13.3
Impact Factor
Theranostics 2026; 16(7):3790-3825. doi:10.7150/thno.123550 This issue Cite
Review
Engineering polysaccharide nanoplatforms for glioblastoma theranostics: Bridging targeted therapy and advanced imaging
1. Department of Radiology, Institution of Radiology and Medical Imaging, Huaxi MR Research Center (HMRRC), Frontiers Science Center for Disease-Related Molecular Network, National Clinical Research Center for Geriatrics, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China.
2. Psychoradiology Key Laboratory of Sichuan Province, West China Hospital, Sichuan University, Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, and Key Laboratory of Transplant Engineering and Immunology, NHC, Chengdu 610041, China.
3. Core Facility of West China Hospital, Sichuan University, Chengdu 610041, China.
4. Animal Imaging Core Facilities, West China Hospital, Sichuan University, China.
5. Xiamen Key Lab of Psychoradiology and Neuromodulation, Department of Radiology, West China Xiamen Hospital of Sichuan University, Xiamen 361021, China.
*Dr. Xiaoming Wang and Qing Yang contributed equally to this study.
Received 2025-8-11; Accepted 2025-12-29; Published 2026-1-14
Abstract
Glioblastoma (GBM) is an aggressive brain tumor characterized by limited therapeutic efficacy and challenges in accurate imaging, largely due to its invasive growth, drug resistance, and the restrictive blood-brain barrier (BBB) hindering the delivery of both therapeutic and diagnostic agents. Current GBM treatments and imaging approaches often suffer from insufficient agent penetration into the tumor. Additionally, they frequently exhibit toxicity or poor signal-to-noise ratios. Polysaccharide (PSC)-based polymers, with their inherent biocompatibility, biodegradability, and versatile chemical modifiability, offer a promising platform to overcome these limitations. These natural polymers can be engineered into sophisticated nanocarriers that enhance BBB traversal, enable targeted tumor accumulation of therapeutic payloads and imaging agents Furthermore, they facilitate controlled drug release and improve diagnostic signal generation. Consequently, PSC-based systems can improve therapeutic efficacy and enhance diagnostic accuracy for tumor visualization. Furthermore, they reduce systemic side effects and support multimodal strategies, ranging from single-modality interventions to integrated theranostic systems. This review aims to comprehensively discuss recent advancements, current challenges, and future perspectives of PSC-based nanomedicines in GBM therapy and imaging.
Keywords: GBM, imaging, nanomedicines, polysaccharide polymer
1. Introduction
GBM presents a formidable clinical challenge due to its invasive growth, inherent resistance to standard therapies, and the complex physiological obstacle posed by the BBB [1] (Figure 1). In high-grade gliomas, the BBB evolves into a heterogeneous blood-brain tumor barrier (BBTB), the integrity of which is a subject of considerable debate and a critical factor for drug delivery. This complexity necessitates a clear distinction between the penetration capabilities of small diagnostic agents versus larger therapeutic nanocarriers. For instance, diagnostic agents like gadolinium-based contrast media or ultra-small imaging nanoparticles (NPs) (e.g., <5 nm) can exploit localized structural compromises in the BBTB. The contrast enhancement observed in T1-weighted magnetic resonance imaging (MRI) directly visualizes regions where the barrier has ruptured, confirming extravasation into the tumor core. However, the level of compromise sufficient for this enhancement is often insufficient for the robust, uniform penetration of larger therapeutic NPs, which typically exceed 50 nm.
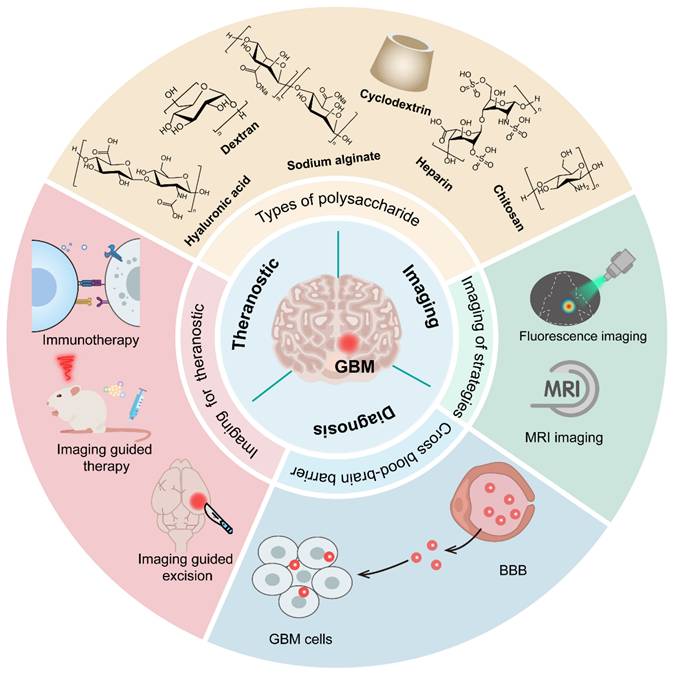
Smart PSC-based nanomedicines (e.g., sodium alginate, chitosan, cyclodextrin, heparin, dextran, etc.) for imaging and therapeutic applications in GBM. Mainly involves (1) crossing the BBB for enhanced accumulation of therapeutic agents and imaging probes at GBM sites; (2) multimodal imaging, including fluorescence imaging and MRI, to support tumor staging, diagnosis, and boundary delineation; (3) theranostic integration, enabling imaging-guided precise treatments (e.g., surgical resection) and immunotherapy.
The extent of BBB compromise in GBM is highly variable. While high-grade gliomas are characterized by neo-angiogenesis and a "leaky" vasculature in the tumor core-driven by elevated vascular endothelial growth factor (VEGF) and reduced tight junction (TJ) proteins-this breakdown is not uniform. Conflicting studies reveal that vessels in the peritumoral region, where recurrence often originates, frequently retain structural components resembling the healthy BBB, such as intact TJs and normal pericyte coverage. This heterogeneity gives rise to the concept of the 'residual BBTB,' which refers to the anatomical and functional regions within and surrounding the malignant tissue, particularly at the invasive margins, where barrier integrity remains partially or fully intact. This residual barrier, composed of tightly controlled endothelial TJs, astrocyte end-feet, and the basal lamina, actively shields infiltrating tumor cells from systemic therapeutics. The presence of this residual BBTB severely restricts the reliability of the Enhanced Permeability and Retention (EPR) effect for GBM therapy. While the passive accumulation of NPs via the EPR effect can occur in the highly disrupted tumor core, it is largely ineffective at the tumor rim, preventing therapeutic agents from reaching the most invasive cell populations. Therefore, reliance on passive delivery is generally insufficient for GBM eradication. Overcoming these obstacles-including inadequate transport across the residual BBTB, poor targeting specificity, and systemic toxicity of potent agents is crucial. This necessitates the development of sophisticated carrier systems capable of precise tumor targeting and controlled payload release.
PSC-based nanoplatforms have emerged as highly promising candidates for addressing these multifaceted challenges in GBM management. PSCs, including widely studied examples like chitosan, hyaluronic acid (HA), dextran, and alginate, as well as various plant-derived PSCs, offer a unique combination of inherent biocompatibility, biodegradability, and abundant reactive groups for versatile chemical modification [2]. These characteristics allow for the rational design of PSC-based carriers that can be functionalized with targeting ligands (e.g., peptides, antibodies) or engineered to respond to specific stimuli within the tumor microenvironment. Such modifications facilitate improved specificity of agent accumulation in GBM cells and enable controlled release of diagnostic or therapeutic payloads. Through their tunable physicochemical properties, PSC-based systems serve as versatile platforms that can be tailored for distinct applications, either as dedicated diagnostic probes or therapeutic carriers [3]. Beyond these single-modality functions, advanced engineering allows for the integration of both imaging agents and therapeutic payloads into a single construct, thereby actualizing true theranostic approaches [4, 5]. It is critical to distinguish these integrated theranostic systems, which simultaneously deliver therapeutic payloads and diagnostic signals, from nanocarriers designed exclusively for a single modality. While single-function systems optimize either sensitivity (imaging) or payload capacity (therapy), true theranostic platforms must balance both, often requiring more complex engineering to prevent signal interference or premature drug release. Consequently, these nanoformulations hold the potential to significantly improve diagnostic accuracy and therapeutic outcomes while minimizing off-target effects. Furthermore, beyond their utility as delivery vehicles, many PSCs possess inherent immunostimulatory properties or can be modified to modulate the tumor immune microenvironment, offering an additional avenue to enhance anti-GBM responses [6].
Given the significant advantages and versatile nature of PSC nanoplatforms in addressing the critical issues faced in GBM diagnosis and treatment, this review aims to comprehensively summarize the recent advancements in the field. We will delve into the various types of PSCs being explored, such as chitosan, HA, dextran, alginate, and other emerging PSCs. The review will detail their rational design strategies and highlight their applications in enhancing imaging modalities for improved GBM diagnosis, augmenting the efficacy of diverse therapeutic strategies (including chemotherapy, gene and RNA therapy, and combination therapies), and developing integrated theranostic systems. By focusing on these aspects, this review seeks to underscore the promise of PSC-based nanomedicines in shaping the next generation of management strategies for GBM.
2. Overcoming the BBB for GBM Therapy: Nanocarrier Strategies and the Role of PSCs
A major obstacle in GBM therapy is the efficient delivery of anticancer agents across the BBB. The tight junctions characteristic of the BBB, formed by endothelial cells, pericytes, and astrocytes, significantly hamper the entry of many therapeutic compounds, particularly large or hydrophilic chemotherapeutics, into the brain parenchyma [7, 8]. This barrier meticulously regulates the passage of substances, protecting the central nervous system (CNS) but concurrently limiting the efficacy of systemic treatments for brain malignancies. Nanocarrier-based systems represent a promising avenue to surmount this biological obstruction, aiming to improve drug concentrations at the tumor site while minimizing systemic toxicity.
Nanocarriers can employ several mechanisms to facilitate their passage across the BBB. In instances where the BBB integrity is compromised, nanocarriers, typically ranging from 1 to 100 nm in size (many clinically relevant NPs are larger than 100 nm), may utilize passive diffusion to enter the brain tissue. This is often the case for regions of disrupted vasculature that are associated with the GBM microenvironment changes (a phenomenon sometimes related to the enhanced permeability and retention, or EPR effect). However, the heterogeneity of BBB disruption in GBM necessitates more sophisticated approaches for consistent and widespread delivery.
For areas where the BBB remains more intact, active transport mechanisms are crucial. These include adsorptive-mediated transcytosis (AMT), which is often triggered by electrostatic interactions between cationic nanocarriers and the negatively charged glycocalyx and phospholipid head groups on the luminal surface of brain capillary endothelial cells. Carrier-mediated transport (CMT) offers another route, utilizing endogenous transporters designed for essential nutrients like glucose, amino acids, and nucleosides, although this pathway is generally more suited for smaller molecules or drugs that structurally mimic these native substrates. A more widely exploited active mechanism for nanocarriers is receptor-mediated transcytosis (RMT). This highly specific pathway involves the binding of ligands, purposefully conjugated to the nanocarrier surface, to receptors abundantly expressed on BBB endothelial cells. Commonly targeted receptors include those for transferrin (TfR), low-density lipoprotein (LDL) receptor-related protein 1 (LRP1), lactoferrin (LfR), and insulin receptors [9]. Upon ligand binding, the nanocarrier is internalized via endocytosis, transported across the endothelial cell, and subsequently exocytosed into the brain interstitium.
