13.3
Impact Factor
Theranostics 2026; 16(7):3507-3540. doi:10.7150/thno.126517 This issue Cite
Review
Recent advances in nanoparticles targeting TGF-β signaling for cancer treatment
1. Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua 321004, China.
2. College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China.
3. Cancer Metastasis Alert and Prevention Center, College of Chemistry, and Fujian Provincial Key Laboratory of Cancer Metastasis Chemoprevention and Chemotherapy, Fuzhou University, Fuzhou 350108, China.
Received 2025-10-10; Accepted 2025-12-15; Published 2026-1-1
Abstract
Multiple therapies blocking TGF-β signaling have been investigated in preclinical and clinical trials over the past few decades; nevertheless, the outcomes of clinical trials are disappointing due to the double-faced systemic effects of TGF-β and the complexity of the tumor microenvironment. Intelligent nanodelivery systems engineered with responsive stimuli and targeting capabilities address the Janus-faced biology of TGF-β through spatially precise inhibition. Nanoparticles targeting TGF-β reciprocally create a positive feedback loop that enhances the penetration and delivery efficiency of nanoparticles because of the role of TGF-β in remodeling the tumor microenvironment. This review first outlines the function of TGF-β signaling, summarizes various tools for suppressing TGF-β signaling and provides an exhaustive emphasis on advanced nanoparticles targeting TGF-β. This review elucidates the symbiotic interplay between TGF-β blockade and nanoparticles, where nanomaterial-based strategies refine the specificity of TGF-β targeting, while the blockade of TGF-β reciprocally enhances the efficiency of nanoparticle-mediated delivery. Additionally, current challenges and future directions are highlighted to guide the future development of TGF-β blockade strategies and nanoparticles for antitumor therapy.
Keywords: nanodrug delivery system, TGF-β blockade, cancer treatment, nanoparticle, nanocarrier
1. Introduction
Transforming growth factor-β (TGF-β) is a secreted cytokine that is involved in various biological activities, including tumor progression and metastasis, immunization inhibition and tissue reconstruction. TGF-β critically governs cellular proliferation, differentiation, apoptosis, and invasion across biological systems but presents a dualistic paradox in disease pathogenesis. During the initial phase of tumor development, it mediates tumor-suppressive effects through the induction of cell cycle arrest and apoptosis under normal physiological conditions [1]. However, in advanced malignancies, TGF-β is transformed into an oncogenic driver, promoting the excessive deposition of extracellular matrix (ECM), angiogenesis, immunosuppression and epithelial-mesenchymal transition (EMT) [2]. Although multiple factors increase the complexity of TGF-β signal transduction, numerous clinical trials have proven the favorable antitumor effect of TGF-β blockade [3,4]. The most popular treatment strategy involves combining TGF-β pathway antagonists (such as antibodies, recombinant proteins, and small-molecule inhibitors) with other agents, including chemotherapeutic drugs [5], immune checkpoint inhibitors (ICIs, e.g., anti-PD-1/PD-L1) [6], and chimeric antigen receptor-modified (CAR)-T cells [7]. However, systemic exposure, off-target effect, and poor tissue penetration of TGF-β pathway antagonists themselves restrict the clinical translation of TGF-β blockade [8].
The advent of nanotechnology has revolutionized strategies for the precise modulation of TGF-β, a Janus-faced cytokine implicated in fibrosis, cancer, and autoimmune diseases. These next-generation nanoplatforms not only potentiate the therapeutic index through pathological site-restricted action but also maintain the physiological homeostasis of TGF-β in nonneoplastic tissues, thereby resolving the conundrum of therapeutic specificity versus systemic preservation of its biological functions.
In other words, the TGF-β signaling pathway offers an innovative direction for designing nanoparticles (NPs) for combination therapy and increasing NP delivery efficiency. The effective delivery of NPs to tumor sites remains a tremendous challenge due to the complex path through which they must traverse the tumor microenvironment (TME), which also affects the therapeutic effect of NPs [9]. TGF-β blockade could enhance delivery and penetration efficiency by weakening stromal barriers, improving vascular permeability and modulating immune responses. Pharmaceutical convergence between nanotechnology and TGF-β-targeted drug delivery systems (DDSs) has shown particular translational promise in multimodal therapeutic architectures that potentiate the effects of conventional chemotherapy, novel ICIs, and revolutionary immune cell therapy [10-12].
This review illuminates the symbiotic relationship evolving at the intersection of TGF-β biology and NPs, where nanotechnology-driven solutions increase the specificity of TGF-β targeting, whereas engineering of the TGF-β pathway reciprocally enhances the efficiency of nanotherapeutic delivery. Central to this discourse are NP systems capable of intrinsically neutralizing TGF-β via ligand scavenging or signaling cascade disruption, alongside advanced nanocarriers engineered for the spatiotemporally controlled delivery of TGF-β pathway antagonists such as monoclonal antibodies, gene-silencing RNAs, and TGF-β ligand traps. Concurrently, TGF-β inhibitors (TGF-βi) are being repurposed to potentiate nanodrug penetration by remodeling pathological microenvironments, such as by decompressing the fibrotic stroma or activating the immunosuppressive TME, thereby establishing a self-reinforcing therapeutic loop. Emerging frontiers further exploit multifunctional nanoplatforms that integrate TGF-β blockade with complementary modalities, including immunotherapies and conventional chemoradiation, capitalizing on the pleiotropic role of TGF-β in orchestrating multimodal antitumor responses. This bidirectional interplay represents a remarkable advancement intumor treatment, where nanotechnology and TGF-β targeting work together to specifically inhibit cancerous areas while protecting healthy tissues, effectively balancing their dual effects.
2. TGF-β signaling and function in tumor cells
TGF-β is a member of the secreted cytokine family, which is composed of three isoforms of TGF-β (TGF-β1, -β2, and -β3), activins, bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs). In particular, TGF-β1 is the most comprehensively and actively researched family member. TGF-β receptors (TβRs) are high-affinity binding proteins located on the cell membrane and can be divided into three subtypes: TβR-I, TβR-II, and TβR-III. Almost all cells in the body can secrete TGF-β, whose receptor also exists on the cell surface [13].
The TGF-β signaling pathway plays an overarching role in diverse biological activities through the canonical suppressor of mothers against decapentaplegic (SMAD) -dependent pathway and non-SMAD pathways. TGF-β initiates key biological events primarily through the phosphorylation of SMAD2 and SMAD3. These transcription factors are pivotal SMAD family members and essential components of the TGF-β signaling cascade [14]. Activated SMAD2 and SMAD3 interact with SMAD4 to form a heterotrimer and enter the nucleus to modulate transcriptional activity. Non-SMAD signaling involves the collective pathways and downstream cascades triggered by TGF-β, including phosphorylation, acetylation, sumoylation, ubiquitination, and protein-protein interactions. For instance, TGF-β is known to initiate the non-SMAD signal-regulated kinases RAS, MEK1/2, and ERK1/2 to complete signal transduction.
TGF-β acts as a tumor suppressor in normal and premalignant cells but undergoes a functional switch to become a tumor promoter in advanced malignancies. In the initial phases of tumor development, the canonical TGF-β/SMAD 4 signaling pathway plays a tumor-suppressive role by inducing cell cycle arrest and apoptosis [15]. SMAD4 plays a critical role at the G1/S checkpoint by inducing cell cycle arrest in the G1 phase through several cyclin-dependent kinase (CDK) inhibitors, including p15, p21, and p27 [16,17]. TGF-β induces intrinsic and extrinsic apoptosis through the modulation of Bcl-2 family members, DAPK, caspases, FAS, and DNA damage-inducible (GADD) 45-β. TGF-β promotes apoptosis primarily through the SMAD-dependent pathway by upregulating proapoptotic genes (e.g., BIM, PUMA, and NOXA), but it can also induce apoptosis via non-SMAD pathways. These pathways include the activation of kinase signaling, such as JNK/p38 MAPK signaling, which in turn modulates the activity and expression of Bcl-2 family proteins [18]. Furthermore, TGF-β signaling initiates key executors such as DAPK, activate the caspase cascade, increase FAS-mediated signaling, and induce the expression of DNA damage-responsive genes (e.g., GADD45β).
In advanced cancer stages, TGF-β facilitates ECM construction, angiogenesis, immunosuppression, EMT, and tumor aggression and metastasis (Figure 1). TGF-β orchestrates a protumorigenic microenvironment by driving ECM remodeling and fibrosis, leading to increased tissue stiffness and enhanced tumor invasion. TGF-β also promotes angiogenesis, ensuring a blood supply for the growing tumor. Crucially, it acts as a master regulator of immunosuppression by impairing the function of cytotoxic T cells and natural killer (NK) cells. Furthermore, TGF-β acts as a primary and crucial EMT inducer in late-stage tumors.
2.1 ECM construction
The TME consists of abnormal vasculature, a high density of tumor ECM and diverse cells (cancer-associated fibroblasts (CAFs), immune cells, endothelial cells, and so forth). The ECM is a dynamic supramolecular network that consists of collagens, fibronectin, elastin, glycosaminoglycans and other proteins and affects the progression and prognosis of tumors. CAFs, as vital facilitators of tumor growth and progression, are identified by the expression of α-smooth muscle actin (α-SMA), vimentin, and collagen I. The activation of the SMAD pathway, mediated via TβRII and TβRI, primarily drives the expression of proteins responsible for collagen deposition and matrix formation [19].
The role of TGF-β in late-stage tumors includes ECM construction, angiogenesis, immunosuppression, and the promotion of EMT. TGF-β: transforming growth factor-β; Tregs: regulatory T cells; Foxp3: forkhead box protein P3; NK cells: natural killer cells; BMP: bone morphogenetic protein; NF-κB: nuclear factor κB; DC: dendritic cell; CAFs: cancer-associated fibroblasts; α-SMA: α-smooth muscle actin; VEGF: vascular endothelial growth factor; ALK: anaplastic lymphoma kinase; EMT: epithelial-mesenchymal transition; ECM: extracellular matrix.
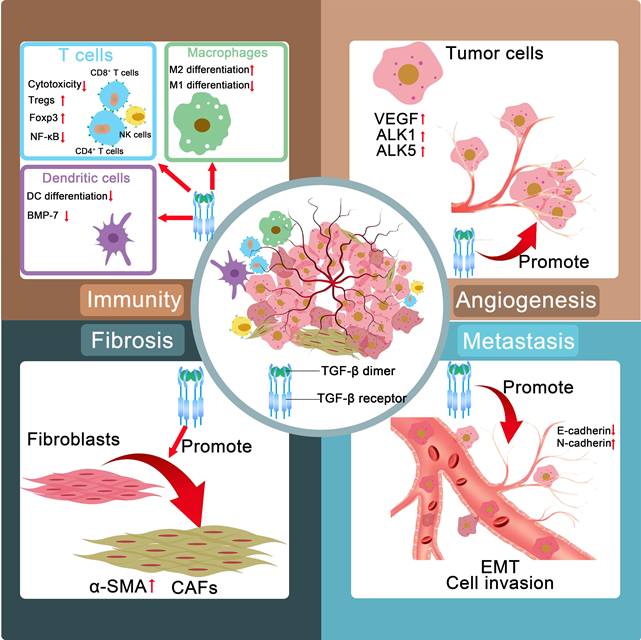
TGF-β induces the differentiation of fibroblasts into activated fibroblasts with increased expression of α-SMA. This marker is widely regarded as the dominant feature associated with CAFs [20]. CAFs are derived from other resident stromal cells, such as epithelial or endothelial cells, through TGF-β-induced EMT. The differentiation of bone marrow-derived cells into CAFs is partially driven by TGF-β. And blockade of TGF-β can prevent mesenchymal stem cells from differentiating into CAFs [21].
2.2 Angiogenesis
The progression of solid tumors relies heavily on the establishment of functional tumor-associated blood vessels, which not only facilitate nutrient and oxygen delivery to proliferating neoplastic cells but also serve as conduits for metastatic dissemination. Notably, this intricate process of tumor vascularization requires coordinated ECM remodeling to provide structural support for the maturation of the nascent vasculature. Emerging evidence positions TGF-β as a pivotal orchestrator of this pathological process through multifaceted regulatory mechanisms.
TGF-β exerts dual regulatory effects on vascular homeostasis by differentially modulating the anaplastic lymphoma kinase (ALK1 and ALK5) signaling pathways, thereby balancing endothelial cell proliferation and stabilization while simultaneously increasing ECM protein deposition to increase vascular integrity. Furthermore, this pleiotropic cytokine demonstrates an immediate proangiogenic capacity by rapidly inducing vascular endothelial growth factor (VEGF) secretion, establishing a hypoxic TME that perpetuates angiogenic signaling cascades [22].
Beyond its immediate effects on endothelial activation, TGF-β coordinates comprehensive vascular maturation through a novel TGF-β-fibronectin signaling axis. This mechanotransduction pathway enhances pericyte-endothelial cell interactions, effectively stabilizing the immature vasculature while promoting the development of perfusion-competent blood vessels capable of sustaining tumor growth [23,24]. This multidimensional regulatory capacity positions TGF-β as a crucial regulator of tumor angiogenesis, simultaneously governing matrix remodeling, growth factor signaling, and vascular structural organization through distinct yet synergistic molecular mechanisms.
2.3 Immunosuppression
TGF-β induces immunosuppressive effects through multiple cell types. In natural killer (NK) cells, it attenuates cytotoxic activity via the dual inhibition of IFN-γ synthesis (through STAT5 pathway blockade) and effector molecule expression (via SMAD3-mediated transcriptional suppression of perforin/granzyme B) [25]. With respect to CD4+ T cells, TGF-β inhibits the differentiation of both Th1 and Th2 lymphocytes while simultaneously facilitating the generation and functional maturation of Th17 cells, Th9 cells, and regulatory T cells (Tregs). Notably, TGF-β is among the most powerful immunomodulatory cytokines involved in orchestrating Th1 cell differentiation and modulating its effector functions, demonstrating a dual regulatory capacity that positions it as a pivotal mediator of immune homeostasis [26]. TGF-β is capable of inducing the differentiation of CD8+ T lymphocytes into forkhead box protein P3 (Foxp3)-expressing Tregs [27]. TGF-β facilitates the phosphorylation of SMAD2 and Akt while concurrently inducing the formation of SMAD2/p-SMAD2 and Akt/p-Akt complexes, ultimately leading to the suppression of monocyte-derived dendritic cell (DC) differentiation. This molecular mechanism contributes significantly to tumorigenesis and malignant progression through the establishment of immunosuppressive microenvironments [28].
In addition, TGF-β drives the expansion and functional maturation of Tregs by transcriptionally activating Foxp3, thereby amplifying immune tolerance within the TME. Concurrently, TGF-β orchestrates macrophage polarization by suppressing nuclear factor κB (NF-κB)-mediated proinflammatory signaling, redirecting these myeloid cells toward a protumorigenic M2 phenotype characterized by arginase-1 overexpression and interleukin-10 secretion [29]. Notably, the synergistic interplay between Treg-derived immunosuppressive cytokines and M2-polarized tumor-associated macrophage (TAM)-secreted trophic factors establishes a self-reinforcing immunosuppressive mechanism, effectively paralyzing cytotoxic T lymphocyte (CTL) activity while promoting tumor immune evasion. The dense ECM driven by TGF-β also restrains the deep penetration of immune cells besides the direct immunosuppressive effect of TGF-β [30].
2.4 Induction of EMT
During the EMT process, epithelial cells acquire mesenchymal characteristics, which enhances their migratory ability, invasiveness, and resistance to apoptosis [31]. In cell culture, epithelial cells stimulated with TGF-β exhibited a loss of E-cadherin expression and an elevated level of N-cadherin expression, along with morphological changes [32,33]. TGF-β is considered among the earliest EMT inducers and has been verified to serve as a crucial modulator of cancer cell progression and metastasis. The TGF-β signaling pathway is predominantly mediated by canonical pathways involving the transcription factors SMAD2, SMAD3, and SMAD4 and multiple noncanonical pathways. An elevated intratumoral concentration of TGF-β induces alterations in the EMT state of tumor cells, which also triggers significant reprogramming of the TME. The metabolism of carcinoma cells is profoundly affected by TGF-β, resulting in the establishment of a hypoxic environment within the TME [34]. Certain carcinoma cells may enter a dormant state, which could serve as a reservoir of therapy-resistant cells, laying the foundation for tumor recurrence [35-41]. Accumulating evidence has indicated that TGF-β induces epithelial tumor cells to acquire a mesenchymal phenotype characterized by increased invasiveness [42]. Thus, high expression of TGF-β1 has been verified to correlate with increased metastasis.
3. Blockade of TGF-β in cancer
In terms of antitumor therapeutic targets, TGF-β pathway antagonists can be classified into seven types: antibodies that inhibit ligand/receptor interactions, small-molecule inhibitors, antisense oligonucleotides, ligand traps, vaccines, natural products and conventional drugs with new applications (Figure 2) [43]. Numerous these agents have been or are being assessed in clinical trials against diverse tumors. The diverse agents are discussed in more detail below.
3.1 Antibodies that inhibit ligand/receptor interactions
Direct blockade of TGF-β ligand-receptor interactions using antibodies has been shown to be a highly specific and promising intervention. By inhibiting signal transduction at its source, these antibodies regulate the activity of the TGF-β pathway, providing therapeutic approaches for related diseases [44-46]. 2G7 and 4A11 are two murine monoclonal antibodies that have been researched in drug-resistant animal models to restore sensitivity to cyclophosphamide (CTX) and cisplatin (CDDP) [47]. The human monoclonal anti-TGF-β antibody LY3022859 suppresses the activation of TβR-I- and TβR-II-mediated signaling by preventing the formation of ligand-receptor complexes. However, a safe and effective dose could not be determined in the phase I study of LY3022859 because the TGF-β receptor is ubiquitously expressed in the body [48].
Molecular mechanisms of action of TGF-β pathway antagonists. TGF-β pathway antagonists can be classified into seven types: antibodies that inhibit ligand/receptor interactions, small-molecule inhibitors, antisense oligonucleotides, ligand traps, vaccines, natural products and conventional drugs with new applications. TGF-β: transforming growth factor-β; TβR: TGF-β receptor; SMAD: suppressor of mothers against decapentaplegic; AMPK: adenosine monophosphate-activated protein kinase; RAS: rat sarcoma; RAF: rapidly accelerated fibrosarcoma; MEK: mitogen-activated protein kinase kinase; ERK: extracellular signal-regulated kinases.
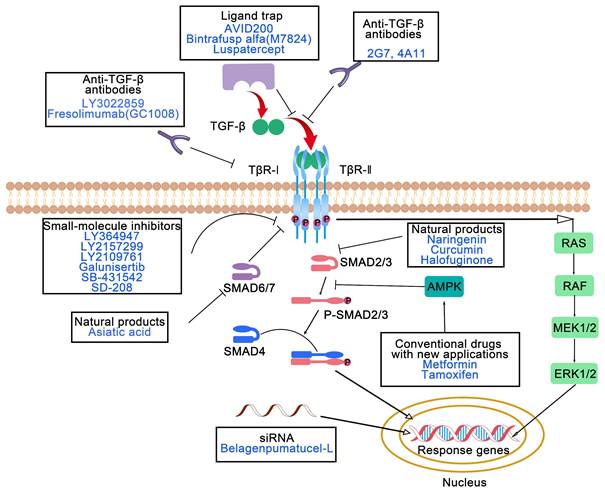
Another human monoclonal antibody, fresolimumab (GC1008), was broadly evaluated in clinical trials of patients with metastatic breast carcinoma [49], renal cell carcinoma [50] and other malignancies [51] to assess the clinical response. Compared with a lower dose of GC1008, a higher dose of GC1008 during radiotherapy was achievable and well tolerated, with efficient immune activation and a prolonged median overall survival among patients with metastatic breast cancer [49]. Additionally, an analysis of functional T cells suggested that combining radiotherapy with GC1008 was ineffective at ameliorating T cell restriction by PD-1 [52], which encouraged the strategy of the combination of dual PD-1 and TGF-β suppression and radiotherapy. In addition, numerous challenges remain in optimizing the affinity, specificity, and therapeutic effect of antibodies [53].
3.2 Small-molecule inhibitors
Numerous small molecules can be classified into three categories according to their structures: pyrazole, imidazole and pteridine compounds. Most TβR-Ⅰ inhibitors are pyrazole derivatives, such as LY364947 (HTS-466284), LY2157299 (galunisertib), and LY2109761. LY2109761 suppresses the TGF-β-induced liver metastasis and progression of pancreatic carcinoma through dual targeting of TβR-I/II activity [54]. Galunisertib is being evaluated in clinical trials for the treatment of myelodysplastic syndrome, advanced hepatocellular carcinoma and metastatic pancreatic cancer. Data from phase II clinical trials (NCT01246986) have revealed that decreased TGF-β1 levels induced by galunisertib are related to longer overall survival in patients with advanced hepatocellular carcinoma [55]. Galunisertib has advanced the most recent clinical trials among all small-molecule TGF-βi and has favorable pharmaceutical properties and selectivity. T LY2157299 was proven to facilitate the antitumor effect of nivolumab (anti-PD-1) in a phase II study (NCT02423343) [56].
TGF-βi based on imidazole and pteridine derivatives have been validated in preclinical studies but have not yet been tested in clinical trials. Both SB-431542 and its derivatives SB-505124 and SB-525334 possess an imidazole scaffold that suppresses the kinase activity of TβR-I, ALK4, ALK5 and ALK7. In preclinical models, SB-431542 has been demonstrated to have antitumor effects on breast carcinoma, colon cancer, osteosarcoma and malignant glioma [57-60]. It abates immune suppression by mitigating the population of Treg cells and rescuing the proliferation of T cells in a murine fibrosarcoma model [61]. The TβR-I kinase inhibitor SD-208 is the only molecule with a pteridine structure. According to a preclinical study in SMA-560 glioma-bearing syngeneic mice, SD-208 prolongs the survival by promoting immune responses [62]. While small-molecule TGF-βi demonstrate remarkable therapeutic potential for improving pharmaceutical properties and synergistic efficacy in combination with ICIs, their clinical treatment remains limited by structural limitations that compromise target specificity and unresolved challenges in pharmacokinetic optimization across diverse TME. Despite the promising preclinical outcomes in terms of metastasis suppression and immune modulation, the long-term therapeutic efficacy of these compounds is unclear because of emerging compensatory signaling pathways and insufficient structural diversity within existing scaffolds to address heterogeneous tumor resistance mechanisms.
