In prediabetic individuals, a 12-week structured exercise intervention restores angiogenic CD31⁺ CD8⁺ T cell populations, linking improved metabolic capacity to vascular protection. This adaptive recalibration of CD8⁺ T cell subsets coincides with enhanced VO₂max and muscle strength, suggesting that exercise aligns immune and metabolic networks to counter cardiometabolic dysfunction [116]. Consistently, in adults newly diagnosed with type 1 diabetes, a 12-week high-intensity interval training re-educates autoimmune CD8⁺ T cell activity by reducing the frequency and proliferation of islet-reactive clones. By restraining pathogenic CD8⁺ responses, exercise may preserve β-cell function and maintain glycaemic stability during the early autoimmune phase [117].
In localized prostate cancer, perioperative yoga training remodulates systemic immunity by increasing circulating CD8⁺ T cells and IFN-γ production while reducing regulatory T cells and myeloid-derived suppressor cells. This coordinated remodeling fosters an antitumor immune phenotype and exemplifies how exercise can modulate effector-suppressor balance [118]. Likewise, in chronic lymphocytic leukemia (CLL), a 16-week physical activity intervention enhances fitness and reduces PD-1 expression on both CD4⁺ and CD8⁺ T cells, alleviating T cell exhaustion. The resulting higher CD4⁺:CD8⁺ ratio and improved cytotoxic competence suggest that sustained exercise reinvigorates modulated T cell functionality to support durable immune control [119].
The impact of various exercise modalities on immune cell alterations, underlying mechanisms, and clinical significance in patients with diverse diseases.
| Exercise intensity | Exercise duration | Disease type | Immune cell changes | Mechanism | Implication | Ref. |
|---|---|---|---|---|---|---|
| Aerobic cycling | 12 months | Lynch syndrome | NK cells↑ CD8+ T cells↑ | / | Enhancing intestinal mucosal immunity | [71] |
| Physical activity 3 times per week | / | Colorectal cancer | CD8+ T cells in tumor front and center↑ | / | Shifting immune profile towards anti-tumorigenic state | [74] |
| Moderate-intensity | 30 minutes | Breast cancer | NK and CD8+ T cells↑ MDSCs↓ | Hemodynamic regulation | Promoting anti-tumorigenic immune response | [16] |
| High-intensity | / | Prostate cancer | NK, NKT-like, and CD8+ T cell concentration↑ | Increasing TNF-α and IL-6 leads to mobilization of immune cells | Improving NK cell cytotoxic activity against cancer cells | [126] |
| Moderate-intensity | 12 weeks | Breast cancer | CD4⁺/CD8⁺, CD3+/CD4+↑ | / | Reducing tumor burden | [76] |
| Moderate-intensity | Six months | Myeloma | T cell exhaustion markers↓ | / | Reducing immune exhaustion | [78] |
| Acute exercise | / | Mammary cancer | CD8+ T cells↑ | Metabolites produced in skeletal muscle | Enhancing effector profile of CD8+ T cells | [79] |
| Moderate-intensity | 1 hour/day 5 days/week | Colon cancer | CD8⁺ TIL infiltration↑ | Decreasing mitochondrial loss and increasing effector function | Elevating IFN-γ and ATP production | [80] |
| Aerobic exercise | 2 weeks | Melanoma | CD8+ T cell infiltration↑ | Modulating ERK5S496 phosphorylation | MHC II upregulation | [82] |
| Voluntary running | 14 or 28 days | Prostate cancer | CD8+ T cells↑ NKG2D receptors↑ | Improving blood perfusion | Regulation of tumor vasculature | [83] |
| Moderate-intensity | 2 weeks | Breast cancer | CD8+ T cells↑ | Extracellular vesicles ignite inflammation | Shifting TNBC tumors towards inflamed phenotype | [122] |
| Moderate-intensity | 45 min/day 5 days/week | Lung cancer | CD8+ T cells↑ Epinephrine↑ | Upregulating CCL5 and CXCL10 | Inhibiting tumor growth | [87] |
| Moderate to high intensity | / | Lymphoma | Mobilization of lymphocytes and NK-cells | Increasing cAMP signaling | β2-adrenergic receptor activation | [88] |
| Moderate intensity | 6 weeks | PDAC | CD8+ T cells infiltration↑ NK cell activity↑ | Engagement of IL-15/IL-15Rα axis | Enhancing sensitivity to chemotherapy | [89] |
| Acute exercise | 1 week | Colorectal cancer | CD8+ cytotoxic T cell infiltration↑ | / | Improving effectiveness of immunotherapy | [91] |
| Moderate to high intensity | 7-14 days | Breast cancer | CD8+ T cells infiltration↑ exhausted T cells↓ | Activating CXCL9/CXCL11-CXCR3 signaling axis | Inducing vascular normalization | [20] |
| Moderate intensity | / | Melanoma | Tumor antigen-specific Tc1↑ | Formating activates Nrf2 to promote Tc1 differentiation | Enhancing Tc1-mediated ICI efficacy | [92] |
| Acute exercise | 20 min | SARS-CoV-2 infection | CD4+ T and CD8+ T cells↑ | Catecholamine and β2-adrenergic receptor activation | Increasing SARS-CoV-2 immunosurveillance | [96] |
| Acute endurance exercise | 45 min | Cerebral palsy | TCRγδ+ T cells↑ CD8+ T cells↑ | Regulation cytokine production and T cell receptor expression | Enhancing immune responses to infections | [103] |
| High-intensity interval training | 3 weeks | Muscle atrophy | M1 and M2 type macrophages↑ | Downregulating TLR4/MyD88/NFκB signaling | Improving muscle repair and physical function | [104] |
| Multimodal exercise | 10 weeks | Multiple Sclerosis | Frequency of Th17 cells↓ | Reducing CD49d expression | Decreasing CNS-homing potential of CD8+ T cells | [105] |
| Endurance exercise | 3 weeks | Pre-diabetic | CD4+/CD8+↑ CD4+/CD3+↑ | Stimulating production and mobilization of naïve T cells | Reversing hallmarks of T cell senescence | [109] |
| High-intensity interval training | 60 min/day 5 days/week | Nonalcoholic steatohepatitis | PD-1+CD8+ T cells↓ | Inhibiting TLR4/MyD88/NFκB signaling pathway | Altering intrahepatic immune cell profile | [112] |
Despite growing interest in this area, clinical evidence remains limited. Ongoing registered trials — including functional characterization of CD8⁺ T cells after supramaximal exercise (NCT06484101), exercise and COVID-19 viral T cell immunity (NCT05019456), and T cell responses to various levels of exercise stress (NCT06638684) — are expected to further clarify how exercise programs influence CD8⁺ T cell dynamics and immune resilience. However, as these trials have not yet reported results, current conclusions remain preliminary.