Beyond these direct transport mechanisms, stimuli-responsive nanocarriers present another sophisticated strategy. These systems are engineered to remain relatively inert and stable in systemic circulation, minimizing premature drug release or off-target interactions. However, these nanocarriers undergo specific changes upon encountering specific triggers within the brain microenvironment. Triggers include the acidic pH characteristic of tumor tissues (around pH 6.5 or lower), altered redox gradients (e.g., elevated glutathione levels), or the presence of specific enzymes like matrix metalloproteinases. Externally applied stimuli, such as ultrasound or magnetic fields, can also induce these changes. These changes can include drug release, conformational alteration leading to enhanced cell uptake, or degradation of the carrier matrix. This targeted response thereby enhances the spatiotemporal control of drug delivery across the BBB or within the brain tissue itself, aiming to maximize therapeutic efficacy while reducing systemic side effects [3].
Among the materials explored for traversing the BBB, PSCs (e.g., chitosan, HA, dextran, alginate) offer distinct advantages due to their inherent biocompatibility, biodegradability, and versatile chemical modifiability [2, 10]. Crucially, specific PSCs leverage distinct transport mechanisms to overcome the BBB and BBTB. Chitosan, a cationic polymer, utilizes AMT; its positive charge facilitates electrostatic interactions with the negatively charged endothelial luminal surface, promoting transcellular passage [3]. Conversely, Hyaluronic Acid (HA) engages RMT by specifically binding to CD44 receptors, which are overexpressed on both activated brain endothelial cells and GBM cells, thereby enabling dual BBB-traversal and tumor targeting. Other PSCs, such as dextran, serve as excellent stealth coatings to prolong circulation or can be functionalized with external ligands (e.g., transferrin, angiopep-2) to induce RMT [9]. By tailoring these physicochemical properties—specifically size (optimally 30-150 nm), surface charge, and ligand density—PSC nanocarriers can be engineered to maximize brain accumulation while minimizing systemic clearance [11, 12].
The successful transit of PSC nanomedicines across the BBB and their subsequent therapeutic action are critically dependent on the meticulous control and optimization of their physicochemical properties and functional attributes. By tailoring nanocarrier size, surface charge, ligand attachments, and responsiveness to stimuli, researchers aim to improve BBB transport, enhance drug accumulation in GBM tissue, and control drug release kinetics, thereby maximizing therapeutic efficacy while minimizing adverse effects [4, 11]. Precise control of nanoparticle size is vital for effective BBB penetration and favorable pharmacokinetics. While smaller PSC-based NPs (e.g., <100 nm) may more readily diffuse through disrupted tight junctions or fenestrations in leaky BBB regions and are generally favored for endocytic uptake, extremely small constructs (<10 nm) can suffer from rapid renal clearance, leading to a significantly reduced circulation time and insufficient opportunity for BBB interaction [12, 13]. Conversely, very large NPs (>200-300 nm) may be rapidly cleared by the RES or may not efficiently cross the BBB even via active transport. Therefore, many PSC nanocarriers are designed with a diameter in the range of 30-150 nm to strike an optimal balance between prolonged systemic residence, avoidance of rapid clearance, and effective brain diffusion or transport.
The surface charge of PSC nanocarriers also significantly influences their interaction with the BBB and systemic fate. Moderately cationic chitosan-based carriers, for example, can leverage their positive charge to encourage AMT. However, an excessively high positive charge might trigger significant nonspecific binding to plasma proteins and blood cells, leading to aggregation, opsonization, and rapid immune clearance, or even endothelial toxicity. Strategies to modulate surface properties and mitigate these issues include coating with neutral, hydrophilic polymers like polyethylene glycol (PEG), known as PEGylation, which creates a steric barrier reducing protein adsorption and prolonging circulation. Partial grafting with other PSCs like HA can also serve a similar purpose or introduce specific targeting functionalities.
The attachment of specific ligands to the surface of PSC nanocarriers is a cornerstone strategy to promote active transport across the BBB via RMT and to enhance tumor cell recognition. Commonly employed ligands include transferrin (Tf), which binds to the highly expressed TfR on brain endothelial cells; angiopep-2, a peptide that targets LRP1; and various antibodies or antibody fragments directed against BBB-specific receptors. For instance, modifications of chitosan or HA with transferrin have been shown to improve NP passage across in vitro and in vivo BBB models. Cell-penetrating peptides (CPPs), such as TAT peptide or penetratin, can also be conjugated to PSC matrices to encourage deeper tissue uptake and accumulation in glioma sites, possibly by transiently modulating tight junction integrity or by promoting direct membrane translocation or enhanced endocytosis. Multiple reports indicate that such peptide-functionalized carriers exhibit greater fluorescence detection in the brain or more potent anticancer responses in glioma models compared to their unmodified counterparts, emphasizing the therapeutic advantage of active ligand conjugation [14, 15].
Incorporating stimuli-responsive elements into PSC nanocarriers allows for controlled drug release or activation specifically at the tumor site, triggered by the unique tumor microenvironment or by external interventions. For pH-responsive platforms, linkages that are stable at physiological pH (7.4) but hydrolyze or alter conformation in the acidic conditions of GBM tissues (pH ~6.5 or lower intracellularly) are employed. This prevents premature drug leakage during circulation and ensures site-specific activation. For example, mesoporous silica NPs coated with PSC layers have demonstrated significantly higher drug release under acidic conditions compared to neutral pH. Magnetic/pH-sensitive graphene oxide-chitosan microspheres loaded with temozolomide (TMZ) showed nearly doubled drug release at pH 4.5 relative to neutral conditions, a release further amplified by a moderate external magnetic field [16]. Redox-sensitive PSC materials leverage the significantly elevated GSH levels found in tumor cells compared to the extracellular environment. These carriers often incorporate disulfide linkages (-S-S-) within their structure or as crosslinkers. These bonds remain stable in the low-GSH environment of the bloodstream but are rapidly cleaved in the high-GSH reductive intracellular milieu of cancer cells, leading to carrier disassembly and burst-like drug release [17]. For instance, disulfide-crosslinked chitosan or dextran NPs can be designed to selectively deliver chemotherapeutic agents upon entering tumor cells. One study utilized a porphyrin-based metal-organic framework (MOF) crosslinked with HA that responded to high GSH levels in GBM tissue, releasing porphyrin-MOF NPs capable of generating reactive oxygen species (ROS) under ultrasound irradiation for sonodynamic therapy, and co-delivering L-cysteine to enhance tumor cell apoptosis. However, variations in redox gradients among individual patients and different tumor regions remain a challenge for achieving reproducible therapeutic outcomes with these systems. Furthermore, a critical limitation observed across chitosan studies is the trade-off between BBB penetration and toxicity. While increasing the degree of deacetylation and positive charge enhances absorptive-mediated transcytosis, it concurrently increases the formation of a protein corona in the bloodstream. This leads to rapid clearance by the reticuloendothelial system (RES) and potential neurotoxicity, a contradiction that explains why high-charge formulations often succeed in vitro but fail to maintain therapeutic concentrations in orthotopic animal models.
Exogenous triggers such as ultrasound (US) and magnetic fields further broaden the therapeutic potential. Focused ultrasound, often in conjunction with microbubbles, can transiently and locally disrupt the BBB, improving NP penetration. US can also stimulate faster drug release from carriers sensitive to mechanical or thermal energy. For instance, PSC-based silica carriers loaded with doxorubicin (DXR) displayed accelerated release upon ultrasonic stimulation, contributing to higher intratumoral accumulation and reduced cardiotoxicity. Magnetic fields can guide PSC-coated magnetic NPs (e.g., iron oxide cores) to the tumor site or trigger drug release. In magnetothermal therapy, these magnetic elements generate localized heat under an alternating magnetic field (AMF), directly damaging glioma cells (typically at 42-45°C) and enabling temperature-dependent drug release from thermosensitive PSC carriers. This hyperthermia can also increase tumor permeability and sensitize cells to chemotherapy or radiotherapy. Iron-containing PSC-NPs can also participate in chemodynamic therapy via the Fenton reaction, where released iron ions catalyze the conversion of endogenous H₂O₂ into highly toxic hydroxyl radicals, specifically within the acidic tumor microenvironment.
Beyond systemic administration, alternative delivery routes are being explored for PSC nanomedicines. Intranasal delivery, for example, exploits the direct nose-to-brain pathways (olfactory and trigeminal nerves) to bypass the BBB to some extent. Chitosan's mucoadhesive properties are particularly beneficial here, prolonging residence time in the nasal cavity and enhancing absorption. Chitosan-decorated poly(lactic-co-glycolic acid) (PLGA) NPs loaded with carmustine (BCNU) nearly tripled brain drug levels in rats via intranasal administration compared to intravenous injection [18]. Similarly, β-cyclodextrin-chitosan coatings on gold-iron oxide NPs demonstrated efficient intranasal uptake and led to marked survival benefits in mouse glioma models [19]. Localized delivery using PSC-based hydrogels implanted into post-resection cavities is another promising strategy. These hydrogels can act as depots for sustained, localized release of chemotherapeutics or immunomodulators, minimizing systemic toxicity and targeting residual tumor cells. For instance, a semi-synthetic PSC hydrogel loaded with ruxolitinib provided sustained release for 14 days and showed strong in vitro GBM cell-growth inhibition [20]. Cellulose nanocrystal hydrogels delivering paclitaxel (PTX) have also demonstrated measurable tumor suppression [21].
GBM is characterized by pronounced inter- and intratumoral heterogeneity, which includes variations in BBB integrity, receptor expression profiles, and microenvironmental conditions across different tumor regions and among patients [22]. Effective therapeutic strategies must therefore be adaptable and capable of addressing drug delivery to both intact and partially leaky BBB regions, as well as targeting diverse tumor cell populations. To enhance specificity and efficacy, dual-targeting approaches are being developed. These equip PSC nanocarriers with one ligand for BBB passage (e.g., transferrin or angiopep-2) and another for binding to receptors overexpressed primarily on tumor cells (e.g., integrin-binding RGD peptides for neoangiature or glioma cells, or CD44-binding HA for glioma cells). This strategy aims to improve drug accumulation and retention specifically within invasive glioma cells, thereby enhancing overall therapeutic efficacy [23, 24]. For example, HA-based nanomicelles modified with angiopep-2 and incorporating a hypoxia-sensitive moiety for triggered release have been developed. One such system, also utilizing Tween 80 for enhanced BBB passage, achieved a 7.6-fold increased transport across an in vitro BBB model compared to formulations lacking Tween 80 [25].
PSC nanocarriers are also being engineered as theranostic platforms, integrating imaging and therapeutic functions into a single complex. Dextran-coated iron oxide NPs delivering antisense oligonucleotides permit concurrent MRI tracking and gene silencing in GBM [26]. Multifunctional chitosan-coated magnetite graphene oxide systems, grafted with gastrin-releasing peptide ligands and loaded with doxorubicin, demonstrated decreased tumor burden under an external magnetic field, showcasing combined targeting, imaging capability, and therapy [27].
PSC nanocarriers are advancing gene and RNA therapy for GBM by securely encapsulating and transporting nucleic acids such as siRNA, miRNA, or plasmid DNA. The inherent biocompatibility of PSCs like chitosan and HA typically reduces immunogenic concerns associated with viral vectors. Surface modifications, including PEGylation or the addition of targeting ligands like transferrin, further extend circulation time, curb nonspecific adsorption, and improve selectivity for glioma cells [28, 29].