3.3 Antisense oligonucleotides
RNA interference (RNAi) is an innovative approach for the specific knockdown of gene expression. Only a few clinical trials in which TGF-β was silenced by a siRNA (siTGF-β) have been conducted for the treatment of non-small cell lung cancer (NSCLC) [63], advanced glioma [64, 65], and malignant melanoma [66]. In a phase II study of belagenpumatucel-L [63], a TGF-β2 antisense gene-modified tumor cell vaccine, a dose-dependent difference in survival was observed, and no significant adverse events were detected among NSCLC patients. RNAi has notable clinical advantages because it involves the use of sequence-specific gene silencing mechanisms to precisely modulate the expression of TGF-β [67]. Nevertheless, the clinical translation of RNAi therapeutics remains limited by intrinsic limitations, including the intrinsic instability of naked RNA molecules in the systemic circulation and inefficient tissue penetration of conventional delivery systems, which collectively contribute to reduced bioavailability and potential off-target effects [68]. Functionalized nanoscale delivery platforms can substantially increase the pharmacokinetic stability of RNAi payloads while enabling ligand-directed tumor targeting through surface engineering modifications, thereby addressing current delivery challenges with increased spatial control over therapeutic agent distribution.
3.4 Ligand traps
In addition to traditional ligands or receptor inhibitors, the design of “ligand traps” capable of efficiently capturing TGF-β ligands has gained attention as a novel strategy for precise therapy [69]. This strategy involves the engineering of receptor domains or fusion proteins that specifically bind to TGF-β ligands, thereby suppressing the downstream signaling pathways. Compared with traditional methods, ligand traps offer advantages such as high affinity, high specificity, and low toxicity, providing an original perspective and the potential to treat TGF-β-related diseases [70-73]. Several TGF-β ligand traps, such as AVID200, bintrafusp alfa (M7824), and luspatercept, can target TGFβ ligands through fusion proteins and prevent ligand-receptor binding. M7824 is a bifunctional fusion protein and is composed of a TGF-β “trap” fused to anti-PD-L1 antibody. Bintrafusp alfa has been investigated in numerous preclinical and clinical trials for treatment of various tumors [74-81]. Compared with monotherapy, dual suppression of PD-L1 and TGF-β by bintrafusp alfa results in effective antitumor effects in a preclinical animal model [80]. A clinical trial (NCT04247282) has indicated that one or two neoadjuvant doses of bintrafusp alfa were well tolerated without adverse reactions or surgical delays [81]. Dual PD-L1 and TGF-β inhibition efficiently enhanced T cell activation in a safe manner, increasing the feasibility of multimechanism neoadjuvant immunotherapy.
3.5 Vaccines
Vaccines targeting the TGF-β pathway, such as gemogenovatucel-T (Vigil, Vital) and belagenpumatucel-L (Lucanix), provide new emerging therapies with superior immunological effects in cancer treatment. Gemogenovatucel-T consists of a plasmid encoding the human immunostimulatory gene GMCSF and a bifunctional shRNA that specifically knocks down furin, TGF-β1 and TGF-β2 [82]. According to completed and ongoing clinical trials of gemogenovatucel-T or combination treatment with ICIs, the safety, efficient immune response, and antitumor effect of gemogenovatucel-T were confirmed in patients with different advanced gynecological cancers [83-88].
3.6 Natural products
Natural molecules derived from plants exhibit promising antitumor effects through the TGF-β signaling. Natural molecules suppressing TGF-β signaling include alkaloids, triterpenoids, diterpenoids, polyphenols, indoles, steroids, anthraquinone, and benzenoids. However, all these agents have been tested in vitro and in vivo but not in clinical trials. Triterpenoids, such as arjunolic acid [89], asiatic acid [90], betulinic acid [91], celastrol [92], and ginsenosides [93, 94], exhibit antitumor effects by suppressing TGF-β signaling. Lian et al. [90] reported that asiatic acid (a SMAD7 agonist) combined with naringenin (a SMAD3 inhibitor) synergistically regulated the TGF-β/SMAD signaling balance and inhibited the invasion and metastasis of melanoma and lung cancer. Diverse investigations have demonstrated that natural products effectively inhibit tumor invasion and metastasis by modulating the TME through the TGF-β signaling pathway. These studies provide experimental basis and potential clinical value of developing natural products that target TGF-β. For instance, curcumin not only suppressed EMT through TGF-β/SMAD2/3 signaling [95] but also reeducated CAFs by reducing α-SMA and COX-2 expression [96]. Halofuginone, a plant alkaloid derivative, inhibited osteosarcoma progression against lung metastases [97] and melanoma against bone metastasis [98] by inhibiting TGF-β/SMAD3 signaling. Salvianolic acid B, obtained from Salvia miltiorrhiza, suppressed TGF-β1-induced EMT and is a promising therapeutic agent for treating NSCLC [99].
3.7 Conventional drugs with new applications
Increasing evidence has verified that some conventional drugs, such as metformin, tamoxifen and pirfenidone, exhibit anti-tumor effect through the regulation of TGF-β signaling. Metformin, a medicine applied to treat type 2 diabetes, suppressed the TGF-β1-induced EMT through the activation of adenosine monophosphate-activated protein kinase (AMPK) in various tumors, such as pancreatic carcinoma [100] and cervical carcinoma [101]. Another study also revealed that metformin decreased the expression of ECM components by modulating PSCs and reeducating TAMs [102]. Tamoxifen, an adjuvant chemotherapeutic, prolonged the survival of patients with almost all stages of estrogen receptor-positive breast carcinoma. Emerging evidence has indicated that the regulation of mitochondrial bioenergetics constitutes an innovative and efficacious approach to achieve the dual suppression of PD-L1 and TGF-β. Zhou et al. reported that tamoxifen efficiently suppressed PD-L1 and TGF-β expression by promoting AMPK phosphorylation [103]. Pirfenidone, an FDA-approved drug for idiopathic pulmonary fibrosis, inhibits tumor metastasis by preventing TGF-β/SMAD signaling in triple-negative breast cancer (TNBC) [104] and reducing collagen and E-cadherin levels through the TGF-β signaling pathway in colorectal carcinoma [105].
4. Strategies for the design of nanodelivery systems targeting TGF-β in cancer therapy
Both TGF-βi and their co-administration with ICIs have been proven to exert therapeutic benefits in clinical trials. However, the clinical translation of TGF-βi such as galunisertib, fresolimumab, and bintrafusp alfa is still hampered by significant limitations, including rapid systemic clearance, off-target toxicity, and insufficient drug penetration. These challenges often result in unsatisfactory efficacy and harmful side effects, hindering successful clinical trial outcomes. Nanodelivery strategies involving the encapsulation of these therapeutics can enhance the precise delivery of TGF-βi with reduced side effects on normal tissues, providing an effective platform to overcome the shortcomings of TGF-βi. On the one hand, TGF-β therapeutic agents have improved the penetration and therapeutic efficacy of antitumor drug-loaded NPs; on the other hand, the emergence of nanodelivery carriers has provided an effective approach to guarantee the biosafety and attenuate the systemic toxicity of TGF-β pathway antagonists for TME modulation and cancer treatment.
Furthermore, NPs with engineered physicochemical properties exploit the enhanced permeability and retention (EPR) effect to achieve passive targeting and employ surface properties for active targeting, promoting specific accumulation and prolonged retention within the TME. This targeted aggregation significantly increases the local drug concentration where it is needed most, while simultaneously sparing normal organs. Moreover, researchers have increasingly shown that inorganic NPs may promote tumor metastasis through TGF-β signaling pathways, revealing the need for future investigations focusing on the potential adverse effects and safety concerns associated with NP applications. Consequently, these advanced strategies are pivotal for enhancing clinical trials for TGF-β blockade, enabling more informative and potentially successful evaluations of these promising agents in cancer treatment.
4.1 Sequential combination of nanoencapsulated TGF-β pathway antagonists and versatile NPs using a two-wave strategy
The convergence of nanotechnology and molecular targeting has catalyzed the development of multifunctional platforms capable of sequential microenvironmental normalization and tumor-specific payload delivery, exemplifying a new frontier in combinatorial therapeutic design. The following investigations adopted a sequential two-wave therapy: initial administration of nanoencapsulated TGF-β pathway antagonists to dismantle biological barriers within the TME through multifaceted mechanisms, followed by targeted delivery of drug-loaded NPs for cancer treatment. Sequential combination treatments involving TGF-β blockade and other therapeutic strategies, including chemotherapy, immunotherapy, and molecular targeted therapy, could allow for controlled spatiotemporal drug release and achieve efficient combination antitumor therapy (Table 1).
4.1.1 Nanodelivery of TGF-β pathway antagonists to increase the efficacy of chemotherapy
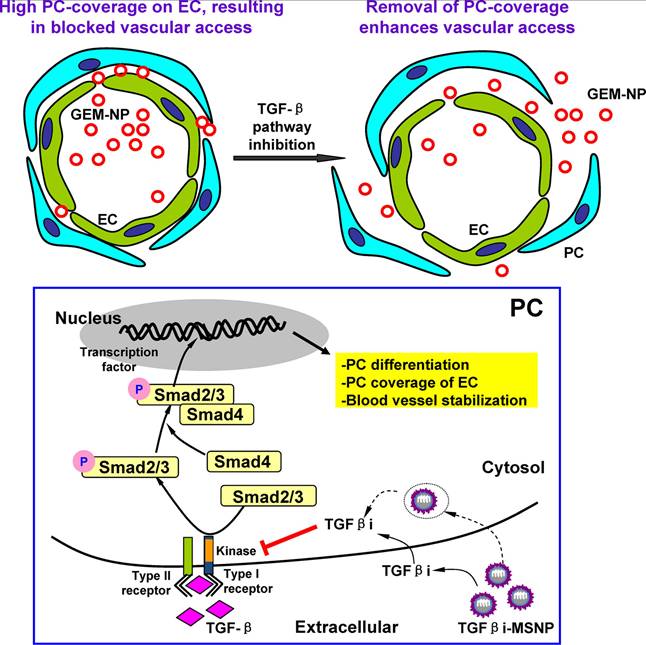
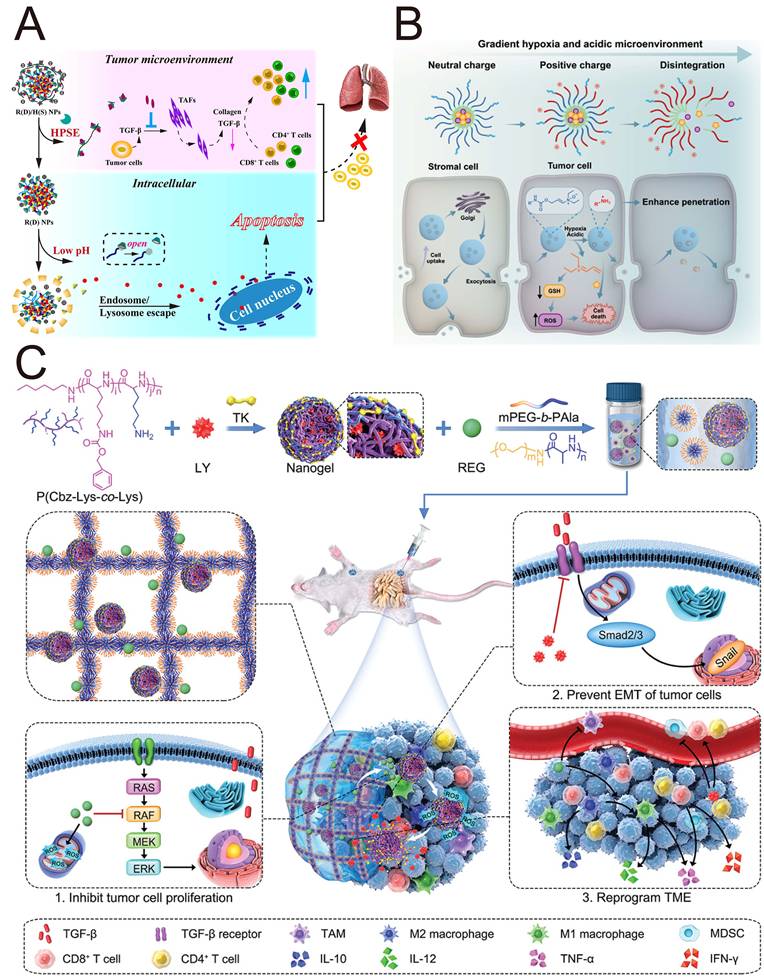
A challenging and central research objective is to improve the efficient delivery of nanomedicine, especially in pancreatic ductal adenocarcinoma (PDAC), in which the tumor comprises approximately 90% of the tumor stroma. The desmoplastic stroma of PDAC contains dense ECM and nontumor cells, especially pericytes. This stromal environment suppresses vascular fenestration and hinders the vascular access of NPs. Meng et al. demonstrated that polyethyleneimine (PEI)/polyethylene glycol (PEG)-coated mesoporous silica NPs (MSNPs) loaded with LY364947 efficiently reduced pericyte coverage of the vasculature by blocking TGF-β. Acting as a "first-wave" NPs, this treatment improved the accumulation of subsequent "second-wave" NPs (Figure 3) [106]. PEI-PEG-encapsulated MSNPs were favorable for the secure delivery of inhibitors due to their ability to maintain monodispersity in biological fluids, as well as their prolonged circulatory half-life benefitting from the PEG coating. According to the results of the in vivo imaging studies, compared with single-wave MSNPs, LY364947-bound MSNPs clearly improved the accumulation and vascular access of “Hard” (PEI-PEG-coated MSNP) or “Soft” (liposome) NPs subsequently injected into BxPC3 xenografts. This sequential combination therapy improved the delivery efficiency of gemcitabine while reducing the systemic toxicity of the chemotherapy.
Another study revealed that the sequential delivery of the natural products α-mangostin and triptolide by two nanoplatforms represents a unique strategy for the treatment of desmoplastic PDAC [107]. First, CAF-targeting CREKA peptide-modified PEG-PLA NPs (CRE-NP(α-M)) loaded with α-mangostin induced CAF deactivation, decreased collagen deposition, normalized the vascular system and improved blood perfusion by suppressing TGF-β/SMAD signaling [107]. Subsequent tumor cell-targeting CRPPR peptide-modified pH-triggered micelles loaded with hydrophobic triptolide (CRP-MC(Trip)) exerted excellent tumor-suppressive effects on a PANC-1/NIH3T3 subcutaneous tumor model with negligible damage to normal organs [107]. In addition to the most representative type of PDAC, chemotherapies for invasive breast cancer, melanoma and other desmoplastic tumors also face the problem of low penetration of chemotherapeutic drugs. The study and application of TGF-βi in other tumor models will offer novel strategies for the combined sequential treatment of desmoplastic tumors with superior therapeutic performance.
4.1.2 Nanodelivery of TGF-β pathway antagonists to enhance the efficacy of molecular targeted therapy
The dense and desmoplastic stroma of PDAC is primarily mediated by TGF-β. Beyond this stromal component, KRAS mutation has been recognized as a pivotal oncogenic driver, accounting for nearly 90% of PDAC cases. TGF-β receptors mediate the phosphorylation of components of the KRAS pathway, whereas KRAS signaling regulates the phosphorylation of SMAD. The dual targeting of TGF-β and KRAS mutations may break through biological barriers and signal transmission for efficient treatment of PDAC. Pei et al. constructed CGKRK-conjugated NPs (Frax-NPCGKRK) for delivery of the antifibrotic agent fraxinellone to suppress TGF-β signaling and developed siRNA-loaded lipid-coated calcium phosphate (LCP) biomimetic NPs (siKras-LCPApoE3) to silence mutant KRAS, which led to the development of a novel sequential targeting strategy [108]. NPs modified with TME-targeting peptide (CGKRK) could accurately deliver fraxinellone to CAFs in the TME, resulting in precise blockade of the TGF-β/SMAD pathway. Additionally, TGF-β blockade led to the inactivation of CAFs, a reduction in M2 macrophage polarization, and normalization of the vascular system in the TME, opening a new avenue for the subsequent delivery of siKras-LCP-ApoE3. This sequential strategy of TGF-β and silencing of mutant KRAS opens broader avenues for the treatment of PDAC.
4.1.3 Nanodelivery of TGF-β pathway antagonists to facilitate immunotherapy
Cancer immunotherapy still faces challenges, such as limited immune responses against antigens and an immunosuppressive TME. Increased levels of immunosuppressive TGF-β might be responsible for poor immune responses. By reprogramming the TME, the Leaf Huang group constructed LPH NPs that delivered siTGF-β to improve the efficacy of immunotherapeutic LCP NP vaccines for advanced melanoma [109]. A mixture of siTGF-β and hyaluronic acid condensed by protamine was coated with PEGylated liposomes through a stepwise self-assembly process to develop LPH NPs.
Sequential combination of nanoencapsulated TGF-β pathway antagonists and versatile NPs by a two-wave strategy.
| Sequential combination strategy | First-wave NPs loaded with TGF-β pathway antagonists | Second-wave NPs | Cancer cell type | Model | Outcome | Ref. |
|---|---|---|---|---|---|---|
| To increase the efficacy of chemotherapy | PEI/PEG-coated mesoporous silica NP loaded with LY364947 | Liposome loaded with gemcitabine | BxPC3 | Female BALB/c nude mice | Two-wave approach improves the delivery efficacy of the gemcitabine by overcoming stromal barrier. | [106] |
| CREKA peptide modified PEG-PLA NPs loaded with α-mangostin | CRPPR peptide modified micelle loaded with triptolide | PANC-1 | BALB/c nude mice | Sequential delivery strategy can reduce ECM production by inactivating CAFs through TGF-β/SMAD signaling, increasing the efficacy of chemotherapy. | [107] | |
| PEGylated liposomes loaded with salvianolic acid B | Docetaxel-loaded PEG-modified liposomes | 4T1, NIH3T3 | Female BALB/c mice | Nanosystem encapsulating salvianolic acid B remodels the TME and enhances the efficacy of NPs loaded with docetaxel. | [112] | |
| Lipid NPs loaded with siTGF-β | Cabazitaxel-loaded albumin NPs | A549 | Female BALB/c nude mice | Combination of chemotherapy and TGF-β blockade perform superior effect against paclitaxel-resistant NSCLC. | [113] | |
| Albumin-bound triphenylphosphine tamoxifen conjugates | Albumin-bound paclitaxel | 4T1 | Female BALB/c mice | Nanosystem loaded with tamoxifen induces codepression of PD-L1 and TGF-β, boosting the chemo-immunotherapy of paclitaxel. | [103] | |
| To enhance the efficacy of molecular targeted therapy | Fraxinellone-loaded CGKRK peptide-modified PEG-PLA NPs | Lipid-coated calcium phosphate NPs for condensing KARS siRNA | PANC-1 | BALB/c nude mice | The pro-blockade of TGF-β leads to the inactivation of CAFs, the reduction of M2 macrophage polarization, and normalization of vascular system, opening the new avenue for the subsequent silencing KRAS mutation. | [108] |
| To facilitate the efficacy of immunotherapy | PEGylated liposome coated with protamine for codelivery of siTGF-β and HA | Mannose-modified lipid-calcium-phosphate loaded with Trp2 peptide and CpG oligonucleotides | B16/F10 | Female C57BL/6 mice | Targeted silencing of TGF-β in TME enhances the efficacy of the vaccine in malignant melanoma. | [109] |
| Fraxinellone | Fraxinellone-loaded AEAA-modified nanoemulsion | BPD6 | Female C57BL/6 mice | Fraxinellone nanoemulsion reverses the immunosuppressive TME and facilitates the therapeutic vaccination in desmoplastic melanoma. | [110] | |
| Combination of TGF-β pathway antagonists and vascular disrupting agent | LY2157299-loaded mPEG5k-b-PLA5k and maleimide- PEG5k-b-PLA5k | Poly(L-glutamic acid)-graft-poly(ethylene glycol)/combretastatin A4 conjugate | 4T1 | Female BALB/c mice | The cooperation of TGF-β inhibition and vascular disrupting agents significantly suppress the tumor growth and metastasis. | [111] |
TGF-β: transforming growth factor-β; NPs: nanoparticles; PEI: polyethyleneimine; PGA: polygalacturonic acid; PLA: polylactide; HA: hyaluronic acid; TME: tumor microenvironment; ECM: extracellular matrix; CAFs: cancer-associated fibroblasts; NSCLC: non-small cell lung cancer.
Schematic illustration of the two-wave strategy for TGF-β suppression and enhanced delivery efficiency of NPs. Adapted with permission from [106]. Copyright 2013, American Chemical Society.
LCP NPs were designed with a calcium phosphate core for the codelivery of a mixture of the Trp2 (SVYDFFVWL) peptide and phosphorylated serine residues together with CpG oligonucleotides (ODNs), whose surfaces were modified with mannose to achieve increased accumulation in the lymph nodes. Considering that TGF-β plays a dual role in different stages of tumors, the authors started the TGF-β siRNA treatment on day 13 to guarantee that the tumors were in a later stage. LPH NPs loaded with siRNA led to approximately 50% knockdown of TGF-β expression, resulting in increased infiltration of CD8+ T cells and decreased levels of Tregs. Compared with the single-vaccine treatment, the combination of LCP NPs and LPH NPs facilitated 52% inhibition of tumor growth. Targeted silencing of TGF-β in advanced tumors resulted in a robust immune response to the vaccine and represents an original combination strategy against immunosuppressive tumors. More importantly, paying attention to the manifestations of the TGF-β inhibition strategy in early tumors will increase therapeutic safety and promote its clinical application.
In another study by the Leaf Huang group, a nanoemulsion (NE) formulation was developed using plutonic F68 and the targeting ligand DSPE-PEG-AEAA on the surface to deliver fraxinellone, which modulates the TME and facilitates the immunotherapeutic efficiency of mannose-modified lipid calcium phosphate NPs containing modified BRAFV600E peptide (pSpSSFGLANEKSI)) and a CpG oligodeoxynucleotide adjuvant in desmoplastic melanoma [110]. In the absence of AEAA modification, compared with nontargeted NE, NE has a more preeminent tumor-targeting ability, indicating that fraxinellone inactivates CAFs. All these mechanistic insights collectively suggest that the spatiotemporal modulation of TGF-β signaling represents an innovative therapeutic approach for cancer management and is capable of simultaneously increasing treatment sensitivity and counteracting immune evasion.