7. Challenges and future perspectives
Despite accumulating evidence that exercise can reshape CD8⁺ T cell-mediated immunity across diverse physiological and pathological settings, several unresolved challenges continue to limit its optimization and translation into clinical practice. These challenges span methodological uncertainties, biological complexities, and barriers to clinical implementation, each constraining the ability to design, monitor, and apply exercise regimens as precise immunomodulatory interventions.
Although exercise is increasingly recognized as a potent modulator of immune homeostasis, the precise “dose” required to maximize CD8⁺ T cell-mediated benefits without inducing adverse effects remains poorly defined. Immune adaptation to exercise follows a non-linear, context-dependent curve in which both insufficient and excessive intensity can blunt or even reverse immunological gains. Preclinical evidence illustrates this complexity. In breast cancer models, only moderate-intensity training, unlike low- or high-intensity regimens, enhanced antitumor immunity by increasing CD8⁺ T cell infiltration and effector function, including elevated proliferation and expression of CD69, IFN-γ, and granzyme B, in parallel with reduced tumor burden [120]. While both low- and moderate-intensity exercise normalized tumor vasculature, only the latter augmented CD8⁺ T cell-mediated responses, indicating that vascular remodeling alone is insufficient to drive immune activation without appropriate intensity-dependent cues. Similar patterns are observed in human studies. In older women at high risk of breast cancer, moderate-intensity aerobic training increased circulating CD8⁺ effector memory T cells and reduced highly differentiated EMRA cells, suggesting attenuation of immunosenescence, with these changes correlating to improved cardiorespiratory fitness and increased β₂-adrenergic receptor expression on central memory CD8⁺ T cells. In contrast, high-intensity interval training decreased total CD4⁺ T cells and naïve subsets, potentially accelerating immune aging [121]. The challenge lies not only in the biological non-linearity of these responses but also in the methodological heterogeneity of defining intensity, ranging from percentage of VO₂max to lactate threshold or perceived exertion, which impairs cross-study comparability and precludes precise meta-analysis. Moreover, physiological workload does not necessarily equate to immunological load, as individuals matched for relative intensity can exhibit divergent T cell mobilization kinetics and cytokine responses depending on baseline inflammatory tone, hormonal milieu, or disease state. Addressing this challenge requires harmonized frameworks that couple continuous physiological monitoring such as heart rate variability, oxygen uptake kinetics, and lactate clearance with longitudinal immune phenotyping, encompassing naïve-to-memory T cell ratios, activation markers including HLA-DR and CD69, and effector molecule production. Stratified multi-centre trials that integrate these metrics, combined with machine learning-based modeling, could generate individualized intensity-response curves, enabling both rigorous cross-study standardization and the development of dynamically adaptive, biomarker-guided exercise prescriptions tailored to specific immune profiles and disease contexts.
The immunological consequences of exercise are profoundly shaped by the cellular and metabolic architecture of the disease microenvironment, making the direction and magnitude of CD8⁺ T cell modulation highly unpredictable. Identical exercise regimens can yield diametrically opposed effects depending on immune composition, stromal organization, vascular integrity, and metabolic constraints. In EO771 breast tumors, exercise alone reduces both CD8⁺ T cell abundance and their proportion among total T cells, yet synergizes with anti-PD-1 therapy to enhance CD8⁺ T cell infiltration [122]. In contrast, B16-F10 melanomas show minimal CD8⁺ T cell modulation with either intervention [123]. These findings highlight that the impact of exercise on cytotoxic T cell responses is context-dependent and may enhance checkpoint blockade efficacy in immunotherapy-refractory tumors. Addressing this challenge requires a shift from one-size-fits-all prescriptions to microenvironment-informed exercise design. Pre-intervention profiling of the immune-metabolic state—quantifying inflammatory mediators such as IL-6 and TNF-α, immune checkpoint expression patterns, oxygenation status, and metabolite availability—could be used to classify patients into predicted responder or non-responder categories. Such biomarker-guided stratification would enable tailoring of exercise parameters, including intensity, duration, and modality, to the underlying immune context. Iterative monitoring during intervention cycles, leveraging circulating immune phenotyping, tumor-derived extracellular vesicle analysis, and non-invasive metabolic imaging, would allow dynamic adjustment of regimens as the microenvironment evolves. Integrating these data streams into predictive computational models could ultimately permit the generation of individualized, adaptive exercise prescriptions that account for microenvironmental heterogeneity and maximize beneficial T cell modulation across diverse disease states.