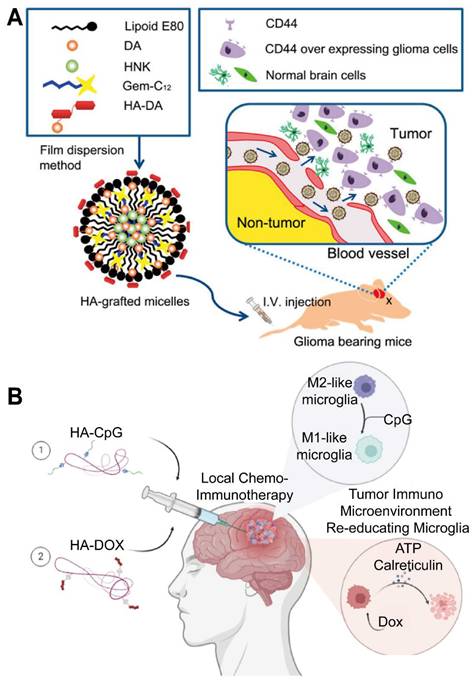
Furthermore, some PSCs (e.g., certain β-glucans, chitosan) possess intrinsic immunostimulatory properties, which can be leveraged to augment the effects of co-delivered immunotherapeutic agents like checkpoint inhibitors, cytokines, or tumor-associated antigens. Such carriers can accumulate in GBM tissue, help activate local anti-tumor immune responses by engaging innate immune cells, and synergize with conventional chemotherapy or radiotherapy. Hybrid constructs incorporating immunomodulators like CpG oligodeoxynucleotides within PSC matrices are also being explored [30].
Ensuring the safety of these nanoplatforms is paramount for clinical translation. This necessitates thorough preclinical evaluation, including comprehensive acute and chronic toxicity studies, immunogenicity assessments, and detailed biodistribution profiles. Noninvasive imaging techniques, such as magnetic resonance imaging (MRI) and near-infrared (NIR) fluorescence imaging, are invaluable tools for monitoring carrier localization, BBB crossing efficiency, tumor accumulation, and overall safety profiles in vivo.
As research in this domain continues to advance, PSC-based nanomedicines hold significant promise for transforming GBM management. They offer a versatile platform that can be engineered for systemic or local administration, employed as standalone delivery systems, or used in combination with other therapeutic modalities such as immunotherapies, focused ultrasound, or radiotherapy [5]. Achieving deeper penetration into the brain parenchyma, beyond the often-compromised tumor core and into invasive tumor margins, requires a holistic approach. This involves capitalizing on passive diffusion where possible, but more critically, harnessing receptor-mediated uptake mechanisms and exploiting environmental triggers or external stimuli to concentrate anticancer agents within GBM cells while minimizing off-target toxicity. The systematic assessment and optimization of all relevant design factors—including PSC type and molecular weight, nanoparticle size and morphology, surface charge and hydrophilicity, ligand choice and density, drug loading capacity, and release kinetics—are crucial for maximizing BBB penetration and subsequent tumor uptake and therapeutic effect [10]. Continued in vivo experiments in increasingly sophisticated and clinically relevant orthotopic GBM models are essential to elucidate how these multifaceted parameters collectively influence therapeutic efficacy and to validate safety. Ongoing efforts will likely focus on refining ligand specificity, developing multi-stimuli responsive systems for even greater spatiotemporal control, integrating combination therapies (e.g., chemo-immunotherapy or chemo-gene therapy), and establishing standardized, reproducible manufacturing and characterization protocols. By meticulously refining each aspect of PSC-driven delivery and rigorously validating their performance, these nanocarriers are poised to remain at the forefront of innovative strategies for crossing the BBB and offering safer, more effective treatments for the formidable challenge of GBM.
3. PSC-Based Strategies for GBM Imaging
PSC-based carriers are increasingly explored for GBM diagnostics, effective imaging. However, is fundamentally limited by the BBB, a highly selective interface that restricts the passage of most agents from systemic circulation into the brain parenchyma [31-33]. Consequently, significant research focuses on engineering nanocarriers, particularly PSCs, to leverage biological transport mechanisms. These biocompatible and structurally versatile macromolecules can be functionalized to cross the BBB and are readily conjugated with imaging agents—such as fluorescent dyes, gadolinium (Gd)-based contrast agents, or NPs—to enable sensitive, noninvasive tracking and improve tumor boundary delineation [34-36].
As detailed in Section 2, PSC nanoplatforms traverse the BBB primarily by leveraging RMT (targeting receptors like TfR1, LRP-1, and GLUT1) or AMT strategies [37-42]. Biomimetic strategies, including hyaluronic acid (HA) coatings to target CD44 on GBM cells [43] or cell-membrane cloaking (e.g., RBCs, EVs) to evade immune detection, also enhance delivery [44]. Additionally, physical methods, notably focused ultrasound (FUS) with microbubbles, can transiently disrupt the BBB to significantly boost nanocarrier accumulation [45].
Once across the BBB, these PSC platforms deliver diagnostic payloads for various imaging modalities, dominated by Magnetic Resonance Imaging (MRI) and optical imaging (OI) due to their clinical maturity and safety (Table 1) [46]. MRI remains the clinical gold standard, providing high-contrast anatomical information. PSCs incorporating superparamagnetic iron oxide NPs (IONPs) or Gd-based agents enable enhanced T1-weighted or T2-weighted imaging, allowing for precise tracking of nanoparticle accumulation and longitudinal monitoring of treatment response [47, 48]. Complementarily, OI, particularly Near-Infrared (NIR) fluorescence, is vital for real-time intraoperative guidance, offering the high spatial resolution needed to accurately delineate tumor margins and ensure maximal safe surgical resection [49, 50].
PSC-Metal Hybrid Imaging Probes
| Approach/Method Name | Nanoparticle Composition | Targeting Moiety | Imaging Modality & Contrast | Therapeutic Agent | Validation Model | Authors |
|---|---|---|---|---|---|---|
| Folic Acid & Hyaluronic Acid Coated SPIONs | SPION core; Hyaluronic acid & Folic acid shell | Hyaluronic acid; Folic acid | MRI (T2) | Intrinsic cytotoxicity | Glioma & adenocarcinoma cells; hepatocytes | Kasprzyk et al. [62] |
| Dextran-Coated SPIONs for RNA Delivery | Iron oxide core; Dextran & Cy5.5 shell | Passive (size-based) | MRI; Optical (Cy5.5) | Antisense oligonucleotide (anti-miR10b) | Orthotopic GBM mouse model | Kim et al. [26] |
| Chitosan-Dextran Hybrid SPIONs (CS-DX-SPIONs) | SPION core; Chitosan-dextran hybrid shell | Chitosan (charge-mediated) | MRI (T2) | None (potential carrier) | U87, C6, HeLa cells; Orthotopic C6 glioma rat model | Shevtsov et al. [66] |
| Magnetic Ternary Nanohybrid (MTN) for MSC Transfection | SPION core; Hyaluronic acid & cationic polymer shell | Hyaluronic acid & Magnetic force (for MSCs) | MRI | Gene delivery (TRAIL plasmid) to MSCs | Human MSCs; U87MG orthotopic xenograft model | Huang et al. [63] |
| Quantum Dot-Biopolymer-Drug Nanohybrids (ZnS@CMC-DOX) | ZnS quantum dot core; Carboxymethylcellulose shell | None | Optical (fluorescence) | Doxorubicin | GBM & healthy cells | Mansur et al. [70] |
| Amino Acid Modified PSC-Capped QDs | AgInS2 quantum dot core; CMCel-L-cysteine or -Poly-L-arginine shell | L-cysteine/poly-L-arginine | Optical (fluorescence) | None | Glioma cells | Carvalho et al. [71] |
| Chlorotoxin-Conjugated Magnetic NPs (IONP-PTX-CTX-FL) | Iron oxide core; PEG, Cyclodextrin, CTX, Fluorescein shell | Chlorotoxin | MRI; Optical (fluorescein) | Paclitaxel | GBM & drug-resistant GBM cells | Mu et al. [69] |
| Mechanism Study of Poly-l-lysine Mediated MNP Uptake | Magnetic NP core; Dextran or Poly-l-lysine shell | Poly-l-lysine | N/A | None | Human glioma & HeLa cells | Siow et al. [68] |
| Low-Molecular-Weight Hyaluronic Acid-SPIONs (Theranostic) | Fe3O4 core; Low-MW hyaluronic acid shell | Low-MW Hyaluronic acid | MRI (T2*) | Intrinsic cytotoxicity (34% inhibition) | U87MG GBM & NIH3T3 fibroblast cells | Chang et al. [64] |
| Low-Molecular-Weight Hyaluronic Acid-SPIONs (Diagnostic) | Fe3O4 core; Low-MW hyaluronic acid shell | Low-MW Hyaluronic acid | MRI (T2*) | None | U87MG GBM & NIH3T3 fibroblast cells | Huang et al. [65] |
Emerging modalities are increasingly integrated into PSC designs to provide functional and quantitative data. Positron Emission Tomography (PET) and Single-Photon Emission Computed Tomography (SPECT) offer exceptional sensitivity and unlimited tissue penetration, overcoming the depth limitations of OI [51]. By stabilizing radiotracers, PSCs can map molecular processes, such as amino acid uptake [18F]-FET PET), to assess tumor metabolism undetected by structural MRI [52, 53]. Hybrid PET/MRI systems combine the anatomical resolution of MRI with the quantitative sensitivity of PET [54]. Furthermore, acoustic modalities offer unique capabilities. Photoacoustic imaging (PAI) leverages PSCs carrying strong light absorbers (e.g., gold NPs) to generate high-resolution, non-invasive images [55]. Ultrasound (US) serves a dual role: beyond its use in FUS-mediated BBB disruption, it can be used for imaging or as an external trigger for controlled drug release from engineered PSC nanobubbles [56, 57].
In summary, while MRI and OI remain the cornerstones of clinical translation for PSC-based imaging, the field is advancing toward sophisticated multimodal systems. These platforms integrate the quantitative metabolic insights of PET/SPECT and the functional capabilities of photoacoustic and ultrasound imaging [58, 59]. The modular nature of PSCs allows for the incorporation of BBB-targeting ligands, multiple imaging moieties, and stimuli-responsive features (e.g., pH/redox sensitivity) to boost signal strength specifically within the tumor. This integrated approach aims to overcome the limitations of single-modality imaging, enabling more precise diagnosis, real-time intervention, and rigorous monitoring of therapeutic outcomes in GBM [60, 61].
3.1 PSC-Based Probes for Magnetic Resonance Imaging
Multiple studies demonstrate that HA-conjugated IONPs exhibit superior T2-weighted contrast compared to non-targeted controls, directly correlated to their accumulation in CD44-overexpressing GBM cells [62]. Another investigation used HA conjugation to low-molecular-weight SPIONs, demonstrating strong T2* signal changes that correlated with significant NP accumulation in GBM cells but minimal uptake in healthy fibroblasts [65]. Shared characterization methods typically include DLS, zeta potential, and various spectroscopic analyses, while in vitro experiments commonly employ U87 glioma cells to confirm preferential internalization [63, 64].