4.1.4 Nanodelivery of TGF-β pathway antagonists combined with vascular disrupting agents
As a promising class of antitumor drugs, vascular disrupting agents (VDAs) directly and precisely destroy tumor blood vessels, inducing secondary tumor necrosis and suppressing cancer progression through the provision of nutrients and oxygen to cancer cells. However, bleeding tissue contains large amounts of TGF-β1, which increases the risk of tumor metastasis. The combination of VDAs and TGF-βi could achieve powerful tumor suppression. Xu et al. selected methoxy-poly(ethylene glycol)-b-poly-(D,L-lactide) (mPEG5k-b-PLA5k) and maleimide-poly(ethylene glycol)-b-poly(D,L-lactide) (MalPEG5k-PLA5k) as carriers to encapsulate LY2157299 (LY) for the fabrication of A15-LY-NPs, whose surface was modified with coagulation-targeting peptide (A15) for accurate and efficient administration of TGF-βi to tumors [111]. Considering the biphasic function of TGF-β in tumor progression, they first examined the effects of LY on early-stage tumors. Bioluminescent signals from in vivo optical imaging revealed that LY-NPs suppressed the establishment of 4T1-luc tumor cell colonies in the lungs and liver, exerting a tumor-suppressive effect on early-stage tumors. By employing a targeted nanoplatform for the precise delivery of TGF-βi, this promising treatment modulates the TGF-β pathway with fewer off-target effects. When further combined with a nanomedicine-loaded VDAs (CA4-NPs), a threefold increase in the intratumoral accumulation of A15-LY-NPs and a 93.7% inhibition of growth were observed in 4T1 tumors. This work presents a targeted strategy for the precise delivery of TGF-βi and a combination strategy for TGF-βi and VDAs.
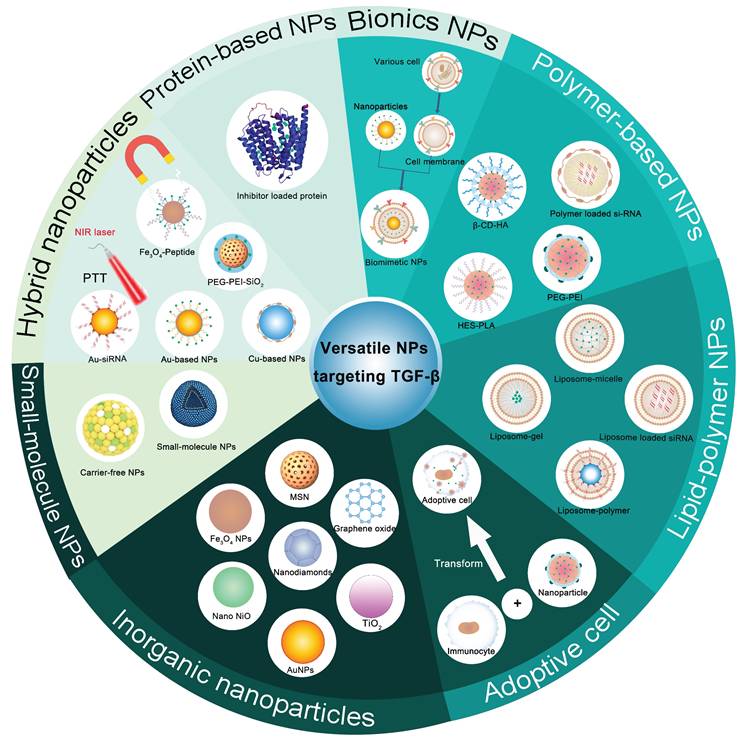
4.2 Recent advances in versatile NPs blocking TGF-β for cancer treatment
The majority of TGF-β therapeutic agents are associated with systemic toxicity and poor treatment outcomes. Targeted delivery of TGF-β therapeutic agents into the TME by versatile NPs (Figure 4) can not only achieve precise delivery of TGF-β pathway antagonists with enhanced therapeutic effects and safety but also open up a promising route for cancer treatment. Versatile NPs targeting TGF-β not only increase the spatial specificity and bioavailability of TGF-βi but also facilitate an improved intratumoral distribution of nanodrugs, thereby addressing key challenges in antitumor therapy, such as stromal barriers and heterogeneous drug delivery. The combination of stimulus-responsive release and stromal modulation represents a promising direction for the next generation of smart nanomedicines, potentially offering new avenues for improving therapeutic outcomes in solid tumors.
4.2.1 Polymer-based NPs
Polymeric NPs with high drug loading capacity and modifiability have emerged as promising carriers to increase the solubility, stability, and targeted delivery of therapeutics, ranging from hydrophobic small molecules and genetic drugs. In addition, responsive polymeric NPs can respond to specific stimuli, releasing their payload in a spatiotemporally controlled manner upon exposure to internal (e.g., pH, enzymes, and redox) or external (e.g., light, temperature, and magnetic fields) stimuli; their tunable properties make them ideal for designing versatile NPs for TGF-β blockade and tumor treatment (Table 2).
4.2.1.1 Enzymatic stimuli
Enzyme-responsive NPs can selectively react with specific enzymes expressed in tumor tissues, contributing to the precise release of therapeutics while minimizing side effects and improving therapeutic effects. Their adaptability makes them promising strategies for personalized and controlled therapy. For the codelivery of a hydrophobic TGF-βi and chemotherapeutic agents into the tumor site, hydroxyethyl starch-polylactide (HES-PLA) core-shell NPs (DOX/LY@HES-PLA) were established with dual α-amylase/pH-responsive properties to codeliver doxorubicin (DOX) and LY2157299 through ultrasonic emulsification and high-pressure homogenization [114]. The hydrophilic shell formed by HES could be disintegrated by endogenous α-amylase, eliminating the detrimental “PEG dilemma” of the hydrophilic layer as a hopeful replacement for PEG and triggering the release of DOX or LY2157299. Compared with the combination of free inhibitor and free DOX, this codelivery nanosystem suppressed tumor growth and inhibited pulmonary metastasis more effectively. The heterogeneous drug distribution of chemotherapeutic drugs is considered a crucial factor contributing to the insufficient efficacy of chemotherapy, which, in turn, promotes EMT and metastasis through the activation of the TGF-β signaling pathway. For instance, DOX, cisplatin, paclitaxel and camptothecin upregulate TGF-β1 expression in various tumor cells. The inhibition of TGF-β could suppress tumor progression and the inadequate efficacy of chemotherapy caused by inhomogeneous drug distribution.
Summary of polymer-based NPs targeting TGF-β for cancer treatment.
| Characteristic | TGF-β pathway antagonists | Nanoparticle/carriers | Tumor cell type | Model | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Enzymatic stimuli | LY2157299 | HES-PLA NPs for codelivery of DOX and LY2157299 | 4T1 | Female BALB/c mice | This codelivery nanosystem eliminates the insufficient chemotherapy promoted metastasis. | [114] |
| LY2109761 | Nanopolyplex self-assembled by DSPE-PEG-plectin-1 peptide and CPI-613 conjugated PEG for delivery of LY2109761 | PANC-1 | BALB/c nude mice | These nanopolyplexes achieve stromal normalization by inhibiting the crosstalk between cancer cells and pancreatic stellate cells, increasing the penetration and anti-tumor efficacy of nanopolyplex. | [118] | |
| SB-431542 | β-cyclodextrin-conjugated heparin and pH-responsive pseudorotaxane for codelivery of DOX and SB431542 | 4T1 | Female BALB/c mice | Dual α-amylase/pH responsive NPs modulate the TME and subsequently amplify the therapeutic effect of DOX. | [119] | |
| LY2157299 | Anti-PD-1 and LY2157299-loaded gelatinase-responsive mPEG-peptide -PCL NPs | H1299 | C57BL/6 mice | Gelatinase-responsive NPs overcome the immunotherapeutic resistance and achieve superior anti-tumor efficacy by cosuppressing PD-1 and TGF-β. | [132] | |
| ROS stimuli | LY2109761 | HCD and FC conjugate shells with BzPGA cores for codelivery of LY210976 and Ce6 | 4T1 | Female BALB/c mice | ROS-responsive core-shell NPs release the LY2109761 in a coordinated manner to achieve timely suppression upon PDT. | [120] |
| siTGF-β | 10-Hydroxycamptothecin grafted polymer core and PEG-PLL-DMMA shell loaded with siTGF-β | B16F10 | C57BL/6 female mice | The pH/ROS-responsive size-loss and charge-reversal nanoplatform improves chemoimmunotherapy by reprogramming the TME. | [122] | |
| pH stimuli | LY2157299 | PEG-PDPA copolymer self-assembled with Ce6 and LY2157299 | CT26 | Male BALB/c mice | The combination of nanoplatform-mediated SDT and TGF-β/SMAD blockade enhances the therapeutic efficacy of anti-PD-L1. | [123] |
| LY2157299 | Polymeric clustered NPs composed of PEG-PCL, PCL and PCL-CDM-PAMAM for codelivery of LY2157299 and siRNA silencing PD-L1 | Panc02 | C57BL/6 mice | Polymeric clustered NPs reverse the immunosuppressive microenvironment and activate the PD-1/PD-L1 checkpoint for enhanced immunotherapy. | [124] | |
| Ingenol-3-mebutate | Single alcoholic hydroxyl (-CH(CH3)-OH polymeric vesicles loaded with ingenol-3-mebutate | Sarcoma-180 | Male C57BL/6 mice | These pH-sensitive polymeric vesicles overcome the hydrophobicity and pH instability of ingenol-3-mebutate, leading to the activation of anti-tumor immunity. | [125] | |
| LY2157299 | Nanomicelles composed of PgA-PAA conjugation for delivery of LY2157299 | HLF | - | These nanomicelles ensure the stability of LY2157299 in acidic pH of gastrointestinal tract. | [126] | |
| Hypoxic stimuli | LY2157299 | Amphiphilic polymer modified with enamine N-oxides and CBP peptide for delivery of LY2157299 and GEM prodrug | KPC, NIH3T3 | Male C57BL/6 mice | These charge-reversal NPs can initiate transcytosis across the ECM barrier to achieve deep penetration in PDAC. | [127] |
| Polymer based hydrogels and nanogels | LY3200882 | Thermosensitive hydrogel incorporating regorafenib and ROS-responsive nanogel loaded with LY3200882 | CT26 | Female C57BL/6 mice | This integrated hydrogel/nanogel system not only restrains the potential tumor metastatic threats induced by regorafenib, but also activates the immune responses by TGF-β blockade. | [128] |
| Gene medicine-loaded polymeric NPs | SB-505124 | β-cyclodextrin-PEI loaded with SB-505124 and the gene encoding murine IL-12 | B16, A549 | Female C57BL/6 mice | This codelivery system facilitates the sustained release of the SB-505124, ensuring efficient transduction of the adenoviral vector encoding the IL-12 gene. | [129] |
| siTGF-β | PLA-based nanovaccine for codelivery of α-Lactalbumin antigens, Toll like receptor ligands, and glutamate chitosan/siTGF-β | 4T1, EO771 | Female BALB/c mice, Female C57BL/6 mice | The synergistic combination of nanovaccine and OX40 suppresses tumor progression by eliciting antigen-specific immunity and silencing TGF-β. | [130] | |
| siTGF-β | Polypeptide loaded with TGF-β and Cox2 siRNA | Hepa1-6 | Female C57BL/6 mice | Gene silencing of TGF-β and Cox2 induces the activation of the immune TME by enhancing T cell penetration. | [133] | |
| Active targeted polymeric NPs | SD-208 | Anti-CD8a F(ab')2 fragments conjugated PEG-PLGA polymeric NPs | MC38, B16 | C57BL/6 mice | These T cell-targeting NPs restore the function of effector T cell and other suppressed immune cells and inhibit the tumor growth. | [131] |
HES: hydroxyethyl starch; PLA: polylactide; DOX: doxorubicin; HCD: hyaluronic acid-aldehyde-monosubstituted β-cyclodextrin; FC: hexadecanol-conjugated ferrocene; BzPGA: benzyl-modified poly(γ-glutamic acid); PgA: poly(glycolic acid); PEI: polyethyleneimine; PGA: polygalacturonic acid; PAA: polyacrylic acid; PEG-PLL-DMMA: polyanion poly(ethylene glycol)-blocked-poly(L-lysine)-modified dimethylmaleic anhydride; PDPA: Poly(2-(diisopropylamino)ethyl methacrylate); PCL: poly(ε-caprolactone); PCL-CDM-PAMAM: poly(amidoamine)-graftpolycaprolactone; GEM: gemcitabine; PEG-PAsp: poly(ethylene glycol)-poly(aspartic acid) block copolymer; SDT: sonodynamic therapy; ROS: reactive oxygen species.
Schematic illustration of multiple NPs targeting TGF-β. NPs: nanoparticles; NIR: near-infrared; PTT: photothermal therapy; PEG: polyethylene glycol; PEI: polyethyleneimine; AuNPs: gold NPs; TiO2: titanium dioxide NPs; Nano NiO: nickel oxide NPs; MSN: mesoporous silica nanoparticle; HES: hydroxyethyl starch; PLA: polylactide; β-CD-HA: hyaluronic acid-aldehyde-monosubstituted β-cyclodextrin.
The TME of PDAC is characterized by a dense and desmoplastic stroma that is largely regulated by TGF-β, which promotes cancer progression, therapeutic resistance, and poor prognosis [115,116]. Recent advances in nanotechnology and stroma-targeting strategies, particularly those designed to modulate TGF-β activity, have provided innovative approaches to overcome these challenges [117]. Based on the high expression of matrix metalloproteinase-2 (MMP-2) by stromal cells and the overexpression of plectin-1 on the surface of pancreatic cancer cells, Li et al. constructed a MMP-2-responsive PPC nanopolyplex to deliver hydrophobic CPI-613 (an antimitochondrial metabolism agent), which self-assembled with the amphiphilic DSPE-PEG-plectin-1 peptide to form a plectin-1-targeting p-PPCL nanopolyplex to deliver the hydrophobic protein LY2109761 [118]. The tumor-responsive nanopolyplex was cleaved by MMP-2 in TEM, triggering the decomposition of the nanoplatform and the release of LY2109761. This “flower-like” nanopolyplex achieved stromal normalization through TGF-β blockade, which increased the accumulation and penetration of the nanopolyplex and enhanced the antitumor effect of free CPI-613 [118]. In normal, healthy tissues, the TGF-β signaling pathway is subject to stringent regulation. TGF-β ligands are synthesized and secreted in an inactive form, remaining latent within the extracellular space. Subsequent activation of these ligands can be mediated by proteolytic cleavage by enzymes such as MMP2 and MMP9. High expression of MMP-2 in tumor tissue is always accompanied by active TGF-β ligands. MMP-2-responsive NPs provide a directional nanoplatform for the accurate delivery of TGF-β pathway antagonists.
The use of grafted polymer nanocarriers to load chemotherapeutic drugs and TGF-βi may amplify the chemotherapeutic effect and suppress tumor metastasis. Zhang et al. [119] constructed hierarchically releasing heparanase/pH-responsive NPs (R(D)/H(S) NPs) based on β-cyclodextrin-conjugated heparin and a pH-responsive pseudorotaxane to encapsulate DOX and SB431542 (Figure 5A). The negative potential of biodegradable heparin prevented SB431542 from being cleared by immune cells, but it could be degraded by heparinase in the TME, triggering the release of SB431542. This study revealed that R(D)/H(S) NPs inactivated CAFs by SB431542, which reduced the expression of collagen I, thereby remodeling the TME and subsequently enhancing the anti-tumor effect of DOX. Enzyme-responsive nanocomplexes protected TGF-βi from being cleared by immune cells and facilitated their entry into cells through lysosomal escape to exert their functions.
4.2.1.2 Reactive oxygen species stimuli
Notably, reactive oxygen species (ROS), a crucial element of PDT, function as key mediators in the activation of latent TGF-β1 in the TME, ultimately causing its downstream detrimental signaling and sacrificing the therapeutic outcome of PDT. Han et al. revealed that PDT led to TGF-β1 accumulation-mediated immunosuppression through increased ROS/TGF-β1/MMP-9 and CD44-mediated local signaling [120]. To opportunely block TGF-β once PDT is performed, they designed core-shell NPs (LC@HCDFC NPs) for the delivery of LY2109761 and chlorin e6 (Ce6, photosensitizer) to complement PDT by blocking TGF-β through the remodeling of immunosuppression [121]. The amphiphilic shells were derived from a conjugate consisting of hyaluronic acid (HA)-aldehyde-monosubstituted β-cyclodextrin (HCD) and hexadecanol-conjugated ferrocene (FC), while the hydrophobic cores were composed of benzyl-modified poly(γ-glutamic acid) (BzPGA)-encapsulated LY210976 and Ce6. HCD conferred exceptional hydrophilicity but also increased CD44 receptor targeting efficiency via HA. Furthermore, the HCDFC inclusion complex facilitated the ROS-responsive release of LY210976, achieving timely inhibition of TGF-β once PDT was administered.
To cope with the immunosuppressive TME and address the low delivery efficiency of NPs, Dai et al. designed a pH/ROS-responsive size-loss and charge-reversal HCPT prodrug nanoplatform to condense siTGF-β for chemoimmunotherapy by reprogramming the immunosuppressive TME [122]. This study leverages a symbiotic interplay between TGF-β inhibition strategies and DDSs. A size-tuneable, ROS-responsive nanoplatform enables the efficient and precise delivery of TGF-βi. Concurrently, the inhibition of TGF-β promotes the deep penetration of nanotherapeutic agents by modulating the TME.
4.2.1.3 pH stimuli
Given the weakly acidic characteristics inherent to the TME, the rational design and engineering of pH-sensitive polymeric NPs emerges as a compelling strategic approach. In addition, electrostatic interactions drive the cellular uptake of positively charged NPs by negatively charged cancer cells. In a groundbreaking study, Huang et al. prepared a pH-responsive positively charged nanodrug via the self-assembly of poly(ethylene glycol)-block-poly(2-diisopropyl methacrylate) diblock copolymer (PEG-PDPA) using a nanoprecipitation method for the codelivery of the sonosensitizer Chlorin e6 (Ce6) and LY2157299 [123]. These positively charged NPs with much smaller particle sizes were conducive to efficient drug accumulation and endocytosis. This platform harnesses SDT to generate cytotoxic ROS bursts and induce robust immunogenic cell death (ICD), which simultaneously releases LY2157299 for the blockade of TGF-β/SMAD signaling and remodeling of the immune microenvironment. This strategy led to a 3.2-fold increase in anti-PD-L1 therapeutic efficacy against colorectal cancer liver metastases in preclinical models [123]. In this study, SDT-mediated ICD, reversion of the immunosuppressive TME by TGF-βi and immune checkpoint blockade constitute an immune cycle for the treatment of immunologically cold tumors.
Hydrophobic polymeric materials with positive charge can be loaded with two functionally distinct drugs via layer-by-layer self-assembly and subsequently delivered to distinct cellular targets within the tumor region. Wang et al. developed pH-sensitive polymeric clustered NPs self-assembled by poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG-PCL), a PCL homopolymer and poly(amidoamine)-grafted polycaprolactone (PCL-CDM-PAMAM) to codeliver LY2157299 and a siRNA targeting the PD-L1 gene for enhanced immunotherapy [124]. Under conditions of acidic tumor extracellular pH, siPD-L1, which is attached to the surface of clustered NPs, is released with PAMAM as size-loss NPs to penetrate deep into the tumor tissue. LY2157299, which is loaded in the core of clustered NPs, exhibited sustained retention within the ECM to target pancreatic stellate cells (PSCs) because of the large size of the NPs. This nanoplatform enables the spatially and temporally controlled release of therapeutic agents, thereby synergistically cosuppressing the TGF-β pathway and the PD-L1 checkpoint for improved antitumor immunotherapy.
Studies of the polymer-based NPs targeting TGF-β for cancer treatment. (A) Schematic illustration of heparanase/pH-responsive NPs for TME modulation. Adapted with permission from [119]. Copyright 2021, American Chemical Society. (B) Schematic illustration of hypoxia/pH-responsive charge-reversal NPs for deep penetration. Adapted with permission from [127]. Copyright 2025, American Chemical Society. (C) Schematic illustration of a hydrogel/nanogel composite for sequential release of drug and synergistic therapy. Adapted with permission from [128]. Copyright 2022, John Wiley and Sons.
The encapsulation of TGF-βi into NPs could overcome the pH instability and hydrophobicity of TGF-βi, thereby facilitating its clinical translation. Yu et al. reported a new TGF-βi, the natural product ingenol-3-mebutate (I3A), which reduced the proportion of Tregs and IL-6 expression and promoted the intratumoral infiltration of CTL [125]. After being encapsulated in polymeric vesicles with 'acidic nuclei' supplied by a single alcoholic hydroxyl (-CH(CH3)-OH), the obtained I3A-PM overcame the hydrophobicity and pH instability of I3A [125]. The development of multiresponsive DDSs will offer a more robust platform for the targeted delivery of these plant-derived hydrophobic drugs.
The development of NPs capable of pH-responsive release under alkaline conditions is equally essential for preventing the degradation of TGF-βi in the acidic gastrointestinal environment. Yu et al. developed an elastic nanocarrier to release LY2157299 in the alkaline pH of the colon through the conjugation of polygalacturonic acid (PgA) and polyacrylic acid (PAA) while resisting the acidic pH of the gastrointestinal tract [126]. These nanomicelles composed of PgA-PAA conjugates exhibited pH-responsive swelling behavior under alkaline conditions, thereby serving as effective stabilizers against the alkaline pH of the colon. Conversely, the aqueous solubility of PAA is limited at low pH, while its inherent mechanical elasticity provides structural support for the formation of nanomicelles, ensuring stability in the acidic pH of the gastrointestinal tract.