While exercise exerts multifaceted benefits on immune surveillance and T cell function, its physiological effects can also generate conditions that transiently or chronically compromise immune homeostasis. Sympathetic activation during acute exercise promotes adrenaline-mediated CD8⁺ T cell trafficking into peripheral tissues and tumor sites, yet this same adrenergic surge can trigger transient elevations in pro-inflammatory cytokines, potentially exacerbating inflammation in vulnerable individuals [88]. Similarly, moderate increases in tissue perfusion enhance oxygen and nutrient delivery, facilitating immune cell migration and antigen presentation, but abrupt hemodynamic fluctuations in patients with advanced atherosclerosis or vascular aneurysms may precipitate cardiovascular events [124]. At the redox level, physiological levels of reactive oxygen species (ROS) generated during exercise act as secondary messengers to promote T cell activation, mitochondrial biogenesis, and adaptive immune responses, whereas excessive ROS induces oxidative stress, DNA damage, and telomere attrition, accelerating immunosenescence [125]. These dualistic effects highlight the need for risk-benefit calibration that considers both the intended immunological gains and systemic physiological vulnerabilities. Beyond biomarker monitoring, incorporating pre-intervention cardiovascular screening, individualized progression in training load, and concurrent use of redox-buffering nutritional strategies may reduce collateral risks while preserving immune benefits.
The translation of exercise-induced T cell modulation into clinical strategies is hindered by significant methodological and population biases. Much of the existing evidence derives from young, healthy individuals or animal models, with underrepresentation of older adults, patients with chronic diseases, and immunocompromised populations—the very groups most likely to benefit from immune support. This gap limits the external validity of current findings. Compounding this is the temporal complexity of T cell kinetics: mobilization, activation, and homing follow tightly regulated time courses, with peak effects often occurring within hours of exercise and rapidly resolving thereafter. Low-frequency sampling in clinical studies fails to capture these transient but functionally critical windows. Furthermore, the overwhelming reliance on peripheral blood measurements overlooks tissue-level dynamics, particularly within TMEs or sites of chronic inflammation, where the immune context may differ fundamentally from systemic circulation. Overcoming these barriers will require the development of high-resolution, minimally invasive monitoring platforms—such as wearable biosensors for cytokines, microfluidic devices for continuous immune cell profiling, and imaging modalities capable of capturing in situ T cell behavior. Longitudinal, disease-specific cohort studies incorporating such technologies will be essential to map the true spatiotemporal landscape of exercise-induced immune adaptation.
Despite promising immunological signals, the pathway from exercise-induced biomarker changes to clinically meaningful outcomes remains incompletely defined. The minimal effective “dose” of exercise necessary to sustain T cell rejuvenation is unknown, and patient adherence is frequently suboptimal, especially among those with physical limitations, fatigue, or treatment-related side effects. Without clear dose-response data, clinicians face difficulty in prescribing exercise regimens with predictable outcomes. More critically, improvements in surrogate immunological endpoints—such as increased naïve T cell frequencies or reduced regulatory T cell proportions—do not consistently correlate with hard clinical endpoints, including progression-free survival, relapse rates, or quality of life. This uncertainty limits exercise adoption as a formal adjunct to therapeutic protocols. Addressing these gaps will require randomized controlled trials that are sufficiently powered and of adequate duration to assess both immunological and clinical endpoints in parallel. Innovative trial designs could embed adaptive intervention arms, testing combinations of exercise with pharmacological or nutritional adjuncts to identify synergistic strategies that optimize patient adherence and maximize translation into durable clinical benefit.
8. Conclusion
The evidence synthesized here positions physical exercise as a systems-level regulator of CD8⁺ T cell biology, linking acute mobilization and trafficking to durable remodeling of subset composition, metabolic resilience and effector potential across both physiological and pathological contexts. By placing CD8⁺ T cells at the core of immune surveillance and tissue protection, this Review integrates mechanistic and translational perspectives to define when and how exercise can be leveraged to reinforce cytotoxic immunity. Mechanistically, convergent neuroendocrine, vascular and metabolic signals recalibrate chemokine gradients, activation thresholds and energetic supply, thereby shaping clonal expansion, memory formation and cytotoxic output. These adaptations encompass the rapid recruitment of activated and differentiated CD8⁺ subsets, preservation of mitochondrial competence, and attenuation of immunosenescence with sustained training, although their magnitude and direction remain highly contingent on host condition and the immune-metabolic architecture of target tissues.
Translating these insights into clinical benefit requires resolution of key uncertainties surrounding dose-response relationships, context-specific outcomes and physiological trade-offs. Exercise intensity and duration do not consistently predict immunological load, and disease-specific microenvironments can invert or obscure expected responses, while the dualistic nature of exercise physiology demands careful calibration to avoid exacerbating inflammation or oxidative stress in vulnerable populations. Overcoming these challenges will depend on integrating continuous physiological telemetry with longitudinal immune phenotyping, stratifying interventions according to baseline immune-metabolic state, and iteratively adapting regimens based on biomarker feedback. Coupling such precision frameworks with rigorous clinical validation could transform exercise from a supportive measure into a modulatory immunotherapy, capable of augmenting CD8⁺ T cell-mediated protection in prevention, treatment and health-span extension.
Abbreviations
MHC: Major histocompatibility complex; FOXP3: Forkhead box p3; CXCR3: C-X-C motif chemokine receptor 3; CCR5: C-C motif chemokine receptor 5; ICAM-1: Intercellular adhesion molecule 1; VCAM-1: Vascular cell adhesion molecule 1; EVs: extracellular vesicles; EM: Effector memory; PDAC: pancreatic ductal adenocarcinoma; VEGF: Vascular endothelial growth factor; TME: Tumor microenvironment; HIIT: High-intensity interval training; NASH: Non-alcoholic steatohepatitis; COPD: Chronic obstructive pulmonary disease; RA: Rheumatoid arthritis; CLL: Chronic lymphocytic leukemia; ROS: Reactive oxygen species.
Acknowledgements
Funding
This work was supported by Guangdong Provincial Science and Technology Project Foundation (2022A0505050038), National Natural Science Foundation of China (81901006, 82372905), Young Top-notch Talent of Pearl River Talent Plan (0920220228), Science and Technology Program of Guangzhou (No.2025A04J3464), Scientific Research Talent Cultivation Project of Stomatological Hospital, Southern Medical University (RC202005) and Science Research Cultivation Program of Stomatological Hospital, Southern Medical University (PY2020002).