Several HA-coated IONP systems feature similar size distributions, generally ranging from 10 to 100 nm, and exhibit low cytotoxicity in vitro. Dual-targeting ligands, such as folic acid, can further enhance receptor-mediated binding to specific GBM subpopulations, although other designs leverage HA alone or in combination with surface peptides to improve BBB crossing or intratumoral penetration [65]. Despite various promising in vitro results, most studies rely on small animal models that may not capture the complexity of human gliomas, highlighting the importance of larger-scale investigations. Exploiting the CD44-mediated uptake and magnetically responsive cores of these HA-based iron oxide probes shows promise for precise tumor visualization, targeted drug delivery, and potentially broader clinical applications. Crucially, a distinction must be made here: while many HA-iron oxide formulations serve solely as passive T2-weighted contrast agents, true theranostic designs further capitalize on the magnetic core for hyperthermia or conjugate chemotherapeutics (e.g., Doxorubicin) to the PSC shell. This transition from a diagnostic probe to a theranostic agent enables the simultaneous monitoring of tumor boundary reduction and therapeutic response. Future work might delineate strategies for integrating multiple targeting moieties or therapeutic payloads to amplify their imaging and cytotoxic effects, but efforts must address safety and pharmacokinetics in clinically relevant settings (Figure 2A-C) [62, 63].
Chitosan and dextran have also been widely adopted to stabilize, functionalize, or crosslink iron oxide cores for MRI-guided GBM interventions. Dextran coatings often confer colloidal stability, reduce cytotoxicity, and allow traceability under MRI. For example, crosslinked dextran-coated IONPs loaded with antisense oligonucleotides enabled image-guided tracking and showed therapeutic potential in orthotopic glioma models. Another study assessed Chitosan-dextran superparamagnetic NPs in rodent glioma models, reporting a uniform particle size of about 55 nm and significant T2 contrast enhancement in the tumor region [64]. These findings indicate that Chitosan can significantly improve cellular uptake and tumor retention, although its elevated charge requires optimization to mitigate potential off-target effects.
Other works have examined chitosan alone as a coating agent for Fe3O4, emphasizing its potential in hyperthermia and its role as a dual-action chemotherapeutic carrier [65, 66]. Comparisons reveal that dextran-stabilized systems frequently exhibit higher stealth characteristics in blood-based assays, whereas chitosan formulations demonstrate stronger cellular internalization. Nonetheless, many investigations use limited in vitro experiments or small-scale animal studies, so the generalizability of robust internalization or hyperthermic outcomes to complex human tumors remains uncertain. Furthermore, specialized clinical equipment may be required for approaches involving strong external magnetic fields or hyperthermic conditions, underscoring the need for continued research on scalability and safety.
Other PSCs including cyclodextrins and CMC, have likewise demonstrated potential to enhance metal-based probes for GBM theranostics. One example employed gold-iron oxide nanohybrids coated with β-cyclodextrin-chitosan polymers for siRNA or miRNA loading, enabling combined optical and magnetic imaging, along with gene-silencing properties. This platform supported fluorescence and MRI-based molecular imaging and showed the capacity to bypass physiological barriers via intranasal delivery. Another approach used cyclodextrin-bearing IONPs conjugated to CTX, reporting improved efficacy against drug-resistant GBM cells and stable dispersion under physiological conditions [67]. These findings highlight cyclodextrins' usefulness in entrapping hydrophobic payloads or enabling host-guest interactions, potentially broadening the range of anticancer agents that can reach the brain.
CMC has been used to cap and functionalize QDs or to dope iron oxide NPs with chemotherapeutic agents [68]. Attaching CMC to ZnS or AgInS2 cores yields nanocrystals with a stable, water-dispersed shell and robust fluorescence, allowing cell labeling and monitoring in glioma models. Similar systems that conjugate DXR to carboxymethylated PSC shells exhibit pH-sensitive drug release, promoting payload release in the acidic tumor environment [69]. Though promising, concerns remain regarding toxic byproducts released during quantum dot or cyclodextrin-based NP degradation, warranting rigorous in vivo toxicity assessments. Ensuring safe accumulation patterns and reliable therapeutic outcomes in more advanced models remains critical before clinical translation.
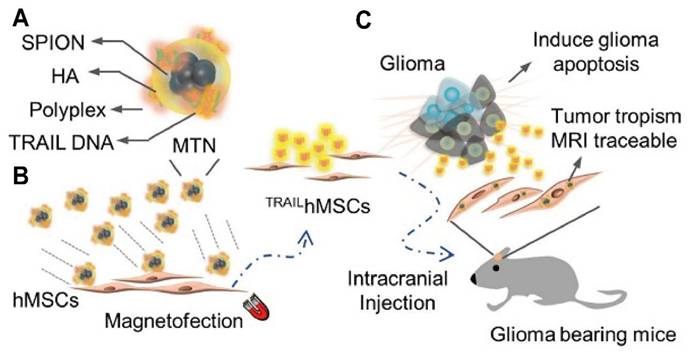
Schematic illustration of the generation and therapeutic application of TRAIL-engineered human mesenchymal stem cells (TRAILhMSCs) for glioma therapy. (A) hMSCs are modified via magnetofection. This process utilizes a magnetic nanoparticle complex consisting of SPIONs, HA, polyplex and plasmid DNA encoding for TRAIL, along with a magnetofection agent (MTN). (B) The resulting TRAILhMSCs are engineered for enhanced tumor tropism and MRI traceability. (C) Following intracranial injection into glioma-bearing mice, TRAILhMSCs migrate towards glioma. The expressed TRAIL protein on the surface of the hMSCs induces apoptosis in glioma cells, leading to tumor regression, while the SPIONs allow for non-invasive tracking of the cells via MRI. Adapted with permission from reference [63]. Copyright 2019 Ivyspring International Publisher (open access).
Less common but still compelling PSCs such as tragacanth and PST001 have also been investigated for metal NP stabilization in GBM applications. One study employed tragacanth to synthesize NiO nanosheets (18-43 nm), which displayed moderate photocatalytic dye degradation (60-82%) and cytotoxic effects against U87MG cells at higher concentrations, with an IC50 of 125 µg/mL [70]. These nanosheets exhibited favorable crystallinity and surface properties, suggesting potential for concurrent imaging based on NiO's optical characteristics, though direct MRI evidence remains limited. Another group developed DXR-loaded carboxymethylated PST001-coated iron oxide NPs that showed improved biocompatibility, enhanced intracellular uptake, and significant reactive oxygen species generation in both two-dimensional and 3D culture models [71]. While these materials demonstrate innovative surface chemistries and promise for GBM therapy, their ability to cross the BBB or deliver clinically meaningful theranostic performance requires further large-scale in vivo testing [72].
Collectively, enlisting these diverse PSCs to coat iron oxide or other metal NPs has enabled significant advancements in imaging contrast, tumor selectivity, and drug delivery. HA-MSA2 systems stand out for their CD44-mediated uptake and strong MRI signals, while chitosan offers higher uptake efficiency through electrostatic interactions, and dextran coatings generally improve circulation time. CD and CMC-based platforms broaden the scope of loaded therapeutics and imaging modalities, although attention to potential toxic byproducts remains paramount. Less common PSCs contribute distinctive surface chemistries but still need substantial in vivo validation to confirm their translational potential. Ongoing efforts must address biodistribution, immunogenicity, and scalability, ensuring that these PSC-based nanosystems can offer safe and effective theranostic solutions for GBM in clinical practice [73].
3.2 PSC-Based Probes for Optical (Fluorescence and Near-Infrared) Imaging
BAA-HA-MSA2-MSA2 remains a promising targeting ligand for GBM therapy, particularly when combined with NIR imaging for enhanced tumor visualization. A multifunctional nanocomplex exemplifying this role integrates B NSs and Au NPs with Ag2S, then caps the construct with BAA-BAA-HA-MSA2-MSA2 [74]. The system leverages the NIR-II fluorescence from Ag2S to enable deep-tumor imaging, while photothermal and sonodynamic activities bolster tumor ablation. The HA coating facilitates tumor-specific accumulation, and US-assisted microbubble disruption transiently opens the BBB, improving localization in GBM tissue. Although researchers observed robust anti-tumor immune responses and immunogenic cell death, the limited number of in vivo subjects constrains broader clinical extrapolation. Nonetheless, integrating HA with NIR-based approaches offers an encouraging direction for tumor-targeted imaging and therapy with greater spatiotemporal precision.
CMC is a versatile PSC matrix that can incorporate QDs for fluorescence-based imaging in GBM cells. For instance, cationic ε-poly-l-lysine-based ZnS QDs assemble with anionic CMC-encapsulated magnetite nanozymes via electrostatic interactions, forming hybrid organic-inorganic stimuli-responsive nanoplexes [75]. Unlike the purely diagnostic QDs discussed previously, these nanoplexes represent a dual-function theranostic platform: the QDs provide bright luminescence for tracking, while the magnetite nanozymes enable concurrent chemodynamic therapy. The QDs provide bright luminescence for imaging in both two-dimensional and 3D spheroid models, while the magnetite nanozymes enable chemodynamic therapy and magnetohyperthermia. Another approach involves supramolecular QD-biopolymer-drug assemblies, where ZnS QDs capped with CMC are conjugated with DXR, facilitating simultaneous bioimaging and chemotherapy. A parallel strategy uses CMC functionalized with cell-penetrating and pro-apoptotic peptides to complex fluorescent AIS semiconductor nanocrystals, resulting in multimodal imaging and potent cytotoxic effects against GBM cells [76]. Although these studies converge on the capacity of CMC to stabilize QDs, NP size and luminescence intensity vary due to differences in QD core composition and the inclusion of therapeutic moieties. A key limitation arises from predominantly in vitro protocols, which may not capture in vivo pharmacokinetics or fully represent the tumor microenvironment. Nonetheless, these findings collectively highlight the potential of CMC-based fluorescent nanoplatforms to advance theranostic applications in GBM.
Dextran, a commonly used PSC for NP coating, enhances the stability, biocompatibility, and imaging capabilities of IONPs. One dextran-based nanosystem featured crosslinked iron oxide cores labeled with Cy5.5 for dual-modal MRI and optical imaging. Its 25 nm cores and crosslinked shell allowed preferential accumulation in orthotopic GBM following intravenous injection, while concurrently delivering antisense oligonucleotides against oncogenic miRNA. Although variability in dextran thickness and crosslinking density can influence biodistribution and clearance, this platform demonstrated transvascular passage and consistent imaging signals in glioma-bearing models. Optimizing coating strategies or further tailoring the shell could improve targeting accuracy while preserving robust imaging contrast.
Chitosan, another abundant PSC, can be adapted for tumor-targeted drug delivery and fluorescence-based imaging. One example employs selenium-Chitosan NPs loaded with TMZ, coated with Eudragit® RS100 polymer, and tracked through encapsulated curcumin [77]. The innate fluorescence of curcumin confirms NP uptake by glioma cells, and Chitosan-based carriers lower the IC50 while downregulating TMZ resistance genes. However, curcumin's photobleaching limits extended imaging capability. Still, combining Chitosan-mediated tumor targeting with fluorescent labeling offers a promising method for monitoring drug release and therapeutic efficacy in GBM [78].
Collectively, PSC-based vehicles composed of HA, CMC, dextran, or chitosan present complementary strategies for coupling robust imaging with tumor-directed therapies in GBM. By incorporating fluorescent or NIR markers, each system enables real-time tumor visualization, more accurate drug delivery, and enhanced assessment of therapeutic effectiveness [79]. Although preclinical studies underscore their promise, variations in particle composition and the complexity of the BBB remain key challenges. Standardizing imaging parameters, addressing scale-up, and evaluating long-term safety are essential next steps in translating these platforms to clinical settings. Continued research may ultimately yield safe, effective, and clinically feasible PSC-based nanotechnologies for targeted GBM therapy.