4.2.1.4 Hypoxic stimuli
The dense ECM mediated by the TGF-β/SMAD signaling pathway causes the collapse of the vascular system, indirectly leading to the formation of a hypoxic acidic and immunosuppressive TME in PDAC. The tunable physical properties of the NPs (e.g., particle size and surface charge) allow for deep penetration and efficient regulation of the TGF-β pathway, making them a powerful nanosystem for TGF-βi delivery. Hypoxia/pH-responsive charge-reversal NPs (GemC18/Gal@CPLNO) with transcytosis functions were constructed by Zhang et al. to adapt to the pathological characteristics of PDAC (Figure 5B) [127]. An amphiphilic polymer with hypoxia-responsive enamine N-oxides and the collagen-binding peptide CBP was synthesized to incorporate LY2157299 and a stearic acid-modified gemcitabine prodrug (GemC18) by self-assembly for stromal reprogramming and immune activation. Enamine N-oxides first achieved hypoxia-responsive transcytosis through self-sacrificial reduction to produce positively charged NPs. Simultaneously, the lysine residues of the polymer triggered cascade transcytosis by turning the neutral charge to a positive charge in response to the acidic TME. These positively charged NPs can initiate transcytosis across the ECM barrier to achieve deep penetration, releasing TGF-βi and other drugs in response to hypoxic and acidic microenvironments.
4.2.1.5 Polymer-based hydrogels and nanogels
Hydrogels and nanogels represent two classes of biomaterials that are extensively utilized for controlled drug delivery. By incorporating nanogels into the three-dimensional network of hydrogels, the hydrogel/nanogel system achieves not only localized drug delivery through hydrogels whose mechanical properties match those of biological tissues but also cascade drug release by nanogels whose core can encapsulate another therapeutic agent. Li et al. constructed a thermosensitive hydrogel (Gel/(REG+NG/LY)) incorporating regorafenib and a ROS-responsive nanogel loaded with LY3200882 for spatiotemporally and sequentially controlled release in colorectal tumors (Figure 5C) [128]. Owing to the prestumoral injection, this injectable hydrogel has fewer systemic side effects. The earlier thermosensitive release of REG increased the production of intracellular ROS after injection, splitting the thioketal linker within the nanogel and leading to the subsequent release of LY3200882. This sequential drug delivery nanosystem not only restrained the potential tumor metastatic threats induced by REG but also increased immune responses to the immunosuppressive TME through TGF-β blockade. This integrated hydrogel/nanogel system enables sequential and cascade drug release, thereby improving the spatiotemporal control of drug delivery and potentially increasing treatment efficacy in complex disease environments such as tumors.
4.2.1.6 Gene medicine-loaded polymeric NPs
Polymeric carriers offer a great opportunity to package hydrophobic TGF-β and negatively charged gene medicines into one system for effective treatment. As TGF-β and IL-12 play diametrically opposing roles within the TME, coupling IL-12 delivery with TGF-β blockade could increase local immune responses to tumors, constituting a particularly potent treatment modality. Jiang et al. designed a versatile polymeric carrier, β-cyclodextrin-PEI, to encapsulate SB-505124 and the gene encoding murine IL-12 for immunotherapy of metastatic malignant melanoma [129]. This nanosystem facilitated the sustained release of SB-505124 while ensuring efficient transduction of the adenoviral vector encoding the IL-12 gene. By incorporating responsive bonds into nanomaterials, responsive sequential release can be achieved for more precise combination treatments.
Multilayered polymeric NPs serve as excellent nanosystems to protect siRNAs targeting TGF-β1 (siTGF-β1) from inchoate decomposition and achieve the sustained release of gene drugs in vivo. Peres et al. constructed a poly(lactic acid) (PLA)-based nanovaccine for the codelivery of α-lactalbumin antigens, Toll-like receptor ligands, and glutamate chitosan/siTGF-β [130]. This nanovaccine combined with the agonist immune checkpoint OX40 synergistically inhibited tumor progression and prolonged overall survival due to antigen-specific adaptive immunity and TGF-β silencing derived from the nanovaccine. This study further confirms that TGF-βi also improve the effectiveness of antigen-presenting cell checkpoint inhibitors, thereby broadening the scope of application of TGF-βi and providing valuable insights for clinical trials involving both ICIs and TGF-βi.
4.2.1.7 Actively targeted polymeric NPs
Polymeric NPs engineered for active targeting can faithfully replicate the protracted growth dynamics of human tumors. T cell targeting NPs that accurately deliver immunomodulatory TGF-βi to tumor sites can improve T cell activity better than free TGF-βi and passive NPs can, with minor side effects. CD8+ T cells targeted polymeric NPs promote the accumulation of the immunomodulatory agent SD-208, restoring the activity of CTL and inhibiting tumor growth. Notably, the treatment achieves these effects at one logarithm lower dosage of anti-PD-1 and anti-SD-208, whereas free small-molecule drugs have no effects at the same dosage [131]. Polymer-based NPs represent among the commonly employed drug delivery systems; however, strategies utilizing actively targeted polymeric systems for the delivery of TGF-βi remain relatively scarce. Further study is warranted to elucidate the relationship between TGF-β and TME-specific overexpression of receptors for the development of actively targeted DDSs.
4.2.2 Inorganic NPs
Over the past few decades, inorganic NPs have presented a remarkable opportunity as drug delivery carriers because of their facile modification, stimuli-responsive drug release mechanisms, and imaging capability. Notably, inorganic NPs can intrinsically modulate the TGF-β signaling pathway, resulting in an antitumor effect through the blockade of TGF-β signaling in cancer or adverse effects on health through the activation of TGF-β (Table 3) [134]. A focus on the biosafety of inorganic NPs and their side effects on normal tissues is crucial for promoting their clinical application.
Summary of inorganic NPs targeting TGF-β.
| Characteristic | Nanoparticle/carriers | Cancer cell type | Model | Outcome | Ref. |
|---|---|---|---|---|---|
| Inorganic NPs suppress the TGF-β signaling pathway | AuNPs | MBT-2 | Female (either C3H/HeN or NOD-SCID) mice | AuNPs attenuate the EMT through the formation of AuNP-TGF-β1 conjugates. | [136] |
| AuNPs | A2780-CP20, CAFs | - | AuNPs with size in 20 nm inactivate the CAFs by blockade of TGF-β1, PDGF, and other markers of CAFs. | [137] | |
| Carbon nanodiamonds | A549 | Nude mice | Nanodiamonds suppress the TGF-β by inducing the lysosomal decomposition of TGF-β receptors. | [138] | |
| Metallofullerenol-based Gd@C82(OH)22 NPs | MDA-MB-231 | Female BALB/c nude mice | Gd@C82(OH)22 NPs eliminate the CSCs and impede the EMT via suppression of HIF-1α and TGF-β. | [139] | |
| TiO2 NPs | A549 | Zebrafish, C57BL/6 mice | TiO2 NPs suppress the EMT progression by binding to the TβRI/II. | [140] | |
| Inorganic NPs activate the TGF-β signaling pathway | Nano-TiO2 | SW480 | - | Nano-TiO2 exhibits the toxic and side effect by activation of the TGF-β/MAPK and Wnt pathways. | [142] |
| Nano NiO | HepG2 | Male wistar rats | Nano NiO promotes the EMT progression and ECM deposition through TGF-β signaling pathway, resulting in hepatic fibrosis. | [143] | |
| TiO2 nanofibers | A549 | BALB/c nude mice | TiO2 nanofibers cause lung epithelial cells to obtain an aggressive tumor phenotype. | [144] | |
| PEI coated MNPs | HeLa | - | PEI-MNPs lead to tumor progression through the induction of autophagy and the activation of the NF-κB and TGF-β. | [145] | |
| Graphene oxide | 4T1 | Female BALB/c mice | GO activates the TGF-β signaling to exert prometastatic effects in a dose-dependent manner. | [146] | |
| AgNPs | - | Male and female ICR mice | Small-sized AgNPs are more active at triggering toxicological or biological responses than large-sized AgNPs. | [147] | |
| AlO NPs | KLN 205, HeLa, A549, SKOV3 | Female DBA/2 mice | Wire shaped AlO, but not spherical shaped AlO NPs, leads to tumor-associated inflammation and metastasis. | [148] |
AuNPs: gold NPs; TiO2: titanium dioxide NPs; NiO NPs: nickel oxide NPs; PEI: polyethyleneimine; MNPs: magnetic NPs; GO: graphene oxide; AlO NPs: aluminum oxide NPs; AgNPs: silver NPs; TGF-β: transforming growth factor-β; CAFs: cancer-associated fibroblasts; EMT: epithelial-mesenchymal transition; ECM: extracellular matrix.
4.2.2.1 Inorganic NPs suppress the TGF-β signaling pathway
By serving as nanocarriers or therapeutic agents, gold NPs (AuNPs) are broadly applied because of their unique physical properties [135]. AuNPs attenuate the EMT through the formation of AuNP-TGF-β1 conjugates, which result in the inactivation of the TGF-β1 [136]. Additionally, in vivo experiments indicated that AuNPs increased tumor immunity by increasing the infiltration of CD4+ and CD8+ T cells and interfering with TGF-β signaling in MBT-2 tumors [136]. Zhang et al. demonstrated that AuNPs with a size of 20 nm inactivated CAFs by regulating the expression of fibroblast activation- or inactivation-associated markers, such as the blockade of TGF-β1, PDGF, and other markers [137]. This study helps us determine the role of AuNPs in interfering with multicellular communication within the TME.
Carbon nanodiamonds, a unique class of carbon NPs, bind to TβR-II, promote its lysosomal degradation and inhibit the transduction of TGF-β signaling [138]. Nanodiamonds suppress tumor invasiveness and induce macrophage polarization toward the antitumor M1 phenotype, indicating potential therapeutic effects on cancer. Metallofullerenol-based Gd@C82(OH)22 NPs, which are virus-like morphological nanocages, exert inherent antitumor effects on triple-negative breast cancer cells, with negligible side effects on normal mammary epithelial cells [139]. Under normoxic and hypoxic conditions, the Gd@C82(OH)22 NPs eliminate CSCs and impede EMT by suppressing the expression of HIF-1α and TGF-β, further emphasizing their therapeutic potential.
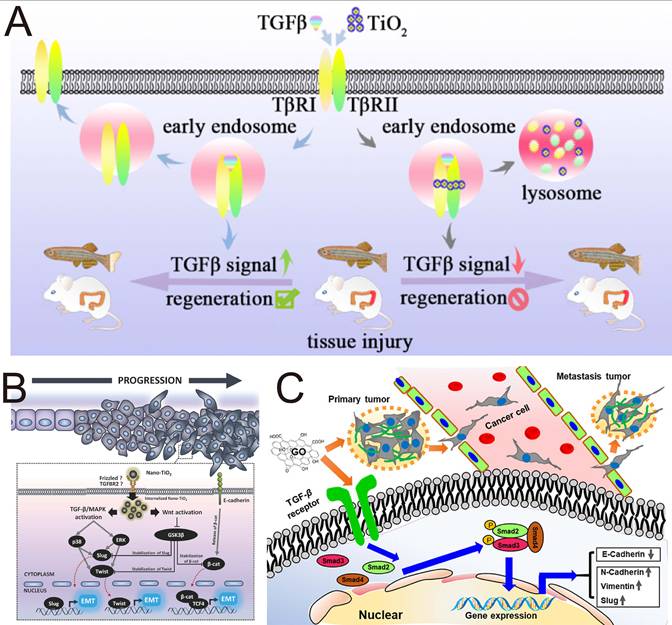
Titanium dioxide NPs (TiO2 NPs) are among the most extensively produced nanomaterials and are widely applied in food production, cosmetics, and pharmaceuticals. Studies have shown that TiO2 NPs are capable of inhibit EMT with negligible cytotoxicity toward human lung epithelial A549 cells (Figure 6A). In terms of mechanism, TiO2 NPs bind to the TGF-β receptors TβRI/II, promote the trapping of TGF-β receptors in the lysosome, and interfere with the phosphorylation of SMAD2/3 [140]. Zinc oxide (ZnO) NPs are unique metal oxide nanomaterials that have garnered significant attention in the biomedical field due to their ability to produce zinc ions (Zn2+) and ROS. Metal ions and ROS act as critical modulators of stem cell physiology and significantly influence their metabolic processes and immunological functions. Recent research indicates that ZnO NPs can efficiently inhibit the production of TGF-β and α-SMA in adipose-derived stem cells, exhibiting significant potential for treating inflammation-related liver fibrosis [141].
4.2.2.2 Inorganic NPs activate the TGF-β signaling pathway
While NPs targeting TGF-β have outstanding tumor-suppressive effects, inorganic NPs can induce prometastasis and side effects by activating TGF-β signaling. Magdiel et al. demonstrated that nano-TiO2, which is universally added to food products, can promote EMT in colorectal carcinoma through both the TGF-β/MAPK pathway and the Wnt pathway (Figure 6B) [142]. Analogously, both silica NPs (nano-SiO₂) and hydroxyapatite NPs (nano-HA), which are commonly utilized as food additives, accumulate in colorectal cancer cells and induce an elongated fibroblast-like morphology. This morphological change is also capable of promoting EMT progression. This study revealed that EMT induction is not merely confined to nano-TiO₂ exposure but may represent a broader nanomaterial-induced phenomenon, indicating the possible toxicity and side effects of absorbable airborne nanomaterials in the induction of EMT progression. Similarly, nickel oxide NPs (Nano NiO) have been shown to induce EMT progression through TGF-β1/SMAD pathway in a hepatoblastoma-derived cell line (HepG2), resulting in hepatic fibrosis [143]. A similar tumor-promoting phenomenon has also been verified in another study investigating TiO₂ nanofibers. TiO2 nanofibers cause lung epithelial cells to obtain an aggressive tumor phenotype with upregulation of HIF-1α, TGF-β and N-cadherin, which serve as angiogenic, profibrotic and EMT markers, respectively [144].
Fe3O4 magnetic NPs (MNPs) arguably rank among the most extensively researched nanomaterials, particularly in cancer theranostics. Using PEI to guarantee MNPs colloidally stable in cell culture media, Man et al. constructed PEI-coated MNPs (PEI-MNPs), which served as a model to determine how the MNPs elicited cellular responses. PEI-MNPs led to tumor progression through the induction of autophagy and the activation of the TGF-β/ NF-κB signaling pathways via the generation of ROS [145].
Depending on many properties, including size, morphology, surface charge, chemical composition and other reactive properties, inorganic NPs might exhibit prometastatic effects and toxicity through TGF-β signaling. Zhu et al. reported that low-dose GO nanosheets with a large size range of 400-900 nm (GO-L) and a small size range of 200-600 nm (GO-S) similarly induced remarkable morphological and structural variations in the tumor cell membrane (Figure 6C), promoting tumor cell migration and invasion by activating TGF-β signaling and increasing the number of TGF-β receptors; although GO nanosheets induced cytotoxicity at relatively high doses [146]. GO activated TGF-β signaling to exert prometastatic effects in a dose-dependent manner. Another study revealed that small-sized (22 nm, 42 nm, and 71 nm) silver NPs (AgNPs) increase the expression of TGF-β, proinflammatory cytokines, and Th1-type and Th2-type cytokines in serum in a concentration-dependent manner and are more active at triggering toxicological or biological responses than large-sized AgNPs (323 nm) [147]. The results obtained by Park et al. proved that orally administered small-sized AgNPs were more capable of inducing organ toxicity and inflammatory responses than large-sized AgNPs were. Aluminum oxide (AlO) NPs are considered potential immunoadjuvants and drug delivery carrier materials. Manshian et al. demonstrated that wire-shaped AlO with a high aspect ratio, but not spherical-shaped AlO NPs, leads to tumor-associated inflammation and metastasis in animal models through the activation of the NLRP3 inflammasome and high expression of TGF-β [148]. All these findings indicate that the side effects of nanomaterials should be considered in the development of nanomedicines. The physicochemical properties of inorganic NPs (e.g., concentration, size, and morphology) are key factors influencing their effects on TGF-β. Future research should focus specifically on how changes in these physicochemical properties affect the bioactivity and safety of NPs.
4.2.3 Hybrid NPs
Owing to the combination of inorganic nanomaterials with organic nanomaterials, hybrid NPs with increased biocompatibility and suspension characteristics have garnered increasing attention in the development of versatile nanosystems for the delivery of TGF-β pathway antagonists (Table 4). Owing to their therapeutic and imaging abilities, controlled release, and versatility, hybrid nanosystems are promising for the theranostics of tumors.
The Janus-faced properties of inorganic NPs in the regulation of TGF-β signaling. (A) Inhibition of EMT by TiO2 NPs. Adapted with permission from [140]. Copyright 2018, American Chemical Society. (B) TiO2 NP-induced cell migration and invasion in intestinal epithelial cancer cells. Adapted with permission from [142]. Copyright 2018, John Wiley and Sons. (C) GO nanosheets promote cancer metastasis by promoting TGF-β signaling-dependent EMT. Adapted with permission from [146]. Copyright 2020, American Chemical Society.
Summary of hybrid NPs, lipid-polymeric NPs and small-molecule NPs targeting TGF-β for cancer treatment.
| Characteristic | TGF-β pathway antagonists | Nanoparticle/carriers | Cancer cell type | Model | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Hybrid NPs | RLX | RLX-SPION | PANC-1 | Male CB17 SCID mice | RLX-2 conjugated SPIONs enhance the chemotherapeutic efficacy of GEM by inhibiting the differentiation of PSCs into CAF-like myofibroblasts. | [149] |
| Anti-TGF-β antibody | Anti-TGF-β and RGD2 conjugated Fe3O4/Gd2O3 hybrid NPs for delivery of sorafenib | MC38 | C57BL/6 mice | This MRI-guided nanosystem achieves synergistic effect of ferroptosis and TGF-β blockade. | [150] | |
| siTGF-β | Angiopep LipoPCB (Temozolomide+BAP/siTGF-β) | GL261 | C57BL/6 mice | Traceable NPs efficiently cross the blood-brain barrier, enabling combined immunotherapy and chemotherapy for intracranial glioblastoma. | [151] | |
| siTGF-β | PEI-PEG nanogels loaded with TGF-β siRNA and Fe3O4 NPs | S180 | Male BALB/c mice | This nanosystem loaded with TGF-β1 siRNA performs MR imaging-guided gene therapy of sarcoma. | [164] | |
| siTGF-β1 | Cysteamine-functionalized AuNPs for delivery of siTGF-β1 | HepG2 | Female athymic nude mice | These AuNP-siRNA nanocomposites deliver siTGF-β1 into implanted tumor cells and protect it from physiological conditions. | [152] | |
| SB525334 | Anti-CD8 VHH nanobody conjugated Amph-NPs for delivery of SB525334 | Lymphocytes | C57BL/6 mice | The targeted nanosystem promotes the delivery of the SB525334 to T cells, while free SB525334 does not affect T cell functionality. | [153] | |
| Maclura tricuspidata extract | Au NPs-conjugated maclura tricuspidata extract (MT-GNPs) | HepG2, SK-Hep-1 | - | MT-GNPs reverse the EMT by TGF-β1 blockade. | [165] | |
| - | cRGD-targeted Au NPs loaded with the gefitinib and IR780 | PC-9GR | BALB/c nude mice | Combination of SDT and low-temperature PTT mediated by hybrid Au NPs overcomes the EGFR-TKI resistance by inactivation of TGF-β/PDLIM5/SMAD resistance pathway. | [166] | |
| LY2157299 | Anti-L1CAM antibody functionalized hybrid NPs intergrating gelatin, protein A, diatomite and Au NPs | SW620 | Athymic nude mice | These hybrid nanosystems achieve targeted precise therapy for treatment of metastatic colorectal cancer. | [167] | |
| Anti-TGF-β antibody | CuS NPs modified with PEG-anti-TGF-β for delivery of ATM inhibitor | HepG2, H22 | Female BALB/c mice | Hybrid CuS NPs modified with anti-TGF-β antibody achieve ATM inhibitor-mediated chemotherapy and CuS-mediated low-temperature PTT with amplified specificity. | [154] | |
| - | Cu2(OH)PO4 NPs coated with PAA | U87MG, U251 and T98G | BALB/c nude mice | The PTT mediated by Cu₂(OH)PO₄ is related to MSH6-CXCR4-TGF-β1 axis. | [155] | |
| SB525334 | GSH-responsive MSN | Panc02 | C57BL/6 mice | Modulating neutrophil polarization via nanomedicine facilitates irreversible electroporation and anti-PD-1-mediated immune checkpoint blockade. | [156] | |
| siTGF-β | PEI-coated MSN for delivery of PD-L1 inhibitor JQ1 and siTGF-β | A375, T lymphocyte cell | - | Hybrid siRNA NPs regulate the TME by protecting tumor escape from immune system. | [168] | |
| Halofuginone | Mesoporous platinum NPs loaded with halofuginone | 4T1 | Female BALB/c nude mice | The halofuginone-loaded theranostic mesoporous platinum nanosystem modulates the ECM with no observed systemic toxicity. | [169] | |
| SB-505124 | SB-505124-loaded PEGylated MSN intergrated with Mn2+ and Fe2+ | CT26 | C57BL/6 mice | Multimetal nanozymes remodel the TME for improving the efficacy of catalytic tumor therapy. | [157] | |
| Curcumin | CaCO3/PDA NPs loaded with curcumin and ropivacaine | 4T1 | BALB/c mice | Blockade of TGF-β inhibits the expression of TRPV1, achieving comfortable immunotherapy with palliative hyperalgesia. | [170] | |
| Lipid-polymeric NPs | SB505124 | Liposomal polymeric gels | - | Female B6 albinomice | Hybrid core-shell nanoplatform enhances tumor immunotherapy against melanomas through the activation of immune responses. | [158] |
| Esomeprazole | Lipid NPs loaded with mRNA and esomeprazole | HeLa, L929 and B16-OVA | Female C57BL/6 mice | Lipid formulations loaded with esomeprazole promote the escape of the mRNA from the endo-/lysosomes and deep penetration of lipid NPs. | [159] | |
| Vactosertib | Vactosertib-loaded liposomes delivering paclitaxel-loaded PEG-PLGA nanospheres | Panc-1 | BALB/c nude mice | The size switchable nanoplatform achieves the sequential cascade release of drugs for enhanced chemotherapy of stroma-rich tumors. | [160] | |
| SB-505124 | Nanoliposome loaded with ICG, SB-505124, and zoledronic acid | 4T1 | Female BALB/c mice | The nanosystem modulates the immunosuppressive microenvironment by combination of photothermal immunotherapy and TGF-β blockade. | [171] | |
| siTGF-β1 | Hybrid NPs composed of PLGA, DSPE-mPEG, and cRGD-conjugated DSPE-PEG | 4T1 | Female BALB/c mice | The nanoplatform remodels the suppressive immune TME to boost the immunotherapy. | [172] | |
| siTGF-β1 | Lipid- PEG NPs | A549, T cells | Female BALB/c nude mice | The siRNA Lipid NPs efficiently defeat paclitaxel-resistant lung cancer. | [173] | |
| Small- molecule NPs | OA | OA NPs and nanogels | 4T1 | Female BALB/c mice | OA nanogels exhibit the highest tumor inhibition efficiency on α-SMA among the different OA formulations. | [161] |
| OA | OA nanogel comprising rose bengal and methylene blue | 4T1 | Female BALB/c mice | Tri-component programmable nanogel inactivate the CAFs via TGF-β blockade for PTT/SDT-assisted immune therapy. | [162] | |
| SB505124 | Self-assembled bioregulators incorporating Ce6, SB505124 and lonidamine | CT26 | BALB/c mice | The combination of PDT and reversal of the immunosuppressive TME synergistically amplify the immunotherapy for treatment of colorectal cancer. | [163] |
RLX: human relaxin-2; SPION: superparamagnetic iron nanocubes; PSC: pancreatic stellate cells; GEM: gemcitabine; RGD2: arginine-glycine-aspartic dimer; siTGF-β1: TGF-β1 siRNA; MRI: magnetic resonance imaging; ATM: ataxia telangiectasia mutated; PEI: polyethyleneimine; PEG: poly(ethylene glycol); PAA: polyacrylic acid; OA: oleanolic acid; MSN: mesoporous silica NPs; PLGA: poly(lactic-co-glycolic acid); ICG: indocyanine green; Ce6: Chlorin e6; TGF-β: transforming growth factor-β; CAFs: cancer-associated fibroblasts; EMT: epithelial-mesenchymal transition; ECM: extracellular matrix; PTT: photothermal therapy; SDT: sonodynamic therapy.