Availability of data and materials
The data generated is included within the manuscript.
Author contributions
LC, and XYZ conceived of the presented idea. LC, XYZ, XC, PL, YFL, WYF, MYZ, and NNL wrote the manuscript. XC, PL, YFL, WYF, MYZ and NNL created the graphs and figures. All authors contributed to and approved the final manuscript.
Competing interests
The authors have declared that no competing interest exists.
References
1. Chopp L, Redmond C, O'Shea JJ, Schwartz DM. From thymus to tissues and tumors: A review of T-cell biology. J Allergy Clin Immunol. 2023;151:81-97
2. Buggert M, Price DA, Mackay LK, Betts MR. Human circulating and tissue-resident memory CD8(+) T cells. Nat Immunol. 2023;24:1076-86
3. Reina-Campos M, Scharping NE, Goldrath AW. CD8(+) T cell metabolism in infection and cancer. Nat Rev Immunol. 2021;21:718-38
4. Pishesha N, Harmand TJ, Ploegh HL. A guide to antigen processing and presentation. Nat Rev Immunol. 2022;22:751-64
5. Cruz FM, Chan A, Rock KL. Pathways of MHC I cross-presentation of exogenous antigens. Semin Immunol. 2023;66:101729
6. Hashimoto K. CD137 as an Attractive T Cell Co-Stimulatory Target in the TNFRSF for Immuno-Oncology Drug Development. Cancers (Basel). 2021 13
7. McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231-47
8. Zielinski CE. Human T cell immune surveillance: Phenotypic, functional and migratory heterogeneity for tailored immune responses. Immunol Lett. 2017;190:125-9
9. Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C. et al. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. 2018;6:87
10. Cox MA, Zajac AJ. Shaping successful and unsuccessful CD8 T cell responses following infection. J Biomed Biotechnol. 2010;2010:159152
11. van Heijst JW, Gerlach C, Swart E, Sie D, Nunes-Alves C, Kerkhoven RM. et al. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient. Science. 2009;325:1265-9
12. Scheffer DDL, Latini A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165823
13. Shao T, Verma HK, Pande B, Costanzo V, Ye W, Cai Y. et al. Physical Activity and Nutritional Influence on Immune Function: An Important Strategy to Improve Immunity and Health Status. Front Physiol. 2021;12:751374
14. Graff RM, Kunz HE, Agha NH, Baker FL, Laughlin M, Bigley AB. et al. β(2)-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav Immun. 2018;74:143-53
15. Simpson RJ, Boßlau TK, Weyh C, Niemiro GM, Batatinha H, Smith KA. et al. Exercise and adrenergic regulation of immunity. Brain Behav Immun. 2021;97:303-18
16. Koivula T, Lempiäinen S, Neuvonen J, Norha J, Hollmén M, Sundberg CJ. et al. The effect of exercise and disease status on mobilization of anti-tumorigenic and pro-tumorigenic immune cells in women with breast cancer. Front Immunol. 2024;15:1394420
17. Batatinha H, Diak DM, Niemiro GM, Baker FL, Smith KA, Zúñiga TM. et al. Human lymphocytes mobilized with exercise have an anti-tumor transcriptomic profile and exert enhanced graft-versus-leukemia effects in xenogeneic mice. Front Immunol. 2023;14:1067369
18. Alley JR, Valentine RJ, Kohut ML. Mitochondrial Mass of Naïve T Cells Is Associated with Aerobic Fitness and Energy Expenditure of Active and Inactive Adults. Med Sci Sports Exerc. 2022;54:1288-99
19. Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC, Kunz H. Exercise and the aging immune system. Ageing Res Rev. 2012;11:404-20
20. Gomes-Santos IL, Amoozgar Z, Kumar AS, Ho WW, Roh K, Talele NP. et al. Exercise Training Improves Tumor Control by Increasing CD8(+) T-cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade. Cancer Immunol Res. 2021;9:765-78
21. Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;17:620-32
22. Schumacher O, Galvão DA, Taaffe DR, Chee R, Spry N, Newton RU. Exercise modulation of tumour perfusion and hypoxia to improve radiotherapy response in prostate cancer. Prostate Cancer Prostatic Dis. 2021;24:1-14
23. Shephard RJ, Verde TJ, Thomas SG, Shek P. Physical activity and the immune system. Can J Sport Sci. 1991;16:169-85
24. Braschler L, Nikolaidis PT, Thuany M, Chlíbková D, Rosemann T, Weiss K. et al. Physiology and Pathophysiology of Marathon Running: A narrative Review. Sports Med Open. 2025;11:10
25. Niedźwiedzka-Rystwej P, Tokarz-Deptuła B, Deptuła W. [Characteristics of T lymphocyte subpopulations]. Postepy Hig Med Dosw (Online). 2013;67:371-9
26. Santamaria J, Darrigues J, van Meerwijk JPM, Romagnoli P. Antigen-presenting cells and T-lymphocytes homing to the thymus shape T cell development. Immunol Lett. 2018;204:9-15
27. Guo M, Liu MYR, Brooks DG. Regulation and impact of tumor-specific CD4(+) T cells in cancer and immunotherapy. Trends Immunol. 2024;45:303-13
28. Zhu X, Zhu J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. Int J Mol Sci. 2020 21
29. Hay ZLZ, Slansky JE. Granzymes: The Molecular Executors of Immune-Mediated Cytotoxicity. Int J Mol Sci. 2022 23
30. Silva-Santos B, Mensurado S, Coffelt SB. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. 2019;19:392-404