4. PSC-Based Systems for GBM Therapy
PSCs are increasingly employed to deliver chemotherapeutic drugs and genetic materials to GBM cells, owing to their biocompatibility, biodegradability, and adaptable chemical structures. These properties support the development of nanocarriers that address critical GBM challenges, including the BBB, tumor heterogeneity, and drug resistance. By modulating parameters such as molecular weight, substitution degree, and surface properties, PSC nanocarriers achieve enhanced stability, targeted delivery, and prolonged circulation, thereby improving the therapeutic index. Their utility spans a wide range of payloads, from conventional anticancer drugs to gene-based treatments, all while mitigating off-target effects. Furthermore, conjugations with PEG or targeting ligands augment immune evasion and strengthen site-specific accumulation. Recent studies have explored various PSC platforms, capitalizing on stimuli-responsive linkers to enable controlled release within the tumor microenvironment. Through these design strategies, PSC-based platforms show promise in addressing GBM's complex pathology and improving patient outcomes.
4.1 Chitosan-based approaches
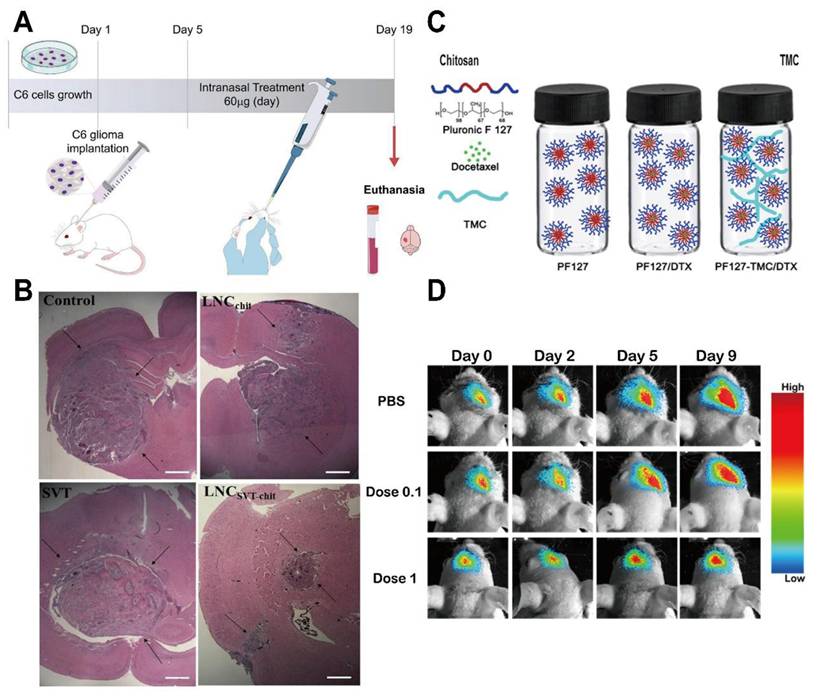
Chitosan-based nanocarriers have garnered attention for intranasal and systemic delivery (Table 2) [80]. For example, nanocapsules utilizing chitosan's mucoadhesive properties significantly reduced glioma size in mouse models (Figure 3A, B) [81]. Other approaches involve thermosensitive hydrogels loaded with drugs like docetaxel (DTX) (Figure 3C), which demonstrated significant inhibition of glioma growth in preclinical models (Figure 3D) [82]. Additionally, drug delivery systems using chitosan have been developed for post-surgical local treatment to prevent glioma recurrence (Figure 4A). In such studies, the drug delivery system is implanted into the tumor remnant cavity after resection (Figure 4B), showing significant inhibition of subsequent tumor growth in an animal model (Figure 4C) [83]. Beyond small molecule agents, chitosan-based systems can also preserve the biological activity of macromolecules. One study demonstrated that trimethyl chitosan (TMC) NPs encapsulating an IGF-1R inhibitor (IGF-Trap) yielded superior intracerebral drug uptake and prolonged survival in mouse glioma models [84].
Additional research underscores the versatility of chitosan-based carriers in accommodating multiple functional elements. Magnetic graphene oxide modified with chitosan and conjugated to gastrin-releasing peptide allowed dual magnetic and receptor-mediated DXR targeting. This system achieved pH-responsive release, significantly lowering the IC50 in GBM cells [27]. Another platform combined silicon NP with a chitosan coating, extending circulation time in tumor-bearing mice and favoring intratumoral accumulation through the EPR effect [85]. Chitosan-coated iron oxide NP similarly demonstrated reduced cytotoxicity in C6 glioma cells compared to uncoated variants, implying that the polymeric layers can mitigate metal-associated toxicity while bolstering anticancer efficacy [86]. Moreover, surface alterations such as chemical modifications to chitosan can improve its solubility at physiological pH. Trimethyl chitosan (TMC), for example, has garnered special interest for its ease of functionalization, enabling ligand attachment that bolsters targeting efficiency and overall therapeutic potential.
Studies have highlighted TMC-based carriers co-functionalized with ligands such as RGD or folate, which anchor selectively to overexpressed receptors in GBM [87]. One investigation described a TMC NP platform functionalized with RGD and L-arginine that directed cytotoxic activity primarily toward cancer cells in a preclinical model, sparing healthy tissues and underlining the specificity conferred by these ligands. Another effort demonstrated folic-acid-conjugated chitosan NP, resulted in improved mucoadhesion and cytotoxic activity in vitro [88]. Additionally, chitosan has been derivatized via N-alkylation to form micelle-like structures, facilitating translocation of macromolecular payloads across the BBB [89].
Chitosan-Based Approaches
| Approach/Method Name | Carrier System | Payload/Active Agent | Targeting/Delivery Strategy | Key Finding/Performance Metric | Validation Model | Authors |
|---|---|---|---|---|---|---|
| MTX-Chitosan-HPMCP NPs | Chitosan-HPMCP NPs | Methotrexate (MTX) | P-gp efflux inhibition; Mucoadhesion | Enhanced cytotoxicity (IC50 = 68.79 vs 80.54 µg/mL for free MTX) | U251MG GBM cells | Naves et al. [84] |
| iNSC-laden Injectable Chitosan Hydrogel | Injectable thermo-responsive hydrogel | Induced neural stem cells (iNSCs) | Post-surgical local cell delivery | 50% increase in median survival vs iNSCs alone | Post-surgical GBM mouse model | King et al. [97] |
| Magnetic GO/Chitosan/Iron Oxide Microspheres | Graphene oxide/chitosan/iron oxide microspheres | Temozolomide (TMZ) | Magnetic field- and pH-sensitive release | Drug release doubled in 90 min with 100 Hz magnetic field | GBM cells (MTT assay) | Ahmadi et al. [16] |
| AT101-conjugated Chitosan Nanobubbles | Chitosan nanobubbles (NBs) | Delivery platform (unloaded) | GPC1 protein targeting via AT101 antibody | Increased specific tumor delivery compared to unconjugated NBs (p=0.02) | U-87 MG xenograft model | Cintio et al. [93] |
| Macroporous Alginate-Chitosan Hydrogel | Macroporous alginate-chitosan hydrogel | Cell trap (no drug) | Physical trapping of GBM cells for subsequent radiotherapy | F98 GBM cells accumulate and are retained within the gel matrix | F98 GBM cells | Parès et al. [99] |
| TPP-conjugated Chitosan NPs | Triphenylphosphonium (TPP+)-conjugated chitosan NPs | Temozolomide (TMZ) | Mitochondrial targeting via TPP+; Intranasal delivery | Entrapment efficiency of 93.59%; Greater nasal mucosal retention | Goat nasal mucosa (ex vivo); In vitro cell lines | Dahifale et al. [91] |
| Chemo-Immunotherapy Crosslinked Hydrogel | Pluronic F-127/Chitosan thermo-responsive hydrogel | Doxorubicin (DOX), BMS-1 | Intratumoral injection for synergistic chemo-immunotherapy | Tumor 43 times smaller than untreated group | GBM tumor-bearing mouse model | Chuang et al. [191] |
| Etoposide-loaded Chitosomes | Chitosomes (chitosan-coated liposomes) | Etoposide | Parenteral administration; Enhanced stability | Heightened efficacy against the U373 GBM cell line | U373 cell line | Gonzalo et al. [85] |
| CRT-functionalized Chitosan/HA Nano-emulsion | Chitosan/hyaluronic acid layered nano-emulsion | Paclitaxel | CRT peptide targeting of transferrin receptor on BBB | 41.5% higher uptake in brain endothelium cells than negative control | bEnd.3 cells (BBB model) | Capua et al. [94] |
| DOX-loaded LLPs in Chitosan/HA/PEI Hydrogel | Liposome-like particles in Chitosan/HA/PEI hydrogel | Doxorubicin (DOX) | Controlled local delivery in resection cavity | Sustained drug release up to 148 hours | GBM spheroids; In vitro cell studies | Adiguzel et al. [98] |
| Gemcitabine Chitosan-coated PLGA NPs | Chitosan-coated PLGA NPs | Gemcitabine (GEM) | Intranasal delivery; Mucoadhesion | Promoted GEM antiproliferative activity and sensitized cells to TMZ | U215 and T98G human GBM cell lines | Ramalho et al. [103] |
| GRP-conjugated Magnetic Graphene Oxide | Chitosan-coated magnetic graphene oxide (mGOC) | Doxorubicin (DOX) | Dual active (GRP peptide) and magnetic targeting | Best potency to suppress tumor growth and prolong animal survival | Orthotopic U87 brain tumor model in mice | Dash et al. [27] |
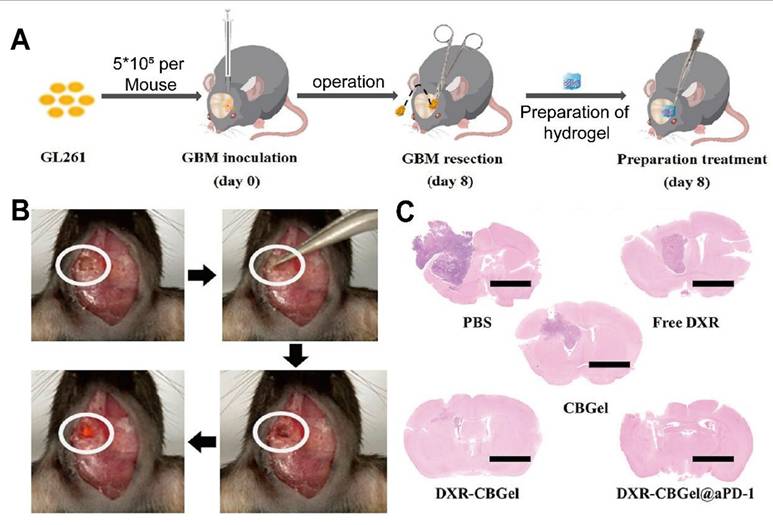
| DXR-loaded Self-crosslinked Hydrogel (DXR-CBGel) | BSA NPs self-crosslinked with chitosan | Doxorubicin (DXR), anti-PD-1 antibody | Localized chemoimmunotherapy as an in situ vaccine | Effectively inhibited cancer recurrence post-surgery | GBM lesions (animal model) | Long et al. [83] |
| Review of Polymeric NPs for GBM | Chitosan, PLGA, PEG, etc. NPs | Various chemotherapeutics | Active targeting with ligands (transferrin, chlorotoxin, etc.) | Review article summarizing multiple strategies | N/A (Review) | Paula et al. [92] |
| 3D Chitosan-Hyaluronic Acid Scaffolds | Chitosan-hyaluronic acid (CHA) porous scaffold | 3D tumor model (no drug) | High-throughput screening (HTS) platform | Produced uniform response (CV < 0.15) and wide screening window (Z' > 0.5) | Three human GBM cell lines | Zhou et al. [108] |
| Curcumin-Chitosan Nano-complex | Curcumin-chitosan nano-complex | Curcumin | Epigenetic modification via gene expression regulation | Significantly increased MEG3 and decreased DNMT gene expression | GBM cell line | Abolfathi et al. [106] |
| Chitosan-functionalized Silicon NPs | Chitosan-coated Silicon NPs (SiNPs) | Silicon NPs (photosensitizer) | Passive targeting via Enhanced Permeability and Retention (EPR) effect | Tumor accumulation increased to 39.55% after 7 days | Nude mice with subcutaneous human GBM | Baati et al. [88] |
| Sialic Acid-functionalized Selenium NPs@Chitosan | Sialic acid-coated, chitosan-stabilized Se NPs | Selenium NPs (Se NPs) | Surface modification with sialic acid for improved stability/activity | Dose- and time-dependent inhibitory effects on T98 GBM cells | T98 and A172 GBM cell lines | Abadi et al. [95] |
| TMZ-loaded Chitosan-based Thermogels | Chitosan-β-glycerophosphate thermogel with SiO2/PCL microparticles | Temozolomide (TMZ) | Local delivery into post-resection cavity | Caused a significant reduction in the growth of tumor recurrences | GBM resection and recurrence mouse model | Gherardini et al. [109] |
In parallel, chitosan's utility extends to functionalization strategies that target specific receptors or enable unique trans-barrier transport. Anti-glypican-1 antibody (AT101)-conjugated chitosan nanobubbles selectively accumulated in glypican-1-overexpressing GBM in a mouse model, significantly augmenting tumor binding relative to unmodified counterparts [90]. Triphenylphosphonium (TPP⁺)-conjugated chitosan formulations likewise improved mitochondrial targeting in GBM cells, thereby enhancing the cytotoxic effectiveness of TMZ. A consecutive layering of chitosan and HA in nano-emulsions, further functionalized with a CRT peptide, yielded increased uptake across BBB-cell models by exploiting transferrin receptor binding [91]. Explorations of sialic-acid-covered chitosan NPs add another dimension by taking advantage of sialic acid's tumor-targeting capabilities and stabilizing effects.