Hybrid NPs composed of magnetic components, such as superparamagnetic iron nanocubes (SPIONs) and Fe3O4 NPs, hold significant potential for magnetic resonance imaging (MRI), hyperthermia of tumors, as ferroptosis inducers, and magnetically driven targeted delivery. Mardhian et al. designed and synthesized human relaxin-2 (RLX)-conjugated SPIONs using biochemical conjugation techniques to suppress the differentiation of PSCs into CAF-like myofibroblasts, which improved the chemotherapeutic efficacy of gemcitabine and decreased collagen deposition in PDAC [149]. Compared with free RLX, RLX-SPION resulted in a greater reduction in α-SMA expression and more efficacious inhibition of TGF-β-induced pancreatic stellate cell differentiation, which was attributed to the multivalent effect of RLX on SPIONs. Recently, the induction of ferroptosis by iron oxide particles in tumor cells has emerged as a prospective strategy to elicit immunogenicity, adaptive immune responses, and therapeutic outcomes. Zhou et al. developed anti-TGF-β antibodies and arginine-glycine-aspartic dimer (RGD2)-conjugated Fe3O4/Gd2O3 hybrid NPs loaded with sorafenib (FeGd-HN@Sorafenib@TGF-β-antibody@RGD2, FG-STR) for MRI-guided TME heating [150]. Synergistic combination of TGF-β blockade and ferroptosis represents an effective strategy for increasing the response rate of poorly immunogenic tumors to ICIs by modulating the TME; however, their side effects on normal tissues still restrict their clinical application. This nanosystem achieves highly efficient MRI-guided combination therapy through the combination of ferroptosis and spatiotemporal TGF-β blockade.
The use of siRNA to regulate the TME for enhanced chemotherapy is promising. Qiao et al. developed a hybrid ROS-responsive nanosystem (Angiopep LipoPCB (Temozolomide+BAP/siTGF-β), ALBTA) with the ability to efficiently cross the blood-brain barrier and escape endosomal/lysosomal degradation for TGF-β RNAi-based immunochemotherapy of glioblastoma (Figure 7A) [151]. Positively charged BAP polymers encapsulating SPIONs were used to complex with siTGF-β and prepare AN@siTGF-βNPs, excluding the “off-target” effect of siTGF-β by the controlled release of siRNA to construct a ROS-labile charge-reversal BAP. Lipid-based envelopes were subsequently coated on AN@siTGF-βNPs, and temozolomide (TMZ) was added to construct LBTA NPs; zwitterionic liposomes protected the siRNA from endosomes/lysosomes. Finally, the peptide angiopep-2 targeting the BBB and glioblastoma cells was conjugated to the NPs to obtain the optimized nanosystem (ALBTA) for accurate MRI and intracranial glioblastoma treatment. This nanosystem crosses the blood-brain barrier via transcytosis and responsively releases drugs and contrast agents based on changes in the local microenvironment, enabling combined immunotherapy and chemotherapy for intracranial glioblastoma.
Au NPs possess extensive biomedical applicability because of their distinct physicochemical properties, such as a tuneable morphology, expansive surface area, amphiphilic nature, and carrier capacity for drug and gene delivery. Wu et al. synthesized positively charged cysteamine-functionalized AuNPs to condense a negatively charged siTGF-β1 and then coated S-PEG on the surface to cover the free cysteamine. These AuNP-siRNA nanocomposites delivered siTGF-β1 into implanted tumor cells and protected them from physiological conditions to knockdown TGF-β1 signaling for oncotherapy [152]. In addition to delivering nucleic acid drugs, ligand-modified amphiphilic gold NPs can also deliver hydrophobic compounds, such as TGF-β1 inhibitors. Another study by Yang et al. revealed that 11-mercaptoundecane sulfonate ligand-modified Au NPs (Amph-NPs) were competent at secluding small-molecule drugs, such as SB525334, within the hydrophobic pockets of their ligand shells [153]. This flexible organic ligand shell terminated by hydrophobic methyl and water-solubilizing sulfonate groups not only allows NPs to embed within lipid bilayers to enter cells without toxicity but also provides a methyl group to serve as a reactive site for the subsequent chemical conjugation of antibody proteins. After being further conjugated with anti-CD8 VHH nanobody, targeting antibodies conjugated with Amph-NPs (VHH-NPs) promoted the delivery of SB525334 to T cells accompanied by increased production of both IFN-γ and TNF-α, while free SB525334 did not influence T cell functionality [153]. These studies might focus on the intrinsic effects of Au NPs on the TGF-β signaling pathway for the further development of Au-based hybrid NPs.
Studies of hybrid NPs, lipid-polymeric NPs and small-molecule NPs targeting TGF-β for cancer treatment. (A) Schematic illustration of the nanotheranostic system (Angiopep LipoPCB (Temozolomide+BAP/siTGF-β), ALBTA) with dual targeting and ROS response to TGF-β RNAi-based immunochemotherapy for glioblastoma. Adapted with permission from [151]. Copyright 2018, John Wiley and Sons. (B) Schematic illustration of the size-switchable lipid‒polymer hybrid nanoplatform (APTEDB-(NS-TAX@Lipo-VAC) against desmoplastic PDAC. Adapted with permission from [160]. Copyright 2021, American Chemical Society. (C) Schematic illustration of the self-assembled carrier-free nanodrug for TGF-β blockade and PDT-amplified immune therapy. Adapted with permission from [163]. Copyright 2022, American Chemical Society.
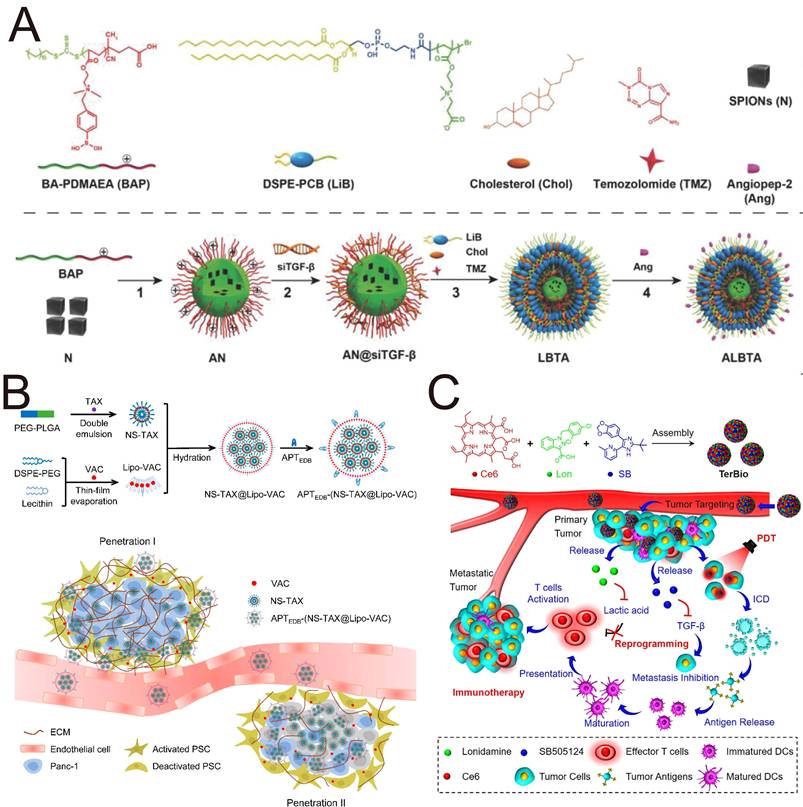
Cu-based nanomaterials, such as copper oxide, Cu metal-organic frameworks, CuS, and Cu2(OH)PO4, exhibit enzyme-mimicking properties, photothermal performance, and cancer imaging ability. Cai et al. synthesized CuS NPs modified with PEG-anti-TGF-β on the surface to deliver an ataxia telangiectasia mutated (ATM) inhibitor for chemotherapy and CuS-mediated photothermal therapy (PTT). Compared with the cellular uptake in LO2 cells and HepG2 cells, the ability of CuS-ATMi@TGF-β NPs to target hepatocellular carcinoma cells was proven by increased red fluorescence [154]. This enhanced cellular uptake and targeting specificity is mediated by anti-TGF-β. In a study of glioblastoma multiforme, Chen et al. developed a “one-for-all” hybrid theranostic agent, Cu2(OH)PO4@PAA, based on polyacrylic acid (PAA)-coated Cu2(OH)PO4 NPs for MRI-guided PTT, which suppressed the MSH6-CXCR4-TGF-β1 pathway and its downstream factors in glioblastoma multiforme [155]. Most investigations have concentrated on increasing the expression of MSH6 to increase the efficacy of temozolomide. Nevertheless, the function and signaling pathway of MSH6 have not yet been considered. This study demonstrated that the oncogenic MSH6-CXCR4-TGF-β1 feedback loop represents a promising therapeutic target in GBM and that the PTT mediated by Cu₂(OH)PO₄ is related to the MSH6-CXCR4-TGF-β1 axis.
Most patients with PDAC are not eligible for surgical resection, and the dense tumor stroma contributes to a poor response to current chemotherapy and radiotherapy. IRE has emerged as a novel ablation modality used in the clinic for PDAC patients, but IRE remains associated with a persistent risk of tumor recurrence. The porous structure and easily modified surface of mesoporous silica NPs (MSNs) provide a robust platform for loading diverse therapeutic agents. Peng et al. fabricated glutathione-responsive degradable mesoporous silica NPs to deliver SB525334 for reprogramming tumor-associated neutrophils against PDAC [156]. The regulation of the immune TME enhanced the therapeutic response of tumors to irreversible electroporation and anti-PD1 therapy. This study addresses the issue of tumor recurrence following irreversible electroporation by blocking TGF-β and PD-1 expression, providing valuable clinical insights for the treatment of PDAC.
MSNs also provide a nanoplatform for multimetal nanozymes with high peroxidase-like and catalase-like activity. Xu et al. constructed SB-505124-loaded PEGylated iron manganese silicate NPs (IMSN-PEG-TI) incorporating Mn2+ and Fe2+ for nanozyme-mediated catalytic therapy [157]. The blockade of TGF-β modulated immune TEM, inducing macrophage polarization towards M1 phenotype. This nanozyme catalyzes the production of H2O2 for ferroptosis mediated tumor therapy. The development of nanozymes to modulate the TME holds significant research value and clinical relevance for improving the efficacy of nanozyme-based catalytic tumor therapy.
4.2.4 Lipid-polymeric NPs
Lipid-polymeric NPs represent a class of nanocarriers characterized by an internal polymeric core enveloped by a multilayered outer lipid shell. This unique architecture endows them with the versatile ability to encapsulate diverse payloads, encompassing both hydrophilic and hydrophobic therapeutic agents. For instance, a hybrid core-shell nanoplatform was developed to codeliver both the hydrophobic inhibitor SB-505124 and the hydrophilic protein cytokine IL-2 for enhanced tumor immunotherapy [158]. The inhibitor-loaded methacrylate-modified β-cyclodextrins and IL-2 were encapsulated in an interlaced polymer matrix with a PEGylated liposomal coating, which enabled the sustained release of TGF-βi and IL-2 for seven days [158]. Blocking TGF-β signaling and stimulating the activation of T cell responses via IL-2 had a synergetic anticancer effect on a B16/B6 melanoma mouse model through the activation of both innate and adaptive immune responses.
Lipid-polymeric NPs have risen as powerful platforms for mRNA delivery; however, the endolysosomal/lysosomal barrier and dense ECM hinder the penetration and delivery efficiency of lipid-polymeric NPs. Young et al. constructed novel lipid formulations (LNP) that leverage esomeprazole (ESO) to promote the escape of mRNA from endo-/lysosomes [159]. As a proton pump inhibitor, ESO hinders the acidification of the endo-/lysosomal pH and destroys membrane integrity, which induces endo-/lysosomal escape. Furthermore, the ESO-loading LNP remodel the ECM through the TGF-β/SMAD signaling pathway, contributing to the deep penetration of both LNP and mRNA. The use of ESO-loading LNP formulations for mRNA delivery allows for both increased local expression of the mRNA and effective immune activation.
The sequential release of TGF-βi and drug is important for the cascade penetration strategy to achieve the anticipated effect. While small NPs exhibit superior penetration into deep tumor regions, their propensity for rapid macrophage-mediated clearance presents a significant therapeutic limitation. An optimal strategy was developed to address this dichotomy using a size-switchable lipid-polymer hybrid nanosystem (APTEDB-(NS-TAX@Lipo-VAC) to achieve drug penetration and the sequential release of vactosertib (VAC) and paclitaxel (TAX) in the desmoplastic stroma (Figure 7B) [160]. A hydrophobic layer of smaller PEG-PLGA nanospheres (NS-TAX) was loaded with TAX and subsequently loaded into larger liposomes, which delivered VAC within a phospholipid bilayer (Lipo-VAC), thereby constructing the nanosystem NS-TAX@Lipo-VAC. The liposomes were functionalized with APTEDB peptide targeting fibronectin extra domain B (EDB), which is highly expressed in the ECM, to achieve targeting and long retention in the tumor. Larger NPs functionalized with APTEDB peptide are targeted precisely to the tumor ECM, and the earlier release of VAC, which is accompanied by the collapse of liposomes, not only decreases the size of the nanosystem but also inactivates PSCs and remodels the ECM via TGF-β blockade to promote the subsequent penetration of TAX, thus astricting pancreatic tumor xenografts more efficiently than free drugs do. This cascade permeation strategy, which employs a stroma-regulable and size-switchable nanosystem, holds significant potential for the treatment of stroma-rich tumors.
4.2.5 Small-molecule NPs
Self-assembled nanodrugs using small-molecule drugs represent a promising alternative that can integrate multiple therapeutic agents within a single platform without additional toxicity or adverse effects associated with conventional carriers. Oleanolic acid (OA), a naturally pentacyclic triterpene found in plants, possesses self-assembly and gelling properties and antitumor effects for the development of carrier-free nanodrugs. Moreover, OA suppressed TGF-β1/SMAD signaling by binding to TβRI, inactivating the CAFs and remodeling the ECM. Thus, we fabricated rigid NPs (OA-NP) and flexible nanogels (OA-NG) to elucidate the effects of elasticity on antitumor effects and ECM reconstruction [161]. Among the different OA formulations, OA-NG strongly inhibited the expression of α-SMA, contributing to prolonged blood circulation and deep penetration of flexible OA-NG. Our group further developed a small-molecule nanodrug based on OA nanogels incorporating the sonosensitizer rose bengal (RB) and the photothermal agent methylene blue (MB) for the inactivation of CAFs via TGF-β blockade and PTT/SDT-assisted immunotherapy [162]. Self-assembled ORM nanogels exhibit three-pronged deep penetration into the TME, in which they cooperate synergistically with SDT or PTT to suppress TGF-β1/SMAD signaling and inactivate CAFs.
The excessive accumulation of TGF-β and lactic acid (LA) is strongly correlated with the immunosuppressive TME. Zhao et al. constructed self-assembled carrier-free bioregulators (TerBio) that incorporate the photosensitizer Ce6, SB505124 and the LA inhibitor lonidamine for immunosuppressive TME remodeling by blocking the activity of TGF-β and LA (Figure 7C) [163]. With a high drug entrapment efficiency, carrier-free TerBio exhibits increased tumor accumulation and deep penetration. The combination of the ICD effect induced by PDT and the immunoactivating TME synergistically amplifies the efficacy of immunotherapy against colorectal cancer. Compared with the use of inert carrier materials, the use of carrier-free nanodrugs with simple compositions through facile preparation can overcome biosafety challenges, such as difficulties in carrier degradation and metabolic clearance.
4.2.6 Cell membrane-coated biomimetic NPs
Cell membrane-coated NPs have risen as a burgeoning nanocarriers (Table 5) because of their improved biocompatibility, lower immunogenicity and precise tumor-targeting capabilities. Characterized by a synthetic or self-assembled nanoparticulate core encapsulated within a naturally derived cell membrane coating, cell membrane-coated NPs can elicit diverse desirable biological effects due to the inherent, multifunctional properties inherited from their source cells.
Immune cell membrane-coated NPs are constructed with the ability to identify antigens for precise targeting, and these NPs have various functions, which are derived mainly from the intrinsic function and surface proteins of immune cells. Building on this principle, Kim et al. developed an innovative therapeutic strategy involving macrophage membrane-coated NPs (Mφ-SDN) loaded with SD-208 [174]. These NPs selectively targeted TAMs, metastasis-associated macrophages (MAMs) and cancer cells, effectively suppressing tumor metastasis and reprogramming the immunosuppressive TME by blocking M2-type macrophage differentiation. Wang et al. fused the macrophage membrane and conjugates of anti-PDL1 and PEGylated phospholipids to coat gold nanocages and loaded LY2157299 into the core, which was developed to form a nanocomposite (GNC-Gal@CMaP) [175]. In response to contributions from the cell membrane and anti-PDL1 modification, GNC-Gal@CMaP exhibited promising tumor-specific targeting ability and could avoid elimination by the reticuloendothelial system. The suppression of TGF-β not only reversed the immunosuppressive TME but also suppressed TGFβ-mediated thermoresistance by downregulating the expression of heat shock proteins 70 and 90 in connection with the thermoresistance of tumor cells. These nanocomposites facilitate low-temperature PTT-induced ICD, promoting the activation of antigen-presenting cells and the subsequent recognition of tumor-specific effector T cells, thereby potently suppressing both primary and metastatic tumor progression through the dual suppression of PD-1/PD-L1 and TGF-β.
Biomimetic Fe3O4 magnetic nanoclusters coated with anti-PD-1 antibody-conjugated leukocyte membranes were constructed for tumor-targeted delivery of the SB-505124 hydrochloride with long circulation (Figure 8A) [176]. Ferroptosis induced by Fe ions and intratumoral H2O2 boosts the immunogenicity, and the combination of ICIs and TGF-βi results in a cyclical immune pattern [176]. Using different tumor models of breast cancer and melanoma, this combination strategy of immunomodulation and ferroptosis was shown to have superior therapeutic performance without tumor recurrence or metastasis.
The application of tumor antigen-primed DC membranes to coat NPs ensures that targeted delivery contributes to the individual capacity of DCs to trigger antitumoral T cell responses. Kim et al. designed lipid biomimetic NPs (T-DCNP) by integrating autologous neoantigen-primed DC membranes with liposomes loaded with SB525334 within their lipid bilayers using a coextrusion strategy [177]. The encapsulation of TGF-βi in the lipid bilayer protected the inhibitor from degradation, with an outstanding ability to reverse the immunosuppressive TME. The tumor-derived antigen-primed DC cell membrane endows T-DCNP with patient-personalized antitumor immune responses for postsurgical immunotherapy, enabling targeted attachment to T cells by effective fusion and native targeting. T-DCNP reshaped the TME with increased infiltration of CD8+ T cells and dual suppression of PD-1 and TGF-β, resulting in significant suppression of the growth of primary and distant tumors and protection against tumor rechallenge.