31. Ribot JC, Lopes N, Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol. 2021;21:221-32
32. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T Cells and Human Disease. Annu Rev Immunol. 2020;38:541-66
33. Zong Y, Deng K, Chong WP. Regulation of Treg cells by cytokine signaling and co-stimulatory molecules. Front Immunol. 2024;15:1387975
34. Kumar BV, Connors TJ, Farber DL. Human T Cell Development, Localization, and Function throughout Life. Immunity. 2018;48:202-13
35. Wei J, Zheng W, Chapman NM, Geiger TL, Chi H. T cell metabolism in homeostasis and cancer immunity. Curr Opin Biotechnol. 2021;68:240-50
36. Jäger A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173-84
37. Kariya T, Ueta H, Xu XD, Koga D, Ezaki T, Yu E. et al. Direct evidence for activated CD8+ T cell transmigration across portal vein endothelial cells in liver graft rejection. J Gastroenterol. 2016;51:985-98
38. Melssen MM, Sheybani ND, Leick KM, Slingluff CL Jr. Barriers to immune cell infiltration in tumors. J Immunother Cancer. 2023 11
39. Mao Y, Zhang J, Zhou Q, He X, Zheng Z, Wei Y. et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024;34:13-30
40. Certo M, Tsai CH, Pucino V, Ho PC, Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. 2021;21:151-61
41. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486-99
42. Aoki K, Kurooka M, Chen JJ, Petryniak J, Nabel EG, Nabel GJ. Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nat Immunol. 2001;2:333-7
43. Nava Lauson CB, Tiberti S, Corsetto PA, Conte F, Tyagi P, Machwirth M. et al. Linoleic acid potentiates CD8(+) T cell metabolic fitness and antitumor immunity. Cell Metab. 2023;35:633-50.e9
44. Biswas PS, Pedicord V, Ploss A, Menet E, Leiner I, Pamer EG. Pathogen-specific CD8 T cell responses are directly inhibited by IL-10. J Immunol. 2007;179:4520-8
45. Fragala MS, Kraemer WJ, Denegar CR, Maresh CM, Mastro AM, Volek JS. Neuroendocrine-immune interactions and responses to exercise. Sports Med. 2011;41:621-39
46. De Ciuceis C, Rizzoni D, Palatini P. Microcirculation and Physical Exercise In Hypertension. Hypertension. 2023;80:730-9
47. Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055-81
48. Feng Y, Feng X, Wan R, Luo Z, Qu L, Wang Q. Impact of exercise on cancer: mechanistic perspectives and new insights. Front Immunol. 2024;15:1474770
49. Hunt RM, Elzayat MT, Markofski MM, Laughlin M, LaVoy EC. Characterization of transitional memory CD4+ and CD8+ T-cell mobilization during and after an acute bout of exercise. Front Sports Act Living. 2023;5:1120454
50. Strömberg A, Mandić M, Wadsworth BJ, Proschinger S, Alam S, Eriksson LM. et al. Functional characterisation of CD8(+) T cells mobilised with acute supramaximal high-intensity interval exercise: implications for immune surveillance. Clin Transl Immunology. 2025;14:e70037
51. Theall B, Stampley J, Cho E, Granger J, Johannsen NM, Irving BA. et al. Impact of acute exercise on peripheral blood mononuclear cells nutrient sensing and mitochondrial oxidative capacity in healthy young adults. Physiol Rep. 2021;9:e15147
52. Hazeldine J, Withnall E, Llibre A, Duggal NA, Lord JM, Sardeli AV. Physical Activity Modifies the Metabolic Profile of CD4(+) and CD8(+) T-Cell Subtypes at Rest and Upon Activation in Older Adults. Aging Cell. 2025;24:e70104
53. Gebhardt K, Hebecker A, Honekamp C, Nolte S, Barthkuhn M, Wilhelm J. et al. Respiratory and Metabolic Responses of CD4 + T Cells to Acute Exercise and Their Association with Cardiorespiratory Fitness. Med Sci Sports Exerc. 2024;56:1882-92
54. Yu X, Pei W, Li B, Sun S, Li W, Wu Q. Immunosenescence, Physical Exercise, and their Implications in Tumor Immunity and Immunotherapy. Int J Biol Sci. 2025;21:910-39
55. Tylutka A, Morawin B, Gramacki A, Zembron-Lacny A. Lifestyle exercise attenuates immunosenescence; flow cytometry analysis. BMC Geriatr. 2021;21:200
56. Bastos MF, Matias MST, Alonso AC, Silva LCR, de Araújo AL, Silva PR. et al. Moderate levels of physical fitness maintain telomere length in non-senescent T CD8+ cells of aged men. Clinics (Sao Paulo). 2020;75:e1628
57. Jahan-Mahin M, Askari R, Haghighi AH, Khaiyat O. The effect of three types of water-based training protocols on thymus atrophy and specific indicators of cellular immune senescence in aged male rats. Biogerontology. 2025;26:44
58. Despeghel M, Reichel T, Zander J, Krüger K, Weyh C. Effects of a 6 Week Low-Dose Combined Resistance and Endurance Training on T Cells and Systemic Inflammation in the Elderly. Cells. 2021 10
59. Park S, Choi BH, Jee YS. Effects of plank exercise on respiratory capacity, physical fitness, and immunocytes in older adults. J Exerc Rehabil. 2023;19:332-8
60. Papp G, Szabó K, Jámbor I, Mile M, Berki AR, Arany AC. et al. Regular Exercise May Restore Certain Age-Related Alterations of Adaptive Immunity and Rebalance Immune Regulation. Front Immunol. 2021;12:639308
61. Tu J. Polyphenols and Exercise in Mitochondrial Biogenesis: Focus on Age-Related CNS Disorders. Mol Neurobiol. 2025;62:15164-88