Study of Chitosan in the treatment of glioma. (A) Nanocapsules coated with Chitosan and loaded with simvastatin are administered intranasally to avoid the BBB and improve drug delivery efficiency. (B) After 14 days of treatment, gliomas in the experimental group were significantly smaller than those in the control group. (C) The thermosensitive hydrogel was prepared with PF127 and N,N, N-TMC as raw materials and loaded with DTX for GBM. (D) PF127-TMC/DTX gel with high concentration can significantly inhibit the growth of glioma cells in vitro. Adapted with permission from reference [81, 82]. Copyright 2022 Elsevier B.V. and 2018 Elsevier Ltd., respectively.
Beyond nano-scale delivery, chitosan-based hydrogels facilitate localized and sustained drug release, particularly beneficial following glioma resection [92]. In situ gelation triggered by temperature changes or crosslinking can achieve prolonged retention of therapeutic agents at the tumor site. One example involved an injectable chitosan hydrogel loaded with tumoricidal neural stem cells (iNSCs), resulting in long-term survival benefits and increased median survival in animal studies relative to direct stem cell injections [93]. Another formulation employed DXR-loaded liposome-like particles (LLP-DOX) in a chitosan/hyaluronic acid/polyethyleneimine (CHI/HA/PEI) matrix, providing gradual drug release and substantially reducing the viability of three-dimensional (3D) GBM spheroids [94]. A further approach used a dual-crosslinked macroporous hydrogel composed of sodium alginate and chitosan, which effectively sequestered GBM cells post-resection in an animal model without overwhelming healthy tissues [95]. Additionally, an albumin-based hydrogel crosslinked with chitosan extended intratumoral retention of Dox in a mouse model, leading to immunogenic cell death and minimal systemic toxicity [96]. Although these matrices require precise tuning of mechanical properties and may rely on suitably sized resection cavities, they offer a promising route to contain residual glioma cells and reduce the risk of recurrence.
Intracavity-filled Chitosan system significantly suppressed tumor recurrence. (A) Study of DOX-loaded bovine serum albumin NPs and chitosan self-crosslinking drug delivery system in glioma recurrence after surgery. (B) Tumor resection was performed on the 8th day after tumor inoculation, and drug delivery systems of different groups were implanted in the tumor remnant cavity. (C) H&E staining on the 18th day of each group showed that the experimental group had significant inhibition on tumor growth after glioma surgery. Adapted with permission from reference [83]. Copyright 2023 American Chemical Society.
Multi-modal or stimuli-responsive chitosan formulations further broaden therapeutic options by merging magnetic, photothermal, or pH-sensitive functionalities. One pH- and magnetically responsive system incorporated iron oxide NPs into a chitosan-graphene oxide matrix, delivering TMZ under external magnetic field stimulation and accelerating drug release within acidic tumor environments [97]. This system functions as a true theranostic platform: the iron oxide core enables T2-weighted MRI tracking of tumor accumulation, while the chitosan shell concurrently manages the stimuli-responsive release of the chemotherapeutic payload. Others employed NIR photothermal conversion using platinum-gold nanorods layered in a chitosan/polycaprolactone scaffold, which yielded extended TMZ release and a high cell-killing rate in GBM cultures under NIR irradiation [98, 99]. In another approach, radiofrequency-triggered hyperthermia in chitosan-coated silicon NPs intensified tumor cell death in preclinical tests. Chitosan is also conducive to photodynamic therapy, exemplified by berberine-based chitosan nanosystems that induced apoptosis in T98G cells while sparing healthy astrocytes [100]. Successful integration of these stimuli hinges on precise field delivery and minimal off-target damage, yet the adaptive nature of chitosan indicates that such hurdles may be overcome with design refinements and advanced instrumentation.
Combining therapeutic modalities appears increasingly feasible with chitosan carriers, which can integrate several agents or strategies in a single platform. For instance, chitosan NPs loaded with gemcitabine heightened the susceptibility of glioma cells to TMZ, achieving synergistic apoptotic effects [101, 102]. Dual photothermal-chemotherapeutic regimens have also been devised. For example, HA-modified chitosan-lipid NPs co-loaded with cisplatin and iron oxide, were guided externally to glioma tissue and activated by NIR light to enhance cytotoxic potency in vivo [103]. Curcumin-chitosan nanocomplexes further exemplify the potential for epigenetic reprogramming, as they modulated DNA methyltransferase gene expression and displayed robust dose- and time-dependent cytotoxicity in GBM cells [104]. While precise attribution of efficacy to each individual component remains challenging, these multi-pronged systems generally yield greater tumoricidal effects than single-agent regimens.
Collectively, these diverse approaches illustrate chitosan's broad applicability to GBM therapy, ranging from NP constructs and hydrogels to complex multi-modal regimens [105]. Three-dimensional tumor models employing chitosan-HA scaffolds emphasize the polymer's promise for both drug delivery and high-throughput drug screening, as the scaffold's pore size modulates GBM cell behavior and resistance [106]. Simultaneously, stealthy chitosan-capped silicon NPs capable of extended circulation underscore emerging strategies to prolong half-life and enhance tumor accumulation [107]. Despite these encouraging findings, many studies rely on limited preclinical models, do not include standard comparators, or assess only short-term outcomes. Moving forward, comprehensive validation in intracranial models, standardized efficacy metrics, and large-scale manufacturing protocols will be vital for translating chitosan-based nanomedicines into effective clinical solutions against GBM.
4.2 HA-based approaches
HA has emerged as a promising PSC-based platform for targeting GBM due to its intrinsic tumor-homing capabilities [108]. By selectively binding these receptors, HA-based carriers facilitate the uptake of chemotherapeutics within tumor tissues while minimizing off-target effects [109]. Moreover, HA's natural biocompatibility, biodegradability, and relatively low immunogenicity support its application in NP formulations for efficient and well-tolerated drug delivery. Preclinical studies show that HA-based nanocarriers accumulate in GBM cells and exploit local microenvironmental conditions to enable targeted release, ultimately improving intracranial drug distribution. Taken together, these advances underscore HA's potential to serve as a versatile vehicle that can cross the BBB and enhance therapeutic specificity (Table 3).
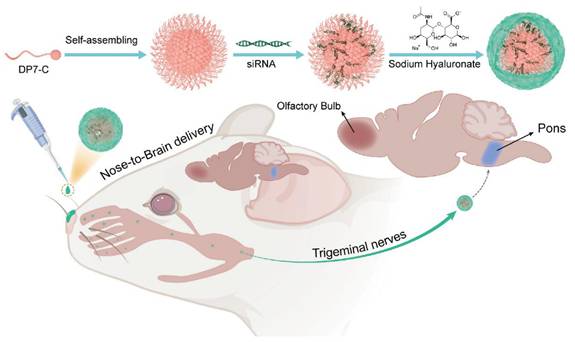
Numerous investigations use HA to functionalize NPs for enhanced drug delivery and imaging. For example, HA-modified copolymers are recognized by CD44 receptors on glioma cells. This interaction leads to cellular uptake in vitro through mediated endocytosis, as depicted in Figure 5A [110]. Once at the target, HA-drug conjugates can also improve the local efficacy of chemotherapy and immunotherapy [111]. To circumvent the BBB, other approaches in animal models show that nasally administered HA-modified nanomicelles can be rapidly transported to the brainstem via the trigeminal pathway, achieving efficient delivery to gliomas (Figure 5B) [112]. Several reports describe magnetic or metallic NP cores coated with HA to achieve tumor selectivity, stability, and biocompatibility. For instance, one study established superparamagnetic iron oxide NPs (SPIONs) stabilized with an HA coating, which provided excellent colloidal stability, improved biocompatibility, robust GBM cell uptake, in vitro, and T2 contrast potential for MRI [62, 89].
HA-modified lipid-based systems have also shown promise in GBM therapy by encapsulating or conjugating various payloads. One investigation encapsulated Dox in HA-liposomes to target CD44-overexpressing GBM cells, revealing a marked increase in tumor growth inhibition in an animal model relative to free Dox [113]. However, comparative evaluations of HA-systems reveal a "binding site barrier" phenomenon. High-affinity HA nanocarriers often bind irreversibly to CD44 receptors at the tumor periphery, effectively preventing penetration into the hypoxic, necrotic core where resistant stem cells reside. This suggests that while HA enhances tumor accumulation, simply maximizing receptor affinity may be counterproductive; optimizing ligand density to allow for detachment and deeper diffusion is likely required for superior efficacy (Figure 6) [114].