Summary of biomimetic NPs, immune cell therapy and protein-based NPs targeting TGF-β for cancer treatment.
| Characteristic | TGF-β pathway antagonists | Nanoparticle/carriers | Cancer cell type | Model | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Cell membrane coated biomimetic NPs | SD-208 | Macrophage membrane-coated NPs loaded with SD-208 | 4T1 | Female BALB/c mice | Macrophage membrane-coated NPs effectively suppress tumor metastasis and reprogramed the immunosuppressive TME by blocking M2-type macrophage differentiation. | [174] |
| LY2157299 | Macrophage membrane and conjugates of anti-PDL1 and PEGylated phospholipids as shell, gold nanocages loading LY2157299 as core | CT26 | BALB/c mice | Nanocomposites exert a promising suppression on both primary and metastatic tumor progression by dual suppression of PD-1/PD-L1 and TGF-β. | [175] | |
| SB-505124 hydrochloride | Fe3O4 nanoclusters coated with anti-PD-1 antibody conjugated leukocyte membrane for delivery of SB-505124 hydrochloride | 4T1, B16F10 | Female BALB/c mice, C57BL/6 mice | Combination strategy of immunomodulation and ferroptosis shows superior therapeutic performance. | [176] | |
| SB525334 | Autologous neoantigens-primed dendritic cell membranes with liposomes for delivery of SB525334 | B16-OVA | C57BL/6 mice | The dual blockade of PD-1 and TGF-β by bionic NPs reshapes the TME and enhances CD8+ T cell infiltration. | [177] | |
| T cell membranes with high expression of TβR | T cell membrane coated CaO2 NPs | B16-F10 | Female C57BL/6 | The T cell-mimicking Ca2+ nanogenerator provides diverse benefits for cytotoxic lymphocyte cell-mediated chemoimmunotherapy. | [178] | |
| LY2157299 | Cancer cell membrane- coated trimetallic nanozyme | LA795 | Male BALB/c mice | Combination of polarization of macrophages by TGF-βi and nanozyme achieves superior therapeutic effects under laser irradiation. | [179] | |
| SB525334 | S. aureus membrane-coated nanosystem with a core of lanthanidedoped scintillators and a hollow macroporous silica shell to entrap SB525334 and Roussin's black salt | CT 26, B16F10, B16F10-OVA | Female BALB/c mice, C57BL/6 mice | The generation of ONOO- works synergistically with GSH-responsive released SB525334, inducing tumor-associated neutrophils polarization toward the anti-tumor N1 phenotype. | [180] | |
| Immune cell therapy | LY2157299 | HES-PCL NPs loaded with LY2157299 and ICG | Raji | BALB/c female mice | The synergistic combination of the nanosystem and CAR-T cells demonstrates higher antitumor activity than CAR-T cell monotherapy. | [181] |
| SB505124 | Nanoemulsion loaded with SB505124 and selenocysteine | MDA-MB-231 | Female nude mice | The pretreatment of nanoemulsion enhances the killing potency and accumulation of NKs for enhanced NK cell adaptive therapy. | [182] | |
| LY2157299 | Chemically prime NK cells by LY2157299- loaded polymeric nanogels | PC-3 | BALB/c nude mice | Reversal of immunosuppressive TME through NK cell-priming activity and blockade of TGF-β performs effective anti-tumor response. | [183] | |
| SB525334 | Anti-Thy1.1 targeted PEGylated liposomes, anti-CD45 targeted PEGylated liposomes | B16F10 | C57BL/6 mice | Two liposomes with two different receptors were developed to deliver SB525334 to T cells in adoptive cell therapy. | [184] | |
| Protein-based NPs | - | Unfolded HAS coated perfluorotributylamine NPs | CT26 | BALB/c mice | The unfolded HSA-coated NPs inhibit the platelet-derived TGF-β, exerting inhibitory effects on tumor metastasis and NK cells immune surveillance. | [185] |
| Tamoxifen | Heptamethine cyanine IR68-tamoxifen conjugate coated albumin | 4T1 | Female BALB/c mice | Tumor-targeting NPs boost radiotherapy by reversing tumor hypoxia as well as by inhibiting PD-L1 and TGF-β. | [187] | |
| Tamoxifen | Albumin coated heptamethine cyanine MHI-tamoxifen conjugate | 4T1 | Female BALB/c mice | Mitochondrial targeted nanosystem through dual inhibition of PD-L1and TGF-β reverses the resistance of photodynamic immunotherapy. | [188] | |
| LY2157299 | Albumin modified by brain-targeting peptide for delivery of LY2157299 and celastrol | GL261 | - | The biomimetic NPs achieve efficient anti-tumor effects against glioma through suppressing the proportion of M2 TAMs and TGF-β1. | [189] |
PEG: poly(ethylene glycol); PD-1: programmed cell death protein 1; PD-L1: programmed cell death ligand 1; ONOO-: peroxynitrite; HES: hydroxyethyl starch; PCL: poly(ε-caprolactone); ICG: indocyanine green; CAR-T: chimeric antigen receptor T cell; TME: tumor microenvironment; NK cells: natural killer cell; HAS: human serum albumin.
Studies of biomimetic NPs, immune cell therapy and protein-based NPs targeting TGF-β for cancer treatment. (A) Schematic illustration of the biomimetic magnetosome for ferroptosis/immunomodulation-mediated tumor treatment. Adapted with permission from [176]. Copyright 2019, American Chemical Society. (B) Schematic illustration of proposed signaling pathway triggered by SSB NMs for NK cell-based cancer immunotherapy. Adapted with permission from [182]. Copyright 2020, American Chemical Society. (C) Schematic illustration of the IR-TAM@Alb nanosystem for enhancing radioimmunotherapy to prevent fibrosis development induced by dual TGF-β/PD-L1 blockade. Adapted with permission from [187]. Copyright 2024, John Wiley and Sons.
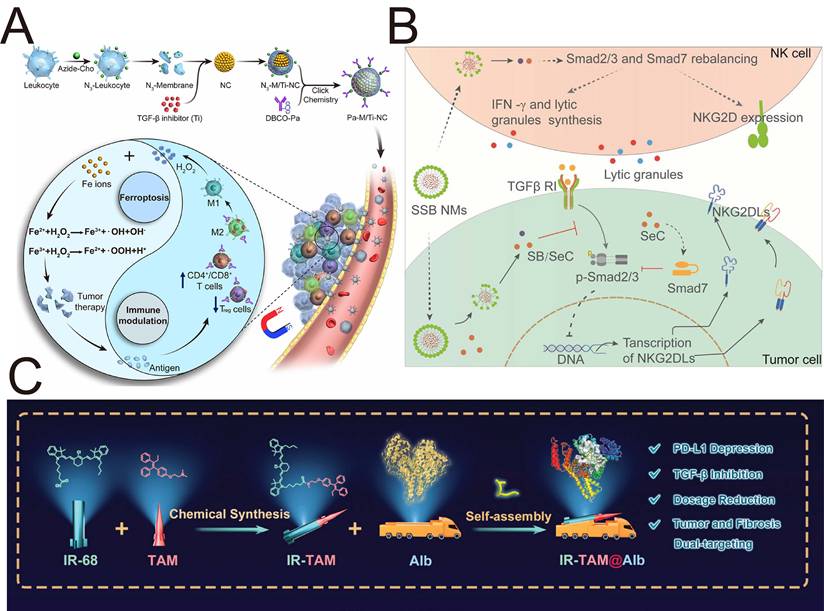
In contrast to the strategies for delivering TGF-βi in the aforementioned studies, a subsequent study by An et al. utilized T cell membranes with high expression of TβR and PD-1 receptors to exhaust TGF-β and block PD-L1 within tumor cells [178]. After being coated with the EL4 T cell membrane, a nanogenerator (CaNP@ECM) based on CaO2 NPs efficiently accumulated at the tumor site through the binding of the adhesion protein LFA-1 expressed on the EL4 cell membrane to inflamed endothelial cells in the TME. This T cell-mimicking Ca2+ nanogenerator provides diverse benefits for cytotoxic lymphocyte cell-mediated chemoimmunotherapy through the simultaneous induction of ICD effects, inhibition of tumor cell glycolysis and reversal of the immunosuppressive TME.
Recent advancements have demonstrated that the tumor cell homing mechanism offers a targeted delivery approach for NPs functionalized with tumor cell membranes. Song et al. developed a tumor cell membrane-coated trimetallic nanozyme (LY-Au@Pt@Rh-CM) composed of three metals (Au, Pt, and Rh) to deliver LY2157299 within the large pore space for photothermal/immunomodulation-boosted catalytic therapy. Owing to the homotypic targeting property of the cancer cell membrane, the nanocomposites achieved the targeted delivery of LY2157299 to tumors without premature leakage. The combination of the polarization of macrophages towards the M1 phenotype by TGF-βi and nanocomposites with dual peroxidase- and catalase-like activities achieved superior therapeutic effects upon laser irradiation [179].
For efficient treatment of colorectal cancer, efficient delivery of therapeutic agents to the intestinal tract is important. Decorating NPs with bacterial membranes helps them cross the intestinal mucus barrier with deep penetration, prolonged retention and controlled release. Li et al. fabricated a S. aureus membrane-coated nanosystem (LNSM-RS) with a core of X-ray-excited lanthanide-doped scintillators and a hollow macroporous silica shell to entrap SB525334 and NO precursors—Roussin's black salt [180]. By precisely targeting expressed Protein A in the TME of colorectal cancer through the S. aureus membrane coating method, LNSM-RS exhibited targeted accumulation and retention within the tumor site in an orthotopic colorectal tumor model, whereas the fluorescence signal of LNS-RS without the S. aureus membrane coating could not be detected during in vivo imaging. The generation of hypertoxic peroxynitrite (ONOO-) by lanthanide-doped scintillators upon X-ray irradiation works synergistically with GSH-responsive released SB525334, inducing tumor-associated neutrophil polarization towards the antitumor N1 phenotype for radiotherapy-based synergistic therapy.
Cell membrane-coated biomimetic NPs with superior biocompatibility and prolonged circulation represent a promising strategy for specifically delivering TGF-βi to tumors. The lipid bilayer also allows for the flexible codelivery of other therapeutic agents. However, this approach faces considerable challenges related to the complexity of manufacturing, including complex isolation and fusion processes, poorly reproducible production, and variability in encapsulation efficiency. Despite these drawbacks, the ability to precisely deliver these critical signaling inhibitors while minimizing systemic toxicity makes cell membrane-coated biomimetic NPs highly attractive platforms in advanced nanomedicine.
4.2.7 Immune cell therapy
Immune cell therapy has risen as a revolutionary strategy against tumors, utilizing the host immune system to defeat cancer cells. In contrast to conventional chemotherapy and radiotherapy, immunotherapy performed superior antitumor treatment with reduced side effects and long-term protection from recurrence. Among the most promising strategies are CAR-T cell therapy, NK cell therapy, and tumor-infiltrating lymphocyte (TIL) therapy. These approaches have shown remarkable efficacy in hematologic malignancies and are being actively explored for solid tumors.
CAR-T cell therapies have garnered enormous attention for treatment of solid tumors; however, the immunosuppressive TME derived from TGF-β and other modulators has limited the therapeutic effects of CAR-T cell therapies. PTT has risen as a minimally invasive strategy for tumor treatment, utilizing the photothermal conversion capabilities of NPs to generate localized hyperthermia upon laser irradiation, improve the tumor stromal barrier, and activate tumor immunity. Tang et al. developed an amphipathic nanodelivery system (HES-PCL NPs) to codeliver the LY2157299 (LY) and the photothermal agent indocyanine green (ICG) for the treatment of lymphoma [181]. The photothermal response of LY/ICG@HESPCL NPs accompanied by the degradation of ICG is beneficial for the responsive release of LY in vivo. TGF-β blockade combined with PTT reverses the TGF-β-mediated suppression of CAR-T cell trafficking by upregulating the expression of CXCL9/10/11 and its cognate receptor CXCR3 while simultaneously mitigating T cell exhaustion and promoting effector memory T cell differentiation [181]. Compared with single CAR-T cells, synergistic therapy with these nanosystems and CAR-T cells resulted in 2.4-fold greater antitumor effects and 2.7-fold greater relapse suppression rates during the treatment period. This study revealed that the combination of TGF-β blockade with PTT enhances the long-term antitumor effect of CAR-T cells.
NK cell adoptive therapy stands as a prospective strategy to circumvent the limitations of traditional therapies; nevertheless, the antitumor effect of NK cells is significantly impacted by the immunosuppressive TGF-β. Liu et al. developed a nanoemulsion system (SSB NMs) via convenient emulsification to encapsulate SB505124 (SB) and selenocysteine (SeC), combining TGF-β blockade and adoptive NK cell-based immunotherapy for TNBC treatment (Figure 8B) [182]. Benefiting from the nanoemulsion, the poor stability and inferior solubility of SB and SeC were attenuated, with good stability for 4 weeks. When SSB NMs were combined with adoptive NK92 cell-based immunotherapy, SSB NMs increased the killing potency and intratumor infiltration of NKs, resulting in the upregulation of NKG2DL and the inhibition of TGF-β/SMAD2/3 signaling, highlighting the application and mechanism of action of NK cells in adaptive therapy for TNBC treatment.
While the above study combines NPs and NK cells to achieve TGF-β blockade and adoptive cell therapy, subsequent work utilizes polymeric NPs encapsulating TGF-βi to chemically reprogram NK cells for adoptive cell therapy. Choi et al. [183] developed polymeric nanogels encapsulating galunisertib to chemically prime NK cells, termed Nano-Chem_NK, leading to the reversion of the immunosuppressive TME for efficient treatment of TGF-β-secreting cancers. This strategy utilized hydrophilic negatively charged HA to encapsulate hydrophobic galunisertib through self-assembly, whose surface was coated with positively charged 25K PEI. These nanogels reverse the suppressive TME by chemically priming NK cells and blocking TGF-β activity.
Screening competent targeting agents for the precise delivery of TGF-βi to transferred T cells may reveal differences in their internalization and therapeutic efficacy. Zheng et al. developed two liposomes, anti-Thy1.1-Lip and anti-CD45-Lip, with two different receptors to deliver the TβR-Ⅰ inhibitor SB525334 to T cells for adoptive cell therapy [184]. Compared with internalized receptor (CD90 or Thy1)-targeted liposomes, T cells preloaded with liposomes in vitro targeting noninternalizing receptor (CD45) induced higher granzyme expression in T cells and greater donor T cell infiltration of B16F10 melanoma tumors [184]. In contrast, when these two different ligands, Thy1 and CD45, were used to direct liposomal TGF-β to lymphocytes, anti-Thy1.1 liposomes enhanced the antitumor effect [184]. These findings potentially underscore the premier donor cell specificity exhibited by Thy1 than by CD45, highlighting the critical necessity of targeting TGF-β specifically to effector T cells rather than focusing on other immune cell populations.
The combination of immune cell therapy with a TGF-β blockade strategy represents an emerging and promising direction for cancer treatment. TGF-β signaling within the TME promotes immunosuppression, contributing to therapeutic resistance and tumor progression. By codelivering TGF-βi alongside adoptive cell therapies, this synergistic approach can potentially reverse immunosuppression, improve T cell activity, and increase tumor infiltration. DDSs offer a powerful platform for this combinatorial strategy, enabling the targeted and controlled release of both therapeutic cells and inhibitors. This integrated nanotechnology-based approach holds significant potential to advance the clinical translation of potent and durable cancer immunotherapies.
4.2.8 Protein-based delivery systems
Protein-based drug carriers, such as albumin, ferritin, legumin and gelatin, have risen as prospective platforms for drug delivery. Protein-based platforms can protect loaded drugs from enzymatic degradation and renal clearance. Albumin, a highly soluble protein, possesses a three-dimensional architecture comprising both hydrophilic and hydrophobic domains. Luo et al. utilized dithiothreitol (DTT) to cut the disulfide bonds of human serum albumin (HSA), and the obtained unfolded HSA was self-assembled with hydrophobic perfluorotributylamine (PFTBA) to develop NPs (PFTBA@HSA) [185]. This unfolded HSA developed using the “unfolding and self-assembly” strategy has a preeminent platelet affinity and delivery capacity, but native HAS does not. Moreover, a dose-dependent elevation in platelet affinity was detected as the degree of unfolding of HSA progressively increased. Compared with other cells, platelets are crucial but devalued carriers of 40-100 times more TGF-β and serve as the dominant source of circulating TGF-β upon exposure to the TME [186]. Engineered HSA-coated PFTBA NPs were meticulously designed to exhibit optimal platelet-targeting capabilities, with robust inhibitory effects on platelet TGF-β-mediated tumor metastasis and NK cell immune surveillance.
Radiotherapy has a limited ability to modulate exogenous hypoxia and the immunosuppressive TME; moreover, the increased expression of PD-L1 and TGF-β after radiotherapy further weakens its antitumor efficacy. Treatment with tamoxifen, an antioestrogen drug designed for estrogen receptor-positive breast cancer, can interfere with mitochondrial metabolism regardless of estrogen receptor status and induce the downregulation of both TGF-β and PD-L1. Thus, Zhou et al. conducted a mitochondrial-targeted nanosystem (IR-TAM@Alb) by utilizing albumin to deliver tamoxifen-IR68 (heptamethine cyanine dyes) conjugate for efficient radiotherapy to prevent fibrosis development (Figure 8C) [187]. This mitochondrion-targeted heptamethine cyanine increase the efficacy of radiotherapy via synergistic integration of dual TGF-β/PD-L1 blockade. Owing to its ability to encapsulate albumin and target mitochondria, this targeted nanosystem significantly suppressed radiation-induced fibroblast activation by blocking TGF-β, whereas the nanosystem without mitochondria-targeting dyes failed to attenuate radiation-induced fibrosis at the same dose.
Protein-based carriers possess superior biocompatibility and low immunogenicity, making them highly appropriate for delivering TGF-β pathway antagonists. Their inherent biodegradability and natural origin minimize adverse immune reactions, which is a significant advantage for sustained therapeutic applications. Furthermore, the functional groups on proteins allow for precise chemical modification, enabling the targeted, controlled release of TGF-β pathway antagonists in specific tissues to reduce systemic side effects. However, structural instability under certain physiological conditions and the risk of enzymatic degradation before the target site is reached remain unknown. Despite these limitations, the versatility and safety profile of protein-based carriers position them as promising vehicles for the optimized delivery of TGF-β therapeutic agent.
5. Challenges and opportunities
Currently, research on TGF-β-targeting NPs predominantly focuses on the tumor-promoting role of TGF-β, the design of multifunctional NPs to block TGF-β for tumor treatment or the examination of how inorganic NPs may exert protumor effects through the TGF-β signaling pathway. Nevertheless, studies addressing the tumor-suppressive function of TGF-β, particularly in the context of NP-based interventions, are scarce. This one-sided approach limits the potential for precise modulation of TGF-β, which remains an underexplored challenge in nanomedicine because of its dual role in different stages of tumors.
Several inorganic NPs, such as nano-TiO2, nano-SiO₂, nano-HA, nano NiO, Fe3O4 magnetic NPs, GO nanosheets, AgNPs, and AlO NPs, have been shown to induce prometastatic effects and side effects through the TGF-β signaling pathway. These NPs induce an aggressive tumor phenotype through EMT and an elongated fibroblast-like morphology with high expression of EMT and profibrotic markers, such as HIF-1α, TGF-β, α-SMA, E-cadherin and N-cadherin. A proactive design approach focusing on the intrinsic properties of the material and surface engineering is essential for mitigating the risk of TGF-β/EMT activation. The hydrophilic PEG layer minimizes protein absorption, preventing the formation of a protein corona that can trigger downstream TGF-β signaling. Coating INPs with cell membranes from red blood cells or platelets can significantly reduce immune recognition and subsequent profibrotic signaling cascades. Standardized in vivo detection methods for early prometastatic signals are crucial for evaluating the safety of inorganic and hybrid NPs. Immunohistochemical staining of primary tumor sections for EMT and pro-fibrotic markers should be executed to distinguish the aggressive phenotype of tumors. Periodically collected blood samples can be analyzed for ctDNA carrying tumor-specific mutations or for the presence of circulating tumor cells that express mesenchymal markers. An increase in the levels of these circulating biomarkers is a strong early indicator of increased metastatic activity.
To date, only one phase I clinical study has investigated the efficacy and biosafety of a polypeptide NP-encapsulated siRNA, STP705, targeting TGF-β1 and COX-2 by subcutaneous administration for localized fat reduction [190], but no clinical trials have been conducted on NPs targeting TGF-β for malignant tumors. Precise modulation of TGF-β's dual functions during tumor progression will boost clinical research and the translation of TGF-β-targeted NPs.
Notably, TGF-β signaling is not uniformly active throughout tumor tissue but displays significant regional variation. Hypoxic cores often exhibit upregulated expression of TGF-β, which induces EMT and stemness. In contrast, invasive tumor cells utilize high TGF-β levels to drive metastasis and immune suppression, whereas perivascular regions may use this pathway for angiogenic signaling. This spatial gradient critically remodels the TME: it recruits and polarizes immunosuppressive cells to specific niches, simultaneously driving the deposition and cross-linking of ECM components by CAFs, leading to heterogeneous stromal stiffness. This physical and biochemical heterogeneity creates a biological barrier, impairing the deep penetration of therapeutics and NPs. Consequently, this landscape highlights a pivotal future direction for the development of intelligent nanodelivery systems that respond to localized TGF-β dynamics caused by hypoxia, enzymes or other stimuli associated with these specific niches.
Additionly, the clinical translation of TGF-β-targeting NPs faces multiple challenges, including unstandardized preclinical assays, manufacturing complexities, regulatory hurdles, and safety concerns. Future research must address three critical challenges and translational bottlenecks to drive the clinical translation of nanomedicine. First, the safety of the material and clear standards for material biodegradation must be prioritized. These standards involve defining acceptable degradation timelines and systematically characterizing and quantifying degradation products, ensuring that they are nontoxic and efficiently cleared. The degradation timelines and systematic characterization and quantification of degradation components should be studied to ensure the biosafety of the material. Currently, only a few studies have systematically investigated the organ-specific toxicity of nanomaterials, and more research should focus on the toxic side effects of carrier materials that promote tumor growth or damage major organs and whether the toxic side effects are caused by the TGF-β-mediated pathway. During the clinical translation of inorganic or hybrid NPs targeting TGF-β, special attention must be given to the potential of these NPs to promote tumor progression via the TGF-β pathway.
Furthermore, preclinical protocols must be rigorously standardized. Longitudinal biodistribution studies should quantify the accumulation of NPs in both tumors and healthy organs over extended periods using established imaging modalities. Comprehensive organ-specific toxicity assessments are crucial for evaluating the expression of immune and fibrotic markers using histopathology. Critically, a dedicated screening platform using diverse in vivo models must be implemented to proactively evaluate the risk of TGF-β inhibition potentially enhancing tumor progression in certain contexts.
Finally, robust and validated manufacturing processes are nonnegotiable for investigational new drug (IND) applications. Numerous novel nanoformulations fail to enter clinical trials because complex, laboratory-scale synthesis cannot be scaled up with sufficient reproducibility, purity, or yield for clinical batches. Therefore, the investigation of scalable, reproducible synthesis methods for the development of multifunctional NPs is crucial for IND applications. Rigorous control of critical quality attributes (CQAs), such as the particle size, polydispersity index, drug loading efficiency, and surface functionalization, is pivotal for advancing the application of TGF-β-targeting nanomedicines in the clinic.