62. Weidenhamer CJ, Huang YH, Natua S, Kalsotra A, Hernández-Saavedra D. Muscle memory of exercise optimizes mitochondrial metabolism to support skeletal muscle growth. Am J Physiol Cell Physiol. 2025;329:C1239-c55
63. Wang Y, Danis A, Wang X, Ren J, Ma T, Wang R. Hispolon reduces mitochondrial dysfunction and improves fibrosis of diabetes nephropathy by activating AMPK signal and inhibiting mPTP opening. Cell Signal. 2025;136:112130
64. Wagner JA, Rosario M, Romee R, Berrien-Elliott MM, Schneider SE, Leong JW. et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest. 2017;127:4042-58
65. Lin JX, Du N, Li P, Kazemian M, Gebregiorgis T, Spolski R. et al. Critical functions for STAT5 tetramers in the maturation and survival of natural killer cells. Nat Commun. 2017;8:1320
66. Bird AW, Yu DY, Pray-Grant MG, Qiu Q, Harmon KE, Megee PC. et al. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature. 2002;419:411-5
67. Přibylová A, Fischer L, Pyott DE, Bassett A, Molnar A. DNA methylation can alter CRISPR/Cas9 editing frequency and DNA repair outcome in a target-specific manner. New Phytol. 2022;235:2285-99
68. Zhu C, Ma H, He A, Li Y, He C, Xia Y. Exercise in cancer prevention and anticancer therapy: Efficacy, molecular mechanisms and clinical information. Cancer Lett. 2022;544:215814
69. He A, Pu Y, Jia C, Wu M, He H, Xia Y. The Influence of Exercise on Cancer Risk, the Tumor Microenvironment and the Treatment of Cancer. Sports Med. 2024;54:1371-97
70. Crespo-Garcia C, Campbell JP, Taaffe DR, Peddle-McIntyre CJ, Jeffery E, Galvao DA. et al. Unleashing anti-tumour immunity: dietary restriction and exercise interventions adjunct to chemotherapy for cancer patients. Exerc Immunol Rev. 2024;30:26-48
71. Deng N, Reyes-Uribe L, Fahrmann JF, Thoman WS, Munsell MF, Dennison JB. et al. Exercise Training Reduces the Inflammatory Response and Promotes Intestinal Mucosa-Associated Immunity in Lynch Syndrome. Clin Cancer Res. 2023;29:4361-72
72. Buzaglo GBB, Telles GD, Araújo RB, Junior GDS, Ruberti OM, Ferreira MLV. et al. The Therapeutic Potential of Physical Exercise in Cancer: The Role of Chemokines. Int J Mol Sci. 2024 25
73. Zhou X, Jiang J, Liu J, Wang Q, Luo Y, Wu L. Aerobic exercise-induced lactate production: a novel opportunity for remodeling the tumor microenvironment. Front Genet. 2025;16:1620723
74. Renman D, Gylling B, Vidman L, Bodén S, Strigård K, Palmqvist R. et al. Density of CD3(+) and CD8(+) Cells in the Microenvironment of Colorectal Cancer according to Prediagnostic Physical Activity. Cancer Epidemiol Biomarkers Prev. 2021;30:2317-26
75. Wennerberg E, Lhuillier C, Rybstein MD, Dannenberg K, Rudqvist NP, Koelwyn GJ. et al. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget. 2020;11:452-61
76. Malicka I, Siewierska K, Olbromski M, Glatzel-Plucinska N, Podhorska-Okolow M, Dziegiel P. et al. The Influence of Physical Training on the Immune System of Rats during N-methyl-N-nitrosourea-Induced Carcinogenesis. J Clin Med. 2022 11
77. Hojman P. Exercise protects from cancer through regulation of immune function and inflammation. Biochem Soc Trans. 2017;45:905-11
78. Joseph JM, Hillengass M, Cannioto R, Tario JD, Wallace PK, Attwood K. et al. T Cell Exhaustion Markers in Multiple Myeloma Patients are Lower After Physical Activity Intervention. Clin Lymphoma Myeloma Leuk. 2024;24:621-8
79. Rundqvist H, Veliça P, Barbieri L, Gameiro PA, Bargiela D, Gojkovic M. et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. Elife. 2020 9
80. Voltarelli VA, Amano MT, Tobias GC, Borges GS, Oliveira da Paixão A, Pereira MG. et al. Moderate-intensity aerobic exercise training improves CD8(+) tumor-infiltrating lymphocytes effector function by reducing mitochondrial loss. iScience. 2024;27:110121
81. Zheng Y, Xu R, Chen X, Lu Y, Zheng J, Lin Y. et al. Metabolic gatekeepers: harnessing tumor-derived metabolites to optimize T cell-based immunotherapy efficacy in the tumor microenvironment. Cell Death Dis. 2024;15:775
82. Savage H, Pareek S, Lee J, Ballarò R, Conterno Minussi D, Hayek K. et al. Aerobic Exercise Alters the Melanoma Microenvironment and Modulates ERK5 S496 Phosphorylation. Cancer Immunol Res. 2023;11:1168-83
83. Esteves M, Silva C, Bovolini A, Pereira SS, Morais T, Moreira Â. et al. Regular Voluntary Running is Associated with Increased Tumor Vascularization and Immune Cell Infiltration and Decreased Tumor Growth in Mice. Int J Sports Med. 2023;44:427-37
84. Mlynska A, Dobrovolskiene N, Suveizde K, Lukaseviciute G, Sagini K, Martin-Gracia B. et al. Exercise-induced extracellular vesicles delay tumor development by igniting inflammation in an immunologically cold triple-negative breast cancer mouse model. J Sport Health Sci. 2025;14:101041
85. Sleijser-Koehorst MLS, Koop MA, Coppieters MW, Lutke Schipholt IJ, Radisic N, Hooijmans CR. et al. The effects of aerobic exercise on neuroimmune responses in animals with traumatic peripheral nerve injury: a systematic review with meta-analyses. J Neuroinflammation. 2023;20:104