Hyaluronic Acid-Based Approaches
| Approach/Method Name | HA-based Core Structure | Therapeutic/Diagnostic Payload | Mechanism/Function | Additional Targeting Moiety | Validation Model | Authors |
|---|---|---|---|---|---|---|
| HA-SPION Dual-Targeted System | Superparamagnetic Iron Oxide Nanoparticle (SPION) | SPION (MRI contrast) | Dual-targeted diagnosis and therapy | Folic acid | Glioma/adenocarcinoma cells (in vitro) | Kasprzyk et al. [62] |
| CHI/HA/PEI Hydrogel with LLP-DOX | Hydrogel (Chitosan/HA/PEI) | Doxorubicin in liposomes | Local, controlled drug release | N/A (local delivery) | GBM spheroids (3D in vitro) | Adiguzel et al. [98] |
| IR780-rGO-HA/DOX Nanoplatform | Reduced Graphene Oxide (rGO) | Doxorubicin; IR780 (photosensitizer) | Multimodal therapy (Chemo/PTT/PDT) | N/A | U87 cells; Xenograft tumor model (in vivo) | Dash et al. [111] |
| ICOVIR17-MSC in sECM | Synthetic ECM (targets endogenous HA) | Oncolytic virus expressing hyaluronidase | Oncolytic virotherapy; ECM degradation | N/A | GBM resection mouse model; PDX xenografts | Martinez et al. [119] |
| RGD-Functionalized HA 3D Scaffold | Biomaterial scaffold | N/A (model system) | 3D in vitro model for studying chemoresistance | RGD peptide (Integrin targeting) | Patient-derived GBM cells | Xiao et al. [118] |
HA-based NPs improve the integration of glioma diagnosis and treatment. (A) HA-modified copolymers are recognized by CD44, which is overexpressed on glioma cells, and are taken up through CD44-mediated endocytosis. (B) HA-modified nanomicelles can be administered nasally and rapidly transported to the pontine through the trigeminal pathway, avoiding the BBB and achieving efficient delivery of gliomas. Adapted with permission from reference [110, 112]. Copyright 2018 American Chemical Society and 2021 Elsevier B.V., respectively.
Schematic illustration of hyaluronan-enveloped peptide nanomicelles for the treatment of glioma. HA-drug conjugate can improve the efficacy of local chemotherapy and immunotherapy for glioma. Adapted with permission from reference [114]. Copyright 2023 Elsevier Ltd.
Genetic material delivery via HA coatings further illustrates the scope of these nanotechnologies. HA-grafted lipid NPs were reported to deliver siRNA against Polo-Like Kinase 1, achieving over 80% gene knockdown and significantly prolonged survival in an orthotopic GBM model [114]. One group developed HA-lipid NP systems designed to deliver RNA interference (RNAi) constructs directly to GBM cells in culture, achieving favorable uptake and silencing efficiency. Another study used a similar HA-lipid platform to deliver miR-181a, prolonging survival in an in vivo tumor model by enhancing accumulation in GBM tissue [115]. Overall, HA-functionalized NPs show considerable promise for improving diagnostic accuracy, therapeutic efficacy, and multimodal approaches in GBM management, though further validation is necessary to confirm their in vivo stability and safety.
Efforts have also focused on HA-based hydrogels designed to provide localized therapy. Injectable or implantable hydrogel composites formed from HA and other polymers offer sustained drug release in situ, aligning mechanical properties with those of brain tissue and improving intracranial drug retention. One group demonstrated CHI/HA/PEI hydrogels that entrapped LLP-DOX, slowing drug release in vitro over 148 hours while preserving low genotoxicity [69]. Another investigation employed a cucumber uril-crosslinked HA hydrogel with 98% water content, facilitating straightforward placement within resection cavities in a preclinical surgical model and enabling therapeutic antibody delivery [116].
Beyond drug delivery, HA-rich scaffolds are employed to investigate cellular behaviors, including GBM invasion, chemoresistance, and proliferation, in physiologically relevant 3D microenvironments. One report systematically varied HA concentration in a soft matrix and identified in a 3D culture system a biphasic effect on patient-derived GBM invasion, with specific HA thresholds maximizing invasive capacity [117]. Parallel findings demonstrated that CD44 binding via HA, together with integrin interactions, can augment resistance to alkylating agents, more accurately mimicking in vivo response patterns than conventional sphere cultures [118]. Despite the promise of these biomimetic approaches, patient-specific variability in HA-driven invasion emphasizes the need for tailored matrix formulations that capture disease heterogeneity while supporting reproducible screening.
Alternate strategies seek to deplete or remodel HA within the tumor microenvironment instead of incorporating it as a scaffold or coating. One study employed an oncolytic adenovirus encoding soluble hyaluronidase, demonstrating in an animal model that localized HA degradation improved viral dispersion and reduced glioma burden [119]. This strategy stands in contrast to formulations that exploit intact HA-CD44 interactions but presents a complementary avenue to counteract the dense ECM barriers limiting drug and gene vector penetration.
Collectively, these findings establish HA as a multifaceted component in the development of next-generation GBM therapies. HA-based NPs leverage CD44 receptor binding to improve drug uptake, provide MRI contrast, and enable synergistic treatments combining photothermal or photodynamic approaches. HA-centered hydrogels and 3D scaffolds can serve as controlled drug depots and physiologically relevant disease models, facilitating localized therapy and elucidating tumor-ECM interactions. In parallel, strategies that degrade HA in the tumor microenvironment attempt to disrupt physical and molecular barriers to treatment, offering a distinct route for enhancing drug penetration. By capitalizing on HA's unique capacity to bind CD44, mimic native ECM, and modulate therapeutic delivery, PSC-based nanomedicines stand poised to expand the therapeutic landscape for GBM [120, 121].
4.3 Dextran-based approaches
Dextran, a PSC composed predominantly of α-1,6-glycosidic bonds, has received growing attention in GBM therapy due to its ease of chemical modification (Table 4). Its backbone carries multiple hydroxyl groups that enable flexible conjugation to drugs, antibodies, or targeting molecules, thereby tailoring dextran-based nanosystems for specific therapeutic interventions [122, 123]. Such modifications can lessen unwanted plasma protein interactions, prolonging circulation times that heighten drug delivery. The polymer's customizable surfaces help NP evade opsonization and clearance, thus providing sufficient time for transport across the BBB or blood-tumor barrier (BTB) [124, 125]. This extended presence elevates drug residence in the bloodstream and subsequent tumor accumulation, particularly where hydrophilic surfaces and versatile chemical derivatization reduce reticuloendothelial uptake [126]. By finely tuning NP size and surface properties, these systems can traverse biological barriers selectively, concentrating anticancer agents at the desired site [127, 128]. Dextran can be equipped with redox- or pH-sensitive linkers to target intracellular drug release in tumor cells, exploiting high GSH levels or acidic compartments for enhanced specificity [125].
Dextran-Based Approaches
| Approach/Method Name | Dextran Formulation | Payload/Active Agent | Primary Function | Validation Model(s) | Key Innovation | Authors |
|---|---|---|---|---|---|---|
| Dextran Sulfate/LMWP NPs | Ionic complex of dextran sulfate and low molecular weight protamine | Nucleic acids | Non-viral gene delivery | GBM cell lines, 3D spheroids, zebrafish models | Cationic polymeric NPs for gene delivery with negligible toxicity and efficient internalization | Esteban et al. [130] |
| Dexamethasone-loaded dex-HEMA Hydrogel | Dextran-hydroxyethyl methacrylate (dex-HEMA) hydrogel | Dexamethasone (micelles) & dexamethasone phosphate (liposomes) | Localized, prolonged anti-edema drug delivery | In vitro release kinetics studies | Biphasic and sequential drug release from an immobilized dual-nanoparticle hydrogel system | Straten et al. [133] |
| Dextran-Coated Iron Oxide NPs (MN-anti-miR10b) | Crosslinked dextran coating on iron oxide NPs | Antisense oligonucleotide (anti-miR10b), Cy5.5 dye | Image-guided RNA delivery | Orthotopic GBM mouse model | Dual-modal (MRI/optical) tracking of a nano-platform delivering RNAi payload across the BBB | Kim et al. [26] |
| PD0721-DOX Antibody-Drug Conjugate | Dextran T-10 as a linker for conjugation | Doxorubicin (DOX) | Targeted chemotherapy (ADC) | EGFRvIII-positive and negative GBM cell lines | Dextran linker to create an scFv antibody-drug conjugate targeting EGFRvIII-expressing cells | Hu et al. [131] |
| Nano-realgar Hydrogel (NRA@DH Gel) | pH-sensitive dextran hydrogel with hyaluronic acid coating | Nano-realgar quantum dots (NRA QDs) | Synergistic chemo-radiotherapy | In situ GL261 GBM mouse model | Hydrogel acts as a sustainable ROS generator to deplete GSH and enhance radiotherapy efficacy | Wang et al. [135] |
| Biomimetic Dextran NPs | pH-sensitive dextran nanoparticle core | ABT-263 and A-1210477 (Bcl-2/Mcl-1 inhibitors) | Brain-targeted combination drug delivery | GBM cell lines, patient-derived cells, orthotopic GBM mouse model | Biomimetic (RBC membrane, ApoE peptide) coating for BBB penetration and synergistic drug delivery | He et al. [132] |
| Chitosan-Dextran SPIONs (CS-DX-SPIONs) | Hybrid chitosan-dextran coating on SPIONs | SPIONs (contrast agent) | MRI contrast enhancement & targeted delivery | Glioma cell lines, orthotopic C6 glioma rat model | Hybrid chitosan-dextran coating enhances cellular uptake and tumor retention for improved MRI contrast | Shevtsov et al. [66] |
| Acetalated Dextran (Ace-DEX) Fibrous Scaffolds | Acetalated dextran biodegradable fibrous implant | Paclitaxel and Everolimus | Interstitial combination chemotherapy | GBM cell lines, orthotopic GBM resection/recurrence mouse models | Synchronized interstitial release of synergistic drugs from scaffolds with tailored degradation rates | Graham-Gurysh et al. [136] |
| Tunable Acetalated Dextran (Ace-DEX) Scaffolds | Acetalated dextran nanofibrous scaffolds | Paclitaxel | Interstitial therapy with controlled release | Orthotopic GBM resection/recurrence and distant metastasis mouse models | Demonstrating that drug release rate is a critical parameter for efficacy in different tumor models | Graham-Gurysh et al. [137] |
Several groups have harnessed dextran in NP systems alongside other polymers or linkers to advance gene and drug therapy. One formulation hinged on ionic complexation of low molecular weight protamine (LMWP) and dextran sulfate to condense nucleic acids in GBM models [129]. These cationic NP demonstrated effective nucleic acid delivery in both two- and 3D cultures, as well as in zebrafish, but detailed validation against human intracranial complexities remains limited. Another dual-sensitive NP employed dendrimer-dextran conjugation via matrix metalloproteinase- and pH-responsive linkers to release DXR in situ [130]. While this approach improved NP retention and penetration in murine glioma, heterogeneous enzymatic activity could affect uniform disassembly in clinical settings.
In a related strategy, dextran T-10 served as a linker to fuse a single-chain antibody (scFv) recognizing EGFRvIII with DXR, generating an antibody-drug conjugate with enhanced cytotoxicity in EGFRvIII-positive glioma cells [131]. Although this design illustrates the impact of receptor-driven targeting, scaling scFv production and ensuring reproducible conjugation chemistry pose nontrivial challenges. Dextran-based NP have also facilitated combination therapies that tackle redundancy in anti-apoptotic pathways. A pH-responsive dextran platform delivered both ABT-263 and an Mcl-1-selective antagonist in orthotopic xenografts, showing superior efficacy while sparing healthy tissue [132]. Collectively, these examples reinforce dextran's adaptability for drug or gene vector delivery, particularly when linkers exploit pH or enzymatic triggers to refine release. Future development must reconcile disparities between preclinical models and human disease, including robust tumor microenvironment simulations critical for eventual clinical translation.