6. Conclusions
It is indispensable to clarify the dual functions of TGF-β in tumors and to utilize nanotechnology for precise delivery for improving both the treatment effectiveness and biosafety of TGF-β therapeutic agent. The targeted and stimuli-responsive NPs improve the treatment effectiveness of TGF-β pathway antagonists while enabling their precise controlled release, which promotes deep penetration of the NPs through the modulation of the TME. This nanoplatform also serves as a robust tool for the combined and sequential administration of TGF-β pathway antagonists with other therapeutic agent, such as chemotherapeutics, phototheranostic agents, ICIs and immune cell therapies. These advances are expected to facilitate the initiation of clinical trials and provide novel strategies for efficient cancer treatment. Notably, inorganic NPs play dual roles in regulating the TGF signaling pathway, as they may suppress or facilitate tumor progression. Attention must be given to the potential adverse effects and biosafety of nanomaterials to accelerate the clinical translation of NPs and TGF-β pathway antagonists.
Acknowledgements
This work was supported by the Open Research Fund of the Key Laboratory of the Ministry of Education for Advanced Catalysis Materials and Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces (KLMEACM202511), Zhejiang Normal University.
Author contributions
Ziying Li: Literature collection, Writing - original draft & editing. Xugang Ji: Formal analysis. Xiaoyi Cong: Data curation. Yu Gao: Conceptualization, Validation. Jianqing Gao: Project administration, Writing -review & editing.
Competing interests
The authors have declared that no competing interest exists.
References
1. Shi X, Yang J, Deng S, Xu H, Wu D, Zeng Q. et al. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J Hematol Oncol. 2022;15:135
2. Huang JJ, Blobe GC. Dichotomous roles of TGF-β in human cancer. Biochem Soc Trans. 2016;44:1441-54
3. Zhou Q, Pan Y, Yang X, Zhao Y, Han G, Pang Q. et al. Neoadjuvant SHR-1701 with or without chemotherapy in unresectable stage III non-small-cell lung cancer: A proof-of-concept, phase 2 trial. Cancer Cell. 2024;42:1258-67
4. Malek E, Rana PS, Swamydas M, Daunov M, Miyagi M, Murphy E. et al. The TGFβ type I receptor kinase inhibitor vactosertib in combination with pomalidomide in relapsed/refractory multiple myeloma: a phase 1b trial. Nat Commun. 2024;15:7388
5. Qiang L, Hoffman MT, Ali LR, Castillo JI, Kageler L, Temesgen A. et al. Transforming Growth Factor-β Blockade in Pancreatic Cancer Enhances Sensitivity to Combination Chemotherapy. Gastroenterology. 2023;165:874-90
6. Liu D, Zhou J, Wang Y, Li M, Jiang H, Liu Y. et al. Bifunctional anti-PD-L1/TGF-βRII agent SHR-1701 in advanced solid tumors: a dose-escalation, dose-expansion, and clinical-expansion phase 1 trial. BMC Med. 2022;20:408
7. Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. 2022;28:724-34
8. Colak S, Ten Dijke P. Targeting TGF-β Signaling in Cancer. Trends in cancer. 2017;3:56-71
9. Raju GSR, Pavitra E, Varaprasad GL, Bandaru SS, Nagaraju GP, Farran B. et al. Nanoparticles mediated tumor microenvironment modulation: current advances and applications. J Nanobiotechnology. 2022;20:274
10. Gupta G, Chellappan DK, Singh SK, Gupta PK, Kesari KK, Jha NK. et al. Advanced drug delivery approaches in managing TGF-β-mediated remodeling in lung diseases. Nanomedicine (Lond). 2021;111:2243-7
11. Jiang J, Zhang Y, Peng K, Wang Q, Hong X, Li H. et al. Combined delivery of a TGF-β inhibitor and an adenoviral vector expressing interleukin-12 potentiates cancer immunotherapy. Acta Biomater. 2017;333:114-23
12. Meshanni JA, Stevenson ER, Zhang D, Sun R, Ona NA, Reagan EK. et al. Targeted delivery of TGF-β mRNA to murine lung parenchyma using one-component ionizable amphiphilic Janus Dendrimers. Nat Commun. 2025;222:1806
13. Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104
14. Katsuno Y, Derynck R. Epithelial plasticity, epithelial-mesenchymal transition, and the TGF-β family. Developmental Cell. 2021;56:726-46
15. Luo K. Signaling Cross Talk between TGF-β/Smad and Other Signaling Pathways. Cold Spring Harb Perspect Biol. 2017;9:a022137
16. Crawford AW, de Bruin HJ. Concentration changes in surface Ca, P, F, Zn, Fe, and Sr during white spot formation. J Dent Res. 1983;62:964-8
17. Belu AM, Davies MC, Newton JM, Patel N. TOF-SIMS characterization and imaging of controlled-release drug delivery systems. Anal Chem. 2000;72:5625-38
18. Tzavlaki K, Moustakas A. TGF-β Signaling. Biomolecules. 2020;10:487
19. Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun. 2018;9:4692
20. Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp Cell Res. 2003;25:90-100
21. Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S. et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257-72
22. Cao SJ, Hong L, Li XQ. Mechanistic studies on the role of TGF-beta1 in angiogenesis through EndMT. Vascular. 2020;29:442-450
23. Ventura E, Weller M, Macnair W, Eschbach K, Beisel C, Cordazzo C. et al. TGF-β induces oncofetal fibronectin that, in turn, modulates TGF-β superfamily signaling in endothelial cells. J Cell Sci. 2018;131:jcs209619
24. Zonneville J, Safina A, Truskinovsky AM, Arteaga CL, Bakin AV. TGF-beta signaling promotes tumor vasculature by enhancing the pericyte-endothelium association. BMC Cancer. 2018;18:670
25. Meadows SK, Eriksson M, Barber A, Sentman CL. Human NK cell IFN-gamma production is regulated by endogenous TGF-beta. Int Immunopharmacol. 2006;30:1020-8
26. Chen W. TGF-β Regulation of T Cells. Annu Rev Immunol. 2023;31:483-512
27. Pomié C, Ménager-Marcq I, van Meerwijk JP. Murine CD8+ regulatory T lymphocytes: the new era. Hum Immunol. 2008;32:708-14
28. Zhong M, Zhong C, Cui W, Wang G, Zheng G, Li L. et al. Induction of tolerogenic dendritic cells by activated TGF-β/Akt/Smad2 signaling in RIG-I-deficient stemness-high human liver cancer cells. BMC Cancer. 2019;33:439
29. Batlle E, Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity. 2019;50:924-40
30. Tauriello DVF, Sancho E, Batlle E. Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22:25-44
31. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-96
32. Du D, Katsuno Y, Meyer D, Budi EH, Chen SH, Koeppen H. et al. Smad3-mediated recruitment of the methyltransferase SETDB1/ESET controls Snail1 expression and epithelial-mesenchymal transition. EMBO Rep. 2018;34:135-55
33. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156-72
34. Wang X, Thiery JP. Harnessing Carcinoma Cell Plasticity Mediated by TGF-β Signaling. Cancers (Basel). 2021;13:3397
35. Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S. et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell. 2017;43:1611-24
36. García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E. et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37-42
37. Du D, Katsuno Y, Meyer D, Budi EH, Chen SH, Koeppen H. et al. Smad3-mediated recruitment of the methyltransferase SETDB1/ESET controls Snail1 expression and epithelial-mesenchymal transition. Embo Rep. 2017;19:135-55
38. Wang X, Eichhorn PJA, Thiery JP. TGF-β, EMT, and resistance to anti-cancer treatment. Semin Cancer Biol. 2023;97:1-11
39. Yao CD, Haensel D, Gaddam S, Patel T, Atwood SX, Sarin KY. et al. AP-1 and TGFß cooperativity drives non-canonical Hedgehog signaling in resistant basal cell carcinoma. Nat Commun. 2020;11:5079
40. Zhao Z, Hao D, Wang L, Li J, Meng Y, Li P. et al. CtBP promotes metastasis of breast cancer through repressing cholesterol and activating TGF-β signaling. Oncogene. 2018;38:2076-91
41. Lee JH, Sánchez-Rivera FJ, He L, Basnet H, Chen FX, Spina E. et al. TGF-β and RAS jointly unmask primed enhancers to drive metastasis. Cell. 2024;187:6182-99
42. Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED. et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374-83
43. Deng Z, Fan T, Xiao C, Tian H, Zheng Y, Li C. et al. TGF-β signaling in health, disease, and therapeutics. Signal Transduct Target Ther. 2024;9:61
44. Yi M, Wu Y, Niu M, Zhu S, Zhang J, Yan Y. et al. Anti-TGF-β/PD-L1 bispecific antibody promotes T cell infiltration and exhibits enhanced antitumor activity in triple-negative breast cancer. J Immuno Ther Cancer. 2022;10:e005543
45. Hinz B. More Velcro for the TGF-β1 straitjacket: A new antibody straps latent TGF-β1 to the matrix. Sci Signal. 2024;17:eado5279
46. Lei Z, Tang R, Wu Y, Mao C, Xue W, Shen J. et al. TGF-β1 induces PD-1 expression in macrophages through SMAD3/STAT3 cooperative signaling in chronic inflammation. JCI Insight. 2024;9:e165544
47. Teicher BA, Holden SA, Ara G, Chen G. Transforming growth factor-beta in in vivo resistance. Cancer Chemother Pharmacol. 1996;37:601-9
48. Tolcher AW, Berlin JD, Cosaert J, Kauh J, Chan E, Piha-Paul SA. et al. A phase 1 study of anti-TGFbeta receptor type-II monoclonal antibody LY3022859 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2017;79:673-80
49. Formenti SC, Lee P, Adams S, Goldberg JD, Li X, Xie MW. et al. Focal Irradiation and Systemic TGF-β Blockade in Metastatic Breast Cancer. Clin Cancer Res. 2018;24:2493-504
50. Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M. et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGF-β) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One. 2014;9:e90353
51. Lacouture ME, Morris JC, Lawrence DP, Tan AR, Olencki TE, Shapiro GI. et al. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor β by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol Immunother. 2015;64:437-46
52. Formenti SC, Hawtin RE, Dixit N, Evensen E, Lee P, Goldberg JD. et al. Baseline T cell dysfunction by single cell network profiling in metastatic breast cancer patients. J Immunother Cancer. 2019;7:177
53. Li T, Wang X, Niu M, Wang M, Zhou J, Wu K. et al. Bispecific antibody targeting TGF-β and PD-L1 for synergistic cancer immunotherapy. Front Immunol. 2023;14:1196970
54. Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G. et al. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7:829-40
55. Giannelli G, Santoro A, Kelley RK, Gane E, Paradis V, Cleverly A. et al. Biomarkers and overall survival in patients with advanced hepatocellular carcinoma treated with TGF-betaRI inhibitor galunisertib. PLoS One. 2020;15:e0222259
56. Sow HS, Ren J, Camps M, Ossendorp F, Ten Dijke P. Combined Inhibition of TGF-beta Signaling and the PD-L1 Immune Checkpoint Is Differentially Effective in Tumor Models. Cells. 2019;8:320
57. Matsuyama S, Iwadate M, Kondo M, Saitoh M, Hanyu A, Shimizu K. et al. SB-431542 and Gleevec inhibit transforming growth factor-beta-induced proliferation of human osteosarcoma cells. Cancer Res. 2003;63:7791-8
58. Tanaka H, Shinto O, Yashiro M, Yamazoe S, Iwauchi T, Muguruma K. et al. Transforming growth factor β signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol Rep. 2010;24:1637-43
59. Chatterjee S, Chatterjee A, Jana S, Dey S, Roy H, Das MK. et al. Transforming growth factor beta orchestrates PD-L1 enrichment in tumor-derived exosomes and mediates CD8 T-cell dysfunction regulating early phosphorylation of TCR signalome in breast cancer. Carcinogenesis. 2021;42:38-47
60. Hjelmeland MD, Hjelmeland AB, Sathornsumetee S, Reese ED, Herbstreith MH, Laping NJ. et al. SB-431542, a small molecule transforming growth factor-beta-receptor antagonist, inhibits human glioma cell line proliferation and motility. Mol Cancer Ther. 2004;3:737-45
61. Premkumar K, Shankar BS. TGF-βR inhibitor SB431542 restores immune suppression induced by regulatory B-T cell axis and decreases tumor burden in murine fibrosarcoma. Cancer Immunol Immunother. 2021;70:153-68
62. Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R. et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954-61
63. Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J. et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24:4721-30
64. Fakhrai H, Mantil JC, Liu L, Nicholson GL, Murphy-Satter CS, Ruppert J. et al. Phase I clinical trial of a TGF-beta antisense-modified tumor cell vaccine in patients with advanced glioma. Cancer Gene Ther. 2006;13:1052-60
65. Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A. et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007;17:201-12
66. Olivares J, Kumar P, Yu Y, Maples PB, Senzer N, Bedell C. et al. Phase I trial of TGF-beta 2 antisense GM-CSF gene-modified autologous tumor cell (TAG) vaccine. Clin Cancer Res. 2011;17:183-92
67. Yuan Y, Cao D, Zhang A, Liu Z, Deng Z, Zhang S. Targeted PLK1 suppression through RNA interference mediated by high-fidelity Cas13d mitigates osteosarcoma progression via TGF-β/Smad3 signaling. J Cell Mol Med. 2024;28:e18400
68. Miyagishi M, Taira K. U6 promoter-driven siRNAs with four uridine 3' overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol. 2002;20:497-500
69. Ali S, Rehman MU, Yatoo AM, Arafah A, Khan A, Rashid S. et al. TGF-β signaling pathway: Therapeutic targeting and potential for anti-cancer immunity. Eur J Pharmacol. 2023;947:175678
70. Kumar R, Grinberg AV, Li H, Kuo T-H, Sako D, Krishnan L. et al. Functionally diverse heteromeric traps for ligands of the transforming growth factor-β superfamily. Sci Rep. 2021;11:18341
71. Kim B-G, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol Oncol. 2021;14:55
72. Aykul S, Maust J, Thamilselvan V, Floer M, Martinez-Hackert E. Smad2/3 Activation Regulates Smad1/5/8 Signaling via a Negative Feedback Loop to Inhibit 3T3-L1 Adipogenesis. Int J Mol Sci. 2021;22:8472
73. Feld J, Navada SC, Silverman LR. Myelo-deception: Luspatercept & TGF-Beta ligand traps in myeloid diseases & anemia. Leuk Res. 2020;97:106430
74. Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH. et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Second-Line Treatment of Patients With NSCLC: Results From an Expansion Cohort of a Phase 1 Trial. J Thorac Oncol. 2020;15:1210-22
75. Tan B, Khattak A, Felip E, Kelly K, Rich P, Wang D. et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Patients with Esophageal Adenocarcinoma: Results from a Phase 1 Cohort. Target Oncol. 2021;16:435-46
76. Lin CC, Doi T, Muro K, Hou MM, Esaki T, Hara H. et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFβ and PD-L1, in Patients with Esophageal Squamous Cell Carcinoma: Results from a Phase 1 Cohort in Asia. Target Oncol. 2021;16:447-59
77. Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M, Kondo S. et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020;8:e000564
78. Doi T, Fujiwara Y, Koyama T, Ikeda M, Helwig C, Watanabe M. et al. Phase I Study of the Bifunctional Fusion Protein Bintrafusp Alfa in Asian Patients with Advanced Solid Tumors, Including a Hepatocellular Carcinoma Safety-Assessment Cohort. Oncologist. 2020;25:1292-302
79. Strauss J, Gatti-Mays ME, Cho BC, Hill A, Salas S, McClay E. et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J Immunother Cancer. 2020;8:e001395
80. Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10:5488
81. Redman JM, Friedman J, Robbins Y, Sievers C, Yang X, Lassoued W. et al. Enhanced neoepitope-specific immunity following neoadjuvant PD-L1 and TGF-β blockade in HPV-unrelated head and neck cancer. J Clin Invest. 2022;132:e161400
82. Senzer N, Barve M, Kuhn J, Melnyk A, Beitsch P, Lazar M. et al. Phase I trial of "bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell" vaccine (FANG) in advanced cancer. Mol Ther. 2012;20:679-86
83. Rocconi RP, Grosen EA, Ghamande SA, Chan JK, Barve MA, Oh J. et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Oncol. 2020;21:1661-72
84. Rocconi RP, Stanbery L, Tang M, Walter A, Monk BJ, Herzog TJ. et al. ENTPD1/CD39 as a predictive marker of treatment response to gemogenovatucel-T as maintenance therapy in newly diagnosed ovarian cancer. Commun Med. 2022;2:106
85. Barve M, Aaron P, Manning L, Bognar E, Wallraven G, Horvath S. et al. Pilot Study of Combination Gemogenovatucel-T (Vigil) and Durvalumab in Women With Relapsed BRCA-wt Triple-Negative Breast or Ovarian Cancer. Clin Med Insights Oncol. 2022;16:11795549221110501
86. Rocconi RP, Stanbery L, Madeira da Silva L, Barrington RA, Aaron P, Manning L. et al. Long-Term Follow-Up of Gemogenovatucel-T (Vigil) Survival and Molecular Signals of Immune Response in Recurrent Ovarian Cancer. Vaccines. 2021;9:894
87. Walter A, Rocconi RP, Monk BJ, Herzog TJ, Manning L, Bognar E. et al. Gemogenovatucel-T (Vigil) maintenance immunotherapy: 3-year survival benefit in homologous recombination proficient (HRP) ovarian cancer. Gynecol Oncol. 2021;163:459-64
88. Oh J, Barve M, Matthews CM, Koon EC, Heffernan TP, Fine B. et al. Phase II study of Vigil® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol Oncol. 2016;143:504-10
89. Elsherbiny NM, Al-Gayyar MM. Anti-tumor activity of arjunolic acid against Ehrlich Ascites Carcinoma cells in vivo and in vitro through blocking TGF-β type 1 receptor. Biomed Pharmacother. 2016;86:28-34
90. Lian GY, Wang QM, Mak TS, Huang XR, Yu XQ, Lan HY. Inhibition of tumor invasion and metastasis by targeting TGF-β-Smad-MMP2 pathway with Asiatic acid and Naringenin. Mol Ther Oncolytics. 2021;87:277-89
91. Chen JL, Tai YS, Tsai HY, Hsieh CY, Chen CL, Liu CJ. et al. Betulinic Acid Inhibits the Stemness of Gastric Cancer Cells by Regulating the GRP78-TGF-β1 Signaling Pathway and Macrophage Polarization. Molecules. 2023;88:1725
92. Jiang Z, Cao Q, Dai G, Wang J, Liu C, Lv L. et al. Celastrol inhibits colorectal cancer through TGF-β1/Smad signaling. Onco Targets Ther. 2019;89:509-18
93. Jiang H, Ma P, Duan Z, Liu Y, Shen S, Mi Y. et al. Ginsenoside Rh4 Suppresses Metastasis of Gastric Cancer via SIX1-Dependent TGF-β/Smad2/3 Signaling Pathway. Nutrients. 2022;91:1564
94. Sun M, Zhuang X, Lv G, Lin Z, Huang X, Zhao J. et al. Ginsenoside CK Inhibits TGF-β-Induced Epithelial-Mesenchymal Transition in A549 Cell via SIRT1. Biomed Res Int. 2021;90:9140191
95. Yin J, Wang L, Wang Y, Shen H, Wang X, Wu L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-β/Smad2/3 signaling pathway. Onco Targets Ther. 2019;12:3893-903
96. Jalilian E, Abolhasani-Zadeh F, Afgar A, Samoudi A, Zeinalynezhad H, Langroudi L. Neutralizing tumor-related inflammation and reprogramming of cancer-associated fibroblasts by Curcumin in breast cancer therapy. Sci Rep. 2023;13:20770
97. Wang D, Tian M, Fu Y, Sun Y, Ding L, Zhang X. et al. Halofuginone inhibits tumor migration and invasion by affecting cancer-associated fibroblasts in oral squamous cell carcinoma. Front Pharmacol. 2022;13:1056337
98. Juárez P, Mohammad KS, Yin JJ, Fournier PG, McKenna RC, Davis HW. et al. Halofuginone inhibits the establishment and progression of melanoma bone metastases. Cancer Res. 2012;72:6247-56
99. Han G, Wang Y, Liu T, Gao J, Duan F, Chen M. et al. Salvianolic acid B acts against non-small cell lung cancer A549 cells via inactivation of the MAPK and Smad2/3 signaling pathways. Mol Med Rep. 2022;25:184
100. Yoshida J, Ishikawa T, Endo Y, Matsumura S, Ota T, Mizushima K. et al. Metformin inhibits TGF-β1-induced epithelial-mesenchymal transition and liver metastasis of pancreatic cancer cells. Oncol Rep. 2020;44:371-81
101. Cheng K, Hao M. Metformin Inhibits TGF-β1-Induced Epithelial-to-Mesenchymal Transition via PKM2 Relative-mTOR/p70s6k Signaling Pathway in Cervical Carcinoma Cells. Int J Mol Sci. 2016;17:2000
102. Incio J, Suboj P, Chin SM, Vardam-Kaur T, Liu H, Hato T. et al. Metformin Reduces Desmoplasia in Pancreatic Cancer by Reprogramming Stellate Cells and Tumor-Associated Macrophages. PLoS One. 2015;10:e0141392