86. Haunhorst S, Bloch W, Ringleb M, Fennen L, Wagner H, Gabriel HHW. et al. Acute effects of heavy resistance exercise on biomarkers of neuroendocrine-immune regulation in healthy adults: a systematic review. Exerc Immunol Rev. 2022;28:36-52
87. Miao SN, Chai MQ, Liu XY, Wei CY, Zhang CC, Sun NN. et al. Exercise accelerates recruitment of CD8(+) T cell to promotes anti-tumor immunity in lung cancer via epinephrine. BMC Cancer. 2024;24:474
88. Smith KA, Batatinha H, Niemiro GM, Baker FL, Zúñiga TM, Diak DM. et al. Exercise-induced β(2)-adrenergic receptor activation enhances effector lymphocyte mobilization in humans and suppresses lymphoma growth in mice through NK-cells. Brain Behav Immun. 2025;128:751-65
89. Kurz E, Hirsch CA, Dalton T, Shadaloey SA, Khodadadi-Jamayran A, Miller G. et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell. 2022;40:720-37.e5
90. Koivula T, Lempiäinen S, Rinne P, Hollmén M, Sundberg CJ, Rundqvist H. et al. Acute exercise mobilizes CD8(+) cytotoxic T cells and NK cells in lymphoma patients. Front Physiol. 2022;13:1078512
91. Gouez M, Rébillard A, Thomas A, Beaumel S, Matera EL, Gouraud E. et al. Combined effects of exercise and immuno-chemotherapy treatments on tumor growth in MC38 colorectal cancer-bearing mice. Front Immunol. 2024;15:1368550
92. Phelps CM, Willis NB, Duan T, Lee AH, Zhang Y, Rodriguez JD. et al. Exercise-induced microbiota metabolite enhances CD8 T cell antitumor immunity promoting immunotherapy efficacy. Cell. 2025
93. Warren K, Thompson N, Wannemuehler M, Kohut M. Antibody and CD8+ T cell memory response to influenza A/PR/8/34 infection is reduced in treadmill-exercised mice, yet still protective. J Appl Physiol (1985). 2013;114:1413-20
94. Warren KJ, Olson MM, Thompson NJ, Cahill ML, Wyatt TA, Yoon KJ. et al. Exercise Improves Host Response to Influenza Viral Infection in Obese and Non-Obese Mice through Different Mechanisms. PLoS One. 2015;10:e0129713
95. Baker FL, Smith KA, Zúñiga TM, Batatinha H, Niemiro GM, Pedlar CR. et al. Acute exercise increases immune responses to SARS CoV-2 in a previously infected man. Brain Behav Immun Health. 2021;18:100343
96. Baker FL, Zúñiga TM, Smith KA, Batatinha H, Kulangara TS, Seckeler MD. et al. Exercise mobilizes diverse antigen specific T-cells and elevates neutralizing antibodies in humans with natural immunity to SARS CoV-2. Brain Behav Immun Health. 2023;28:100600
97. Daynes E, Evans RA, Greening NJ, Bishop NC, Yates T, Lozano-Rojas D. et al. Post-Hospitalisation COVID-19 Rehabilitation (PHOSP-R): a randomised controlled trial of exercise-based rehabilitation. Eur Respir J. 2025 65
98. Felismino ES, Santos JMB, Rossi M, Santos CAF, Durigon EL, Oliveira DBL. et al. Better Response to Influenza Virus Vaccination in Physically Trained Older Adults Is Associated With Reductions of Cytomegalovirus-Specific Immunoglobulins as Well as Improvements in the Inflammatory and CD8(+) T-Cell Profiles. Front Immunol. 2021;12:713763
99. Cao Dinh H, Bautmans I, Beyer I, Onyema OO, Liberman K, De Dobbeleer L. et al. Six weeks of strength endurance training decreases circulating senescence-prone T-lymphocytes in cytomegalovirus seropositive but not seronegative older women. Immun Ageing. 2019;16:17
100. LaVoy EC, Hussain M, Reed J, Kunz H, Pistillo M, Bigley AB. et al. T-cell redeployment and intracellular cytokine expression following exercise: effects of exercise intensity and cytomegalovirus infection. Physiol Rep. 2017 5
101. Zanetti HR, Cruz LG, Lourenço CL, Neves Fde F, Silva-Vergara ML, Mendes EL. Non-linear resistance training reduces inflammatory biomarkers in persons living with HIV: A randomized controlled trial. Eur J Sport Sci. 2016;16:1232-9
102. Dirksen C, Hansen BR, Kolte L, Haugaard SB, Andersen O. T-lymphocyte subset dynamics in well-treated HIV-infected men during a bout of exhausting exercise. Infect Dis (Lond). 2015;47:919-23
103. Kruse A, Imery I, Corell L, Hjalmarsson E, Fernandez-Gonzalo R, Von Walden F. et al. Circulating immune cell populations at rest and in response to acute endurance exercise in young adults with cerebral palsy. Dev Med Child Neurol. 2024;66:902-9
104. Luo L, Liu M, Xie H, Fan Y, Zhang J, Liu L. et al. High-Intensity Interval Training Improves Physical Function, Prevents Muscle Loss, and Modulates Macrophage-Mediated Inflammation in Skeletal Muscle of Cerebral Ischemic Mice. Mediators Inflamm. 2021;2021:1849428
105. Proschinger S, Belen S, Adammek F, Schlagheck ML, Rademacher A, Schenk A. et al. Sportizumab - Multimodal progressive exercise over 10 weeks decreases Th17 frequency and CD49d expression on CD8(+) T cells in relapsing-remitting multiple sclerosis: A randomized controlled trial. Brain Behav Immun. 2025;124:397-408
106. Pool AJ, Whipp BJ, Skasick AJ, Alavi A, Bland JM, Axford JS. Serum cortisol reduction and abnormal prolactin and CD4+/CD8+ T-cell response as a result of controlled exercise in patients with rheumatoid arthritis and systemic lupus erythematosus despite unaltered muscle energetics. Rheumatology (Oxford). 2004;43:43-8
