13.3
Impact Factor
Theranostics 2026; 16(7):3556-3576. doi:10.7150/thno.123005 This issue Cite
Research Paper
Characterization and validation of a bone metastatic castration-resistant prostate cancer model as a nanomedicine evaluation platform
1. Polymer Therapeutics Laboratory, Príncipe Felipe Research Center (CIPF), Valencia, Spain.
2. Centro de Investigación médica en Red Cáncer (CIBERONC), Madrid, Spain.
3. Institute for Experimental Immunology and Imaging, University Hospital, University of Duisburg-Essen, Essen, Germany.
4. Institute for Virology, University Hospital, University of Duisburg-Essen, Essen, Germany.
5. Leibniz-Institut für Analytische Wissenschaften - ISAS - e.V., Dortmund, Germany.
6. Department of Bioengineering, Graduate School of Engineering, The University of Tokyo, Tokyo, Japan.
7. Department of Pathology, Consorcio Hospital General Universitario de Valencia, Valencia, Spain.
Abstract
Rationale: Bone metastases - common in metastatic castration-resistant prostate cancer (mCRPC) - lead to severe complications and currently suffer from limited therapeutic options. Poor solubility, systemic toxicity, and therapeutic resistance hamper conventional approaches, such as docetaxel (Dtx) treatment. Nanomedicine-based strategies - including polymer-drug conjugates - can help overcome said limitations through enhanced tumor targeting and reduced unwanted side effects in healthy tissues.
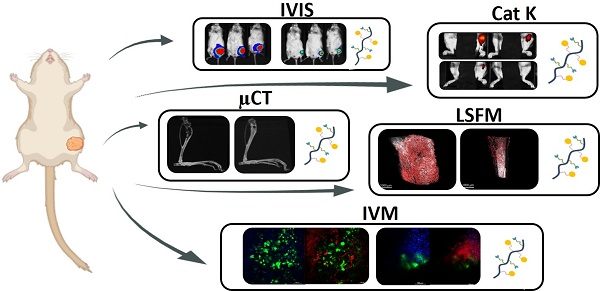
Methods: An intratibial bone mCRPC mouse model - used to recapitulate tumor growth and microenvironmental dynamics - was developed and characterized. A poly-L-glutamic acid (PGA)-Dtx) conjugate synthesized to enhance Dtx delivery and efficacy was also characterized in terms of size, zeta potential, drug loading, and pH-dependent release. In vivo evaluations included tumor growth monitoring by bioluminescence imaging, cathepsin K activity from tumor by fluorescence imaging, bone damage evaluation by micro-computed tomography, tumor vasculature by light-sheet fluorescent microscopy, cell population at tumor site by histology, modulation of blood cell populations by tumor and treatment by hematology, and biodistribution of PGA-Dtx using fluorescent imaging and intravital microscopy.
Results: Our intratibial bone mCRPC model supported reliable tumor establishment, progressive osteolytic damage and vascularization, and systemic inflammation. PGA-Dtx displayed optimal properties (6.6 nm size, -24.1 mV zeta potential, 3.3 mol % drug loading) and supported lower but sustained Dtx release at acidic pH. The enhanced tumor accumulation following PGA-Dtx administration significantly suppressed tumor growth in vivo, normalized cathepsin K activity levels, and reduced bone damage while avoiding the systemic toxicity associated with free Dtx.
Conclusions: Our intratibial bone mCRPC mouse model provides a robust platform for studying PCa bone metastases and evaluating nanomedicine efficacy. PGA-Dtx displays promise as a safe and effective therapy for mCRPC, offering improved drug delivery and reduced systemic side effects, which supports the translational potential of polymer-drug conjugates in mCRPC management.
Keywords: prostate cancer, bone metastasis, nanomedicine, polymer-drug conjugates, docetaxel
 Global reach, higher impact
Global reach, higher impact