103. Zhou Z, Luo W, Zheng C, Wang H, Hu R, Deng H. et al. Mitochondrial metabolism blockade nanoadjuvant reversed immune-resistance microenvironment to sensitize albumin-bound paclitaxel-based chemo-immunotherapy. Acta Pharm Sin B. 2024;14:4087-101
104. Luo D, Zeng X, Zhang S, Li D, Cheng Z, Wang Y. et al. Pirfenidone suppressed triple-negative breast cancer metastasis by inhibiting the activity of the TGF-β/SMAD pathway. J Cell Mol Med. 2023;27:456-69
105. Jamialahmadi H, Nazari SE, TanzadehPanah H, Saburi E, Asgharzadeh F, Khojasteh-Leylakoohi F. et al. Targeting transforming growth factor beta (TGF-β) using Pirfenidone, a potential repurposing therapeutic strategy in colorectal cancer. Sci Rep. 2023;13:14357
106. Meng H, Zhao Y, Dong J, Xue M, Lin YS, Ji Z. et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano. 2013;7:10048-65
107. Feng J, Xu M, Wang J, Zhou S, Liu Y, Liu S. et al. Sequential delivery of nanoformulated alpha-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials. 2020;241:119907
108. Pei Y, Chen L, Huang Y, Wang J, Feng J, Xu M. et al. Sequential Targeting TGF-beta Signaling and KRAS Mutation Increases Therapeutic Efficacy in Pancreatic Cancer. Small. 2019;15:e1900631
109. Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. 2014;8:3636-45
110. Hou L, Liu Q, Shen L, Liu Y, Zhang X, Chen F. et al. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics. 2018;8:3781-96
111. Xu Y, Lv J, Liu Y, Du J, Luo C, Wang Y. et al. Coagulation-Targeted TGF-β Signaling Pathway Inhibitor Nanomedicine for Inhibiting the Growth and Lung Metastasis of Breast Cancer. Nano Lett. 2025;25:504-13
112. Chen Y, Hu M, Wang S, Wang Q, Lu H, Wang F. et al. Nano-delivery of salvianolic acid B induces the quiescence of tumor-associated fibroblasts via interfering with TGF-β1/Smad signaling to facilitate chemo- and immunotherapy in desmoplastic tumor. Int J Pharm. 2022;623:121953
113. Tan T, Feng Y, Wang W, Wang R, Yin L, Zeng Y. et al. Cabazitaxel-loaded human serum albumin nanoparticles combined with TGFβ-1 siRNA lipid nanoparticles for the treatment of paclitaxel-resistant non-small cell lung cancer. Cancer Nanotechnology. 2023;14:70
114. Zhou Q, Li Y, Zhu Y, Yu C, Jia H, Bao B. et al. Co-delivery nanoparticle to overcome metastasis promoted by insufficient chemotherapy. J Control Release. 2018;275:67-77
115. Bertrand-Chapel A, Caligaris C, Fenouil T, Savary C, Aires S, Martel S. et al. SMAD2/3 mediate oncogenic effects of TGF-β in the absence of SMAD4. Commun Biol. 2022;5:1068
116. Chen H-d, Ye Z, Hu H-f, Fan G-x, Hu Y-h, Li Z. et al. SMAD4 endows TGF-β1-induced highly invasive tumor cells with ferroptosis vulnerability in pancreatic cancer. Acta Pharmacol Sin. 2023;45:844-56
117. Wang X, Istvanffy R, Ye L, Teller S, Laschinger M, Diakopoulos KN. et al. Phenotype screens of murine pancreatic cancer identify a Tgf-α-Ccl2-paxillin axis driving human-like neural invasion. J Clin Invest. 2023;133:e166333
118. Li Y, Zhao Z, Liu H, Fetse JP, Jain A, Lin CY. et al. Development of a Tumor-Responsive Nanopolyplex Targeting Pancreatic Cancer Cells and Stroma. ACS Appl Mater Interfaces. 2019;11:45390-403
119. Zhang J, Zuo T, Yang J, Hu Z, Wang Z, Xu R. et al. Hierarchically Releasing Bio-Responsive Nanoparticles for Complete Tumor Microenvironment Modulation via TGF-beta Pathway Inhibition and TAF Reduction. ACS Appl Mater Interfaces. 2021;13:2256-68
120. Han L, Huang X, Zhao B, Zhu H, Wang R, Liu S. et al. TGF-β1 mediates tumor immunosuppression aggravating at the late-stage post-high-light-dose photodynamic therapy. Cancer Immunol Immunother. 2023;72:3079-95
121. Han L, Huang X, Zhu H, Wang R, Zhao B, Liu S. et al. Programmed cyclodextrin-based core-shell nanoparticles for cooperative TGF-β blockade to reverse immunosuppression post photodynamic therapy. Chem Eng J. 2023;455:140830
122. Dai L, Li X, Zheng X, Fu Z, Yao M, Meng S. et al. TGF-β blockade-improved chemo-immunotherapy with pH/ROS cascade-responsive micelle via tumor microenvironment remodeling. Biomaterials. 2021;276:121010
123. Huang S, Ding D, Lan T, He G, Ren J, Liang R. et al. Multifunctional nanodrug performs sonodynamic therapy and inhibits TGF-β to boost immune response against colorectal cancer and liver metastasis. Acta Biomater. 2023;134:538-52
124. Wang Y, Gao Z, Du X, Chen S, Zhang W, Wang J. et al. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci. 2020;8:5121-32
125. Yu M, Zhao M, Yu R, Chu S, Xu J, Xia M. et al. Nanotechnology-mediated immunochemotherapy with Ingenol-3-Mebutate for Systematic Anti-tumor Effects. J Control Release. 2019;304:242-58
126. Hanafy N, Quarta A, Ferraro M, Dini L, Nobile C, De Giorgi M. et al. Polymeric Nano-Micelles as Novel Cargo-Carriers for LY2157299 Liver Cancer Cells Delivery. Int J Mol Sci. 2018;19:748
127. Zhang S, You H, Fan H, Chen Y, Song H, Zhao Z. et al. Transcytosis-Triggering Nanoparticles for Overcoming Stromal Barriers and Reversing Immunosuppression in Pancreatic Cancer Combinatorial Therapy. Nano Lett. 2025;25:2949-59
128. Li Z, Xu W, Yang J, Wang J, Wang J, Zhu G. et al. A Tumor Microenvironments-Adapted Polypeptide Hydrogel/Nanogel Composite Boosts Antitumor Molecularly Targeted Inhibition and Immunoactivation. Adv Mater. 2022;34:e2200449
129. Jiang J, Zhang Y, Peng K, Wang Q, Hong X, Li H. et al. Combined delivery of a TGF-beta inhibitor and an adenoviral vector expressing interleukin-12 potentiates cancer immunotherapy. Acta Biomater. 2017;61:114-23
130. Peres C, Matos AI, Carreira B, Moura LIF, Kleiner R, Vaskovich-Koubi D. et al. Multifunctional Nanovaccine Sensitizes Breast Cancer to Immune Checkpoint Therapy. Adv Funct Mater. 2024;34:2401749
131. Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB. et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun. 2017;8:1747
132. Yen YT, Zhang Z, Chen A, Qiu Y, Liu Q, Wang Q. et al. Enzymatically responsive nanocarriers targeting PD-1 and TGF-β pathways reverse immunotherapeutic resistance and elicit robust therapeutic efficacy. J Nanobiotechnology. 2025;23:124
133. Kim W, Ye Z, Simonenko V, Shahi A, Malikzay A, Long SZ. et al. Codelivery of TGFβ and Cox2 siRNA inhibits HCC by promoting T-cell penetration into the tumor and improves response to Immune Checkpoint Inhibitors. NAR Cancer. 2024;6:zcad059
134. Zhang Q, Chang X, Wang H, Liu Y, Wang X, Wu M. et al. TGF-β1 mediated Smad signaling pathway and EMT in hepatic fibrosis induced by Nano NiO in vivo and in vitro. Environ Toxicol. 2019;35:419-29
135. Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int J Mol Sci. 2018;19:1979
136. Tsai YS, Chen YH, Cheng PC, Tsai HT, Shiau AL, Tzai TS. et al. TGF-beta1 conjugated to gold nanoparticles results in protein conformational changes and attenuates the biological function. Small. 2013;9:2119-28
137. Zhang Y, Elechalawar CK, Hossen MN, Francek ER, Dey A, Wilhelm S. et al. Gold nanoparticles inhibit activation of cancer-associated fibroblasts by disrupting communication from tumor and microenvironmental cells. Bioact Mater. 2021;6:326-32
138. Liu C, Hu X, Li X, Zhou Y, Wang H, Fan C. et al. Reprogramming of cancer invasiveness and macrophage education via a nanostructured antagonist of the TGFβ receptor. Mater Horiz. 2019;6:1675-81
139. Liu Y, Chen C, Qian P, Lu X, Sun B, Zhang X. et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat Commun. 2015;6:5988
140. Li X, Song L, Hu X, Liu C, Shi J, Wang H. et al. Inhibition of Epithelial-Mesenchymal Transition and Tissue Regeneration by Waterborne Titanium Dioxide Nanoparticles. ACS Appl Mater Interfaces. 2018;10:3449-58
141. Park N, Kim KS, Lee S, Choi JH, Na K. Enhanced stem cell-mediated therapeutic immune modulation with zinc oxide nanoparticles in liver regenerative therapy. Biomaterials. 2025;320:123232
142. Setyawati MI, Sevencan C, Bay BH, Xie J, Zhang Y, Demokritou P. et al. Nano-TiO2 Drives Epithelial-Mesenchymal Transition in Intestinal Epithelial Cancer Cells. Small. 2018;14:e1800922
143. Zhang Q, Chang X, Wang H, Liu Y, Wang X, Wu M. et al. TGF-beta1 mediated Smad signaling pathway and EMT in hepatic fibrosis induced by Nano NiO in vivo and in vitro. Environ Toxicol. 2020;35:419-29
144. Medina-Reyes EI, Delgado-Buenrostro NL, Déciga-Alcaraz A, Freyre-Fonseca V, Flores-Flores JO, Hernández-Pando R. et al. Titanium dioxide nanofibers induce angiogenic markers and genomic instability in lung cells leading to a highly dedifferentiated and fibrotic tumor formation in a xenograft model. Environ Sci-Nano. 2019;6:286-304
145. Man S, Li M, Zhou J, Wang H, Zhang J, Ma L. Polyethyleneimine coated Fe3O4 magnetic nanoparticles induce autophagy, NF-κB and TGF-β signaling pathway activation in HeLa cervical carcinoma cells via reactive oxygen species generation. Biomater Sci. 2020;8:201-11
146. Zhu J, Li B, Xu M, Liu R, Xia T, Zhang Z. et al. Graphene Oxide Promotes Cancer Metastasis through Associating with Plasma Membrane To Promote TGF-beta Signaling-Dependent Epithelial-Mesenchymal Transition. ACS Nano. 2020;14:818-27
147. Park EJ, Bae E, Yi J, Kim Y, Choi K, Lee SH. et al. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol. 2010;30:162-8
148. Manshian BB, Poelmans J, Saini S, Pokhrel S, Grez JJ, Himmelreich U. et al. Nanoparticle-induced inflammation can increase tumor malignancy. Acta Biomater. 2018;68:99-112
149. Mardhian DF, Storm G, Bansal R, Prakash J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J Control Release. 2018;290:1-10
150. Zhou R, Liu Y, Wang Z, Lv J, Liao W, Shen Z. et al. Nanoparticle-Based MRI-Guided Tumor Microenvironment Heating via the Synergistic Effect of Ferroptosis and Inhibition of TGF-β Signaling. Adv Healthc Mater. 2023;12:e2300176
151. Qiao C, Yang J, Shen Q, Liu R, Li Y, Shi Y. et al. Traceable Nanoparticles with Dual Targeting and ROS Response for RNAi-Based Immunochemotherapy of Intracranial Glioblastoma Treatment. Adv Mater. 2018;30:e1705054
152. Wu J, Liu B, Wu H, Wu Y, Zhang W, Zhao S. et al. A Gold Nanoparticle Platform for the Delivery of Functional TGF-beta1 siRNA Into Cancer Cells. J Biomed Nanotechnol. 2016;12:800-10
153. Yang YS, Moynihan KD, Bekdemir A, Dichwalkar TM, Noh MM, Watson N. et al. Targeting small molecule drugs to T cells with antibody-directed cell-penetrating gold nanoparticles. Biomater Sci. 2018;7:113-24
154. Cai H, Dai X, Guo X, Zhang L, Cao K, Yan F. et al. Ataxia telangiectasia mutated inhibitor-loaded copper sulfide nanoparticles for low-temperature photothermal therapy of hepatocellular carcinoma. Acta Biomater. 2021;127:276-86
155. Chen Y, Liu P, Sun P, Jiang J, Zhu Y, Dong T. et al. Oncogenic MSH6-CXCR4-TGFB1 Feedback Loop: A Novel Therapeutic Target of Photothermal Therapy in Glioblastoma Multiforme. Theranostics. 2019;9:1453-73
156. Peng H, Shen J, Long X, Zhou X, Zhang J, Xu X. et al. Local Release of TGF-β Inhibitor Modulates Tumor-Associated Neutrophils and Enhances Pancreatic Cancer Response to Combined Irreversible Electroporation and Immunotherapy. Adv Sci (Weinh). 2022;9:e2105240
157. Xu B, Cui Y, Wang W, Li S, Lyu C, Wang S. et al. Immunomodulation-Enhanced Nanozyme-Based Tumor Catalytic Therapy. Adv Mater. 2020;32:e2003563
158. Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R. et al. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumor immunotherapy. Nat Mater. 2012;11:895-905
159. Kim YA, Jeong H, Kim H, Lee S, Kim KS, Na K. Lipid nanoparticles with prazole adjuvant to enhance the efficacy of mRNA cancer vaccines. J Control Release. 2025;383:113756
160. Zhao X, Yang X, Wang X, Zhao X, Zhang Y, Liu S. et al. Penetration Cascade of Size Switchable Nanosystem in Desmoplastic Stroma for Improved Pancreatic Cancer Therapy. ACS Nano. 2021;15:14149-61
161. Li Z, Zheng Y, Shi H, Xie H, Yang Y, Zhu F. et al. Convenient Tuning of the Elasticity of Self-Assembled Nano-Sized Triterpenoids to Regulate Their Biological Activities. ACS Appl Mater Interfaces. 2021;13:44065-78
162. Li Z, Shi H, Xie H, Yang Y, Zheng Y, Chen H. et al. Tri-component programmable nanoregulator with Three-pronged penetration boosts immunotherapy of Triple-Negative breast cancer. Chem Eng J. 2022;439:135712
163. Zhao LP, Zheng RR, Kong RJ, Huang CY, Rao XN, Yang N. et al. Self-Delivery Ternary Bioregulators for Photodynamic Amplified Immunotherapy by Tumor Microenvironment Reprogramming. ACS Nano. 2022;16:1182-97
164. Peng Y, Gao Y, Yang C, Guo R, Shi X, Cao X. Low-Molecular-Weight Poly(ethylenimine) Nanogels Loaded with Ultrasmall Iron Oxide Nanoparticles for T(1)-Weighted MR Imaging-Guided Gene Therapy of Sarcoma. ACS Appl Mater Interfaces. 2021;13:27806-13
165. Park SY, Kim B, Cui Z, Park G, Choi YW. Anti-Metastatic Effect of Gold Nanoparticle-Conjugated Maclura tricuspidata Extract on Human Hepatocellular Carcinoma Cells. Int J Nanomedicine. 2020;15:5317-31
166. Lv W, Wu H, Zhang Y, Li H, Shu H, Su C. et al. cRGD-targeted gold-based nanoparticles overcome EGFR-TKI resistance of NSCLC via low-temperature photothermal therapy combined with sonodynamic therapy. Biomater Sci. 2023;11:1677-91
167. Delle Cave D, Mangini M, Tramontano C, De Stefano L, Corona M, Rea I. et al. Hybrid Biosilica Nanoparticles for in-vivo Targeted Inhibition of Colorectal Cancer Growth and Label-Free Imaging. Int J Nanomedicine. 2024;19:12079-98
168. Lucena-Sánchez E, Hicke FJ, Clara-Trujillo S, García-Fernández A, Martínez-Máñez R. Nanoparticle-Mediated Tumor Microenvironment Remodeling Favors the Communication with the Immune Cells for Tumor Killing. Adv Therap. 2024;7:202400004
169. Zhang J, Xu Z, Li Y, Hu Y, Tang J, Xu J. et al. Theranostic mesoporous platinum nanoplatform delivers halofuginone to remodel extracellular matrix of breast cancer without systematic toxicity. Bioeng Transl Med. 2023;8:e10427
170. Li Y, Wang D, Sun J, Hao Z, Tang L, Sun W. et al. Calcium Carbonate/Polydopamine Composite Nanoplatform Based on TGF-β Blockade for Comfortable Cancer Immunotherapy. ACS Appl Mater Interfaces. 2024;16:3187-201
171. Cao Y, Wen E, Chen Q, Li X, Wang Z. Multifunctional ICG-SB@Lip-ZA Nanosystem Focuses on Remodeling the Inflammatory-Immunosuppressive Microenvironment After Photothermal Therapy to Potentiate Cancer Photothermal Immunotherapy. Adv Healthc Mater. 2025;14:e2402211
172. Phung CD, Nguyen BL, Jeong JH, Chang JH, Jin SG, Choi HG. et al. Shaping the "hot" immunogenic tumor microenvironment by nanoparticles co-delivering oncolytic peptide and TGF-β1 siRNA for boosting checkpoint blockade therapy. Bioeng Transl Med. 2023;8:e10392
173. Zeng Z, Zeng X, Li X, Feng Y, Kan Y, Liu X. et al. The Efficacy and Safety of Polyethylene Glycol Cholesterol- and Tocopherol Polyethylene Glycol 1000 Succinate-Modified Transforming Growth Factor β1 Small Interfering RNA Lipid Nanoparticles in the Treatment of Paclitaxel-Resistant Non-Small-Cell Lung Cancer. Pharmaceutics. 2024;16:16010075
174. Kim J, Kim M, Yong SB, Han H, Kang S, Lahiji SF. et al. Engineering TGF-beta inhibitor-encapsulated macrophage-inspired multi-functional nanoparticles for combination cancer immunotherapy. Biomater Res. 2023;27:136
175. Wang S, Song Y, Cao K, Zhang L, Fang X, Chen F. et al. Photothermal therapy mediated by gold nanocages composed of anti-PDL1 and galunisertib for improved synergistic immunotherapy in colorectal cancer. Acta Biomater. 2021;133:621-32
176. Zhang F, Li F, Lu GH, Nie W, Zhang L, Lv Y. et al. Engineering Magnetosomes for Ferroptosis/Immunomodulation Synergism in Cancer. ACS Nano. 2019;13:5662-73
177. Kim D, Choi J, Jin D, Xu E, Lee J, Byun J. et al. Hybrid lipid nanoparticles with tumor antigen-primed dendritic cell membranes for post-surgical tumor immunotherapy. J Control Release. 2025;379:537-48
178. An J, Guo R, Liu M, Hu H, Zhang H. Multi-modal Ca(2+) nanogenerator via reversing T cell exhaustion for enhanced chemo-immunotherapy. J Control Release. 2024;372:715-27
179. Song G, Shao X, Qu C, Shi D, Jia R, Chen Y. et al. A large-pore mesoporous Au@Pt@Rh trimetallic nanostructure with hyperthermia-enhanced enzyme-mimic activities for immunomodulation-improved tumor catalytic therapy. Chem Eng J. 2023;477:147161
180. Li H, Zeng J, You Q, Zhang M, Shi Y, Yang X. et al. X-ray-activated nanoscintillators integrated with tumor-associated neutrophils polarization for improved radiotherapy in metastatic colorectal cancer. Biomaterials. 2025;316:123031
181. Tang Y, Yao W, Hang H, Xiong W, Mei H, Hu Y. TGF-β blocking combined with photothermal therapy promote tumor targeted migration and long-term antitumor activity of CAR-T cells. Mater Today Bio. 2023;134:100615
182. Liu C, Lai H, Chen T. Boosting Natural Killer Cell-Based Cancer Immunotherapy with Selenocystine/Transforming Growth Factor-Beta Inhibitor-Encapsulated Nanoemulsion. ACS Nano. 2020;14:11067-82
183. Choi SH, Cho HB, Choi JH, Kim HJ, Jang HJ, Cho S. et al. Nano-chemical priming strategy to enhance TGF-beta resistance and anti-tumor activity of natural killer cells. J Control Release. 2024;367:768-78
184. Zheng Y, Tang L, Mabardi L, Kumari S, Irvine DJ. Enhancing Adoptive Cell Therapy of Cancer through Targeted Delivery of Small-Molecule Immunomodulators to Internalizing or Noninternalizing Receptors. ACS Nano. 2017;11:3089-100
185. Luo L, Zhang B, Tao F, Chen Z, Ye Q, Zhao X. et al. Perfluorotributylamine-Loaded Albumin Nanoparticles Downregulate Platelet-Derived TGFβ to Inhibit Tumor Metastasis. ACS Nano. 2023;17:15388-400
186. Karolczak K, Watala C. Blood Platelets as an Important but Underrated Circulating Source of TGF-β. Int J Mol Sci. 2021;22:4492
187. Zhou Z, Jiang X, Yi L, Li C, Wang H, Xiong W. et al. Mitochondria Energy Metabolism Depression as Novel Adjuvant to Sensitize Radiotherapy and Inhibit Radiation Induced-Pulmonary Fibrosis. Adv Sci (Weinh). 2024;11:e2401394
188. Jiang X, Yi L, Li C, Wang H, Xiong W, Li Y. et al. Mitochondrial Disruption Nanosystem Simultaneously Depressed Programmed Death Ligand-1 and Transforming Growth Factor-β to Overcome Photodynamic Immunotherapy Resistance. ACS Nano. 2024;143:3331-48
189. Zhu S, Sun F, Zhao P, Liang G, Sun X, Zeng L. et al. Brain-targeting biomimetic nanoparticles for codelivery of celastrol and LY2157299 for reversing glioma immunosuppression. Int J Pharm. 2022;619:121709
190. Nestor MS, Hetzel J, Awad N, Bhupalam V, Lu P, Molyneaux M. A Novel Injectable Polypeptide Nanoparticle Encapsulated siRNA Targeting TGF-β1 and COX-2 for Localized Fat Reduction II: Phase I Clinical Trial. J Cosmet Dermatol. 2025;24:e16722
Author contact
![]() Corresponding authors: Jianqing Gao, Institute of Pharmaceutics, Zhejiang Province Key Laboratory of Anti-Cancer Drug Research, State Key Laboratory of Advanced Drug Delivery and Release Systems, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China; orcid.org/0000-0003-1052-7060; Email: gaojianqingedu.cn. Ziying Li, Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, 321004, China; orcid.org/0000-0002-6443-492X; Email: ziyingli108com; ziyingliedu.cn.
Corresponding authors: Jianqing Gao, Institute of Pharmaceutics, Zhejiang Province Key Laboratory of Anti-Cancer Drug Research, State Key Laboratory of Advanced Drug Delivery and Release Systems, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China; orcid.org/0000-0003-1052-7060; Email: gaojianqingedu.cn. Ziying Li, Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, 321004, China; orcid.org/0000-0002-6443-492X; Email: ziyingli108com; ziyingliedu.cn.
 Global reach, higher impact
Global reach, higher impact