Beyond NPs, dextran can be formulated into hydrogels or scaffolds for localized drug delivery in GBM. A dextran-hydroxyethyl methacrylate (dex-HEMA) hydrogel embedded with dexamethasone (DXM) micelles and dexamethasone phosphate liposomes produced a biphasic release in vitro, with freely diffusing dexamethasone for up to two weeks before the gel degraded and released the phosphate form [133]. This release profile surpasses conventional steroid regimens in sophistication but requires careful crosslink density calibration to accommodate in vivo variability. Another injectable system combined Dox-loaded ferritin with oxidized dextran to create Schiff base bonds, gradually discharging intact ferritin particles in an animal model [134]. Further expansion of dextran hydrogels includes pH-sensitive designs that encapsulate realgar quantum dots (QDs) and a metabolic inhibitor, achieving synergistic action with radiotherapy in preclinical studies [116]. Additionally, acetalated dextran (Ace-DEX) offers tunable degradation rates, allowing controlled local release of agents such as PTX, everolimus, and Dox in tailored scaffolds. Slow-release versions proved valuable for tumor resection sites, whereas faster release was advantageous for micrometastatic disease, though polymer synthesis variability may challenge clinical standardization. These hydrogel and scaffold concepts underscore dextran's versatility for sustained, site-specific therapy in GBM, particularly when coupled with radiosensitizing strategies. Comparative analyses under uniform intracranial models can elucidate the most effective release profiles, but rodent surgical procedures may not capture the full spectrum of human tumor infiltration. Ensuring reproducible polymer manufacturing and precisely tuning drug release are crucial for regulatory viability [135, 136].
Concurrently, iron oxide platforms coated or crosslinked with dextran have gained traction for imaging and combined treatment. Several investigations in animal models have shown that dextran-coated iron oxide NPs can load antisense oligonucleotides, cross the BBB, and accumulate in tumor sites, monitored by MRI and optical methods. Nonetheless, such platforms frequently demand additional surface modifications to maintain colloidal stability and thwart off-target uptake.
Parallel work highlights dextran's potential to enhance imaging resolution and hyperthermia. For instance, a dextran@Fe3O4 NP formulation improved susceptibility-weighted imaging of glioma vasculature in an animal model [137]. Another approach leveraged dextran-coated SPIONs for hyperthermia in animal studies, optimizing dosing regimens to counter organ-level accumulation, though significant uptake still occurred in the spleen, liver, and lungs [138]. Dextran-shelled magnetite NPs have also mediated radiosensitization in preclinical models, likely by boosting reactive oxygen species during X-ray exposure, but the enhancement can vary across different radiation modalities [139]. In animal models, chitosan-dextran hybrids exhibited improved MRI-based tumor delineation yet faced suboptimal penetration into certain regions of the tumor. Altogether, these findings affirm dextran's stabilizing role, its capacity to improve contrast performance, and its promise for tackling GBM through combination treatments, albeit with persisting hurdles in heterogeneity and off-target distribution. Progressing to large-animal or clinically oriented imaging protocols is paramount for validating dextran-coated platforms in real-world scenarios.
Overall, dextran-based systems—from NP carriers to hydrogel scaffolds and iron oxide platforms—showcase exceptional utility in addressing the multifactorial challenges of GBM. Dextran's adjustable functionalization and capacity for stimulus-sensitive release can collectively reinforce strategies to cross the BBB and concentrate therapeutics at tumor sites. Stimuli-responsive linkers harnessing pH or redox gradients, as well as ligands that promote receptor-mediated uptake, further improve therapeutic efficiency. Despite the promise shown in various in vivo models, methodological disparities—ranging from radiation modes to resection techniques—must be harmonized to support clinical integration. Ongoing work should focus on refining large-scale manufacturing, ensuring consistent biodistribution, and clarifying long-term safety before dextran-based approaches can seamlessly transition into standard of care.
4.4 Alginate-based approaches
Alginate, derived from the cell walls of marine brown algae, is frequently used in prolonged drug release strategies for GBM. Its linear structure, comprising β-D-mannuronic acid and α-L-guluronic acid residues, undergoes ionic crosslinking and various chemical modifications to yield biocompatible, biodegradable, and minimally immunogenic hydrogels and NP. Because alginate hydrogels can form under physiological conditions, they can fill irregular tumor resection cavities or be injected for localized drug delivery, and their mucoadhesive and bioadhesive properties can improve drug penetration and retention in GBM regions. This PSC's ability to entrap and gradually release therapeutics helps sustain effective drug concentrations in the tumor bed over prolonged intervals, and adjusting the molecular weight and mannuronic-to-guluronic acid ratio can confer pH- or redox-responsiveness. Further functionalizing alginate with specific ligands enables active targeting of glioma cells, relying on receptor overexpression to concentrate drugs in tumors while minimizing collateral damage in healthy tissue. Although some of these approaches await further validation, they illustrate alginate's versatility as a platform material. While primarily utilized for therapeutic delivery and tissue engineering in these studies, its physicochemical properties also support the potential future engineering of combined theranostic systems.
This capacity for controllable gelation and sustained release is particularly beneficial for local GBM treatment, as shown by two complementary investigations. The first study developed a semi-synthetic PSC molecularly imprinted polymer (MIP) hydrogel comprising calcium-crosslinked alginate-poly(N-isopropylacrylamide) graft copolymer, which demonstrated thermo-thickening and shear-thinning behavior with high drug entrapment efficiency (84.59% for ruxolitinib) over a 14-day release period [20]. This formulation significantly inhibited GBM cell proliferation (U251 and A172) in vitro by reducing colony formation and slowing wound healing, although in vivo evidence remains limited. In contrast, a second study employed an injectable alginate-based hydrogel containing a sonosensitizer prodrug and semiconducting polymer NPs, which generated singlet oxygen upon US irradiation and cleaved singlet oxygen-sensitive linkers to release a pathway inhibitor [140]. Beyond direct tumor cell destruction, this approach showed potential for triggered, localized treatments in preclinical models. While both hydrogels demonstrate potential efficacy against GBM, variability in matrix composition and mechanisms of activation suggests the need for standardized comparative evaluations.
Several investigations also highlight how alginate scaffolds improve the physiological relevance of GBM models by offering 3D architectures that mirror tumor morphology and microenvironmental cues. One study optimized a 5 wt% gelatin and 5 wt% sodium alginate hydrogel, confirmed by rheological and spectroscopic analyses, to enhance GBM cell viability in vitro [141]. Within this matrix, tumor spheroids maintained strong proliferative capacity, and administering a CD73 inhibitor reduced VEGF and HIF1-α expression. This sensitivity to targeted therapy underscores the platform's potential for screening new anti-GBM compounds. A parallel 3D co-culture approach used alginate fibers as porogens to incorporate endothelial cells into a PEG hydrogel, enabling spatial compartmentalization that simulates in vivo tumor-endothelial interactions [142]. Compared to simpler models, this setup promoted GBM cell proliferation while preserving endothelial phenotype in vitro.
For larger-scale applications, a microscale alginate tube (AlgTube) system supported long-term culturing of GBM tumor-initiating cells with minimal loss of stem cell markers. This platform sustained approximately 700-fold expansion over 14 days and achieved a high volumetric yield (~3.0 × 10^8 cells/mL). Unlike other 3D methods that may compromise yield over time, the AlgTube approach enabled multiple passages while maintaining essential phenotypes, indicating utility in testing therapies that require large numbers of GBM cells. Taken together, these scaffold-based models better recapitulate tumor-like conditions and offer feasibility for high-throughput experiments, although further validation in organotypic or patient-derived xenograft settings remains necessary [143].
Alginate's versatility also extends to capturing or immobilizing GBM cells in macroporous matrices, which can concentrate therapeutic agents in the tumor area without harming nearby healthy tissue. One study engineered a sodium alginate hydrogel with pore diameters up to 225 μm, showing enhanced capture and retention of F98 GBM cells in vitro, especially when functionalized with RGD peptides targeting integrins common in GBM [144]. Another investigation employed a similarly macroporous alginate-RGD matrix to sequester F98 cells for irradiation, achieving complete cell eradication within the scaffold in vitro while preserving mechanical properties [145]. These approaches rely on physical confinement of infiltrative tumor cells, thereby allowing high-dose treatment in confined regions. They differ in methodologies for pore characterization and imaging, yet both illustrate the promise of trapping GBM cells in local matrices. Future research should combine these capture-based platforms with other targeted modalities, examining how pore geometry and biochemical cues affect treatment responses in vivo.
4.5 Heparin-based approaches
Increasing evidence underscores the potential of heparin-based platforms for GBM therapy by enhancing drug delivery and therapeutic efficacy in GBM. These platforms can serve integrated roles in diagnosis and treatment; for instance, a lactoferrin-heparin (Lf-heparin) complex achieved oral bioavailability and selective transport across the BBB in a preclinical animal model. As illustrated in Figure 7A, this orally administered complex is absorbed in the small intestine and transported across the BBB to the glioma, where it limits tumor angiogenesis by disrupting VEGF-VEGFR interactions [146]. This targeted distribution curtailed angiogenic progression and supported less invasive administration routes. Another novel heparin-based nanoparticle (DNPH) was developed for simultaneous magnetic resonance imaging (MRI) and tumor targeting (Figure 7B), showing effective enrichment at glioma sites in an animal model (Figure 7C) [147]. For instance, heparin-polyethyleneimine (HPEI) NP were engineered to encapsulate shRNA targeting COUP-TFII, an angiogenic transcription factor in gliomas, regulating tumor vascularization in an orthotopic mouse model [148]. The incorporation of heparin facilitated nucleic acid loading while minimizing toxicity. Similarly, researchers synthesized a heparin-taurocholate conjugate (LHT7) to address limitations of unfractionated heparin [149], showing in U87 GBM and human umbilical vein endothelial cells that LHT7 reduced cell viability and endothelial sprouting while suppressing phosphorylation of phospho-ERK and phospho-VEGFR2 [150]. Another approach integrated a heparin-containing polymer with US-targeted microbubble destruction for the delivery of cilengitide (CGT), resulting in lower renal clearance, enhanced tumor retention of the payload, and extended median survival in a mouse model when combined with US-mediated delivery [151, 152]. Collectively, these advances highlight the capacity of PSC conjugates to bolster drug loading, mitigate rapid clearance, and improve tumor specificity. Further refinements may optimize these constructs by adjusting heparin chain length or incorporating additional targeting ligands to accelerate clinical translation.
Integrated study of heparin-based nanomaterials in glioma diagnosis and treatment. (A) The lactoferrin-heparin coupling is absorbed through the gastrointestinal tract and transported across the BBB to the brain glioma site through transferrin-mediated transport, which limits tumor angiogenesis by disrupting VEGF-VEGFR interactions. (B) A novel DNPH for simultaneous MRI and tumor targeting. (C) DNPH showed effective enrichment at glioma sites. Adapted with permission from reference [146, 147]. Copyright 2023 Elsevier B.V. and 2013 Springer Science+Business Media New York, respectively.