107. Feng J, Zhang Q, Chen B, Chen J, Wang W, Hu Y. et al. Effects of high-intensity intermittent exercise on glucose and lipid metabolism in type 2 diabetes patients: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2024;15:1360998
108. Messonnier LA. Physical Exercise or Activity and Energy Balance or Metabolism in the Context of Health and Diseases. Nutrients. 2023 15
109. Philippe M, Gatterer H, Burtscher M, Weinberger B, Keller M, Grubeck-Loebenstein B. et al. Concentric and Eccentric Endurance Exercise Reverse Hallmarks of T-Cell Senescence in Pre-diabetic Subjects. Front Physiol. 2019;10:684
110. Curran M, Campbell J, Drayson M, Andrews R, Narendran P. Type 1 diabetes impairs the mobilisation of highly-differentiated CD8+T cells during a single bout of acute exercise. Exerc Immunol Rev. 2019;25:64-82
111. Salman F, Erten G, Unal M, Kiran B, Salman S, Deniz G. et al. Effect of acute maximal exercise on lymphocyte subgroups in type 1 diabetes. Acta Physiol Hung. 2008;95:77-86
112. Tsutsui Y, Mori T, Yoshio S, Sato M, Sakata T, Yoshida Y. et al. Exercise changes the intrahepatic immune cell profile and inhibits the progression of nonalcoholic steatohepatitis in a mouse model. Hepatol Commun. 2023 7
113. Shen H, He J, Ling X, Liu C, Wang Y, Zhang X. et al. Wheel-Running Exercise Protects Ovariectomized Mice from Bone Loss via IFN-γ-Mediated Suppression of the NF-κB and MAPK Pathways. Oxid Med Cell Longev. 2022;2022:2030818
114. Boßlau TK, Wasserfurth P, Reichel T, Weyh C, Palmowski J, Nebl J. et al. 12-week combined strength and endurance exercise attenuates CD8(+) T-cell differentiation and affects the kynurenine pathway in the elderly: a randomized controlled trial. Immun Ageing. 2023;20:19
115. Graff RM, Jennings K, LaVoy ECP, Warren VE, Macdonald BW, Park Y. et al. T-cell counts in response to acute cardiorespiratory or resistance exercise in physically active or physically inactive older adults: a randomized crossover study. J Appl Physiol (1985). 2022;133:119-29
116. Baker CJ, Min D, Marsh-Wakefield F, Siwan E, Gerofi J, Wang X. et al. Circulating CD31(+) Angiogenic T cells are reduced in prediabetes and increase with exercise training. J Diabetes Complications. 2024;38:108868
117. Quickfall M, Cocks M, Long HM, Di Rosa F, Andrews R, Narendran P. et al. EXTOD-Immune: a randomised controlled trial to investigate whether a remotely monitored, home-based exercise intervention can reduce disease activity in people with type 1 diabetes. BMJ Open Sport Exerc Med. 2024;10:e002144
118. Kaushik D, Shah PK, Mukherjee N, Ji N, Dursun F, Kumar AP. et al. Effects of yoga in men with prostate cancer on quality of life and immune response: a pilot randomized controlled trial. Prostate Cancer Prostatic Dis. 2022;25:531-8
119. Crane JC, Gordon MJ, Basen-Engquist K, Ferrajoli A, Markofski MM, Lee CY. et al. Relationships between T-lymphocytes and physical function in adults with chronic lymphocytic leukemia: Results from the HEALTH4CLL pilot study. Eur J Haematol. 2023;110:732-42
120. Gomes-Santos IL, Kumar AS, Hausmann F, Meyer MN, Shiferaw SZ, Amoozgar Z. et al. Exercise intensity governs tumor control in mice with breast cancer. Front Immunol. 2024;15:1339232
121. Niemiro GM, Coletta AM, Agha NH, Mylabathula PL, Baker FL, Brewster AM. et al. Salutary effects of moderate but not high intensity aerobic exercise training on the frequency of peripheral T-cells associated with immunosenescence in older women at high risk of breast cancer: a randomized controlled trial. Immun Ageing. 2022;19:17
122. Mlynska A, Dobrovolskiene N, Suveizde K, Lukaseviciute G, Sagini K, Gracia BM. et al. Exercise-induced extracellular vesicles delay tumor development by igniting inflammation in an immunologically cold triple-negative breast cancer mouse model. J Sport Health Sci. 2025: 101041.
123. Buss LA, Williams T, Hock B, Ang AD, Robinson BA, Currie MJ. et al. Effects of exercise and anti-PD-1 on the tumour microenvironment. Immunol Lett. 2021;239:60-71
124. Williamson PN, Docherty PD, Yazdi SG, Khanafer A, Kabaliuk N, Jermy M. et al. Review of the Development of Hemodynamic Modeling Techniques to Capture Flow Behavior in Arteries Affected by Aneurysm, Atherosclerosis, and Stenting. J Biomech Eng. 2022 144
125. Wan Y, Wang J, Chen M, Wang J, Nan F, Huang H. et al. Dual roles of IRE1α inhibition in reversing mitochondrial ROS-induced CD8(+) T-cell senescence and exerting direct antitumor effects in multiple myeloma. J Immunother Cancer. 2025 13
126. Schauer T, Djurhuus SS, Simonsen C, Brasso K, Christensen JF. The effects of acute exercise and inflammation on immune function in early-stage prostate cancer. Brain Behav Immun Health. 2022;25:100508
Author contact
![]() Corresponding author: Li Cui, Email: licuiedu.cn.
Corresponding author: Li Cui, Email: licuiedu.cn.
 Global reach, higher impact
Global reach, higher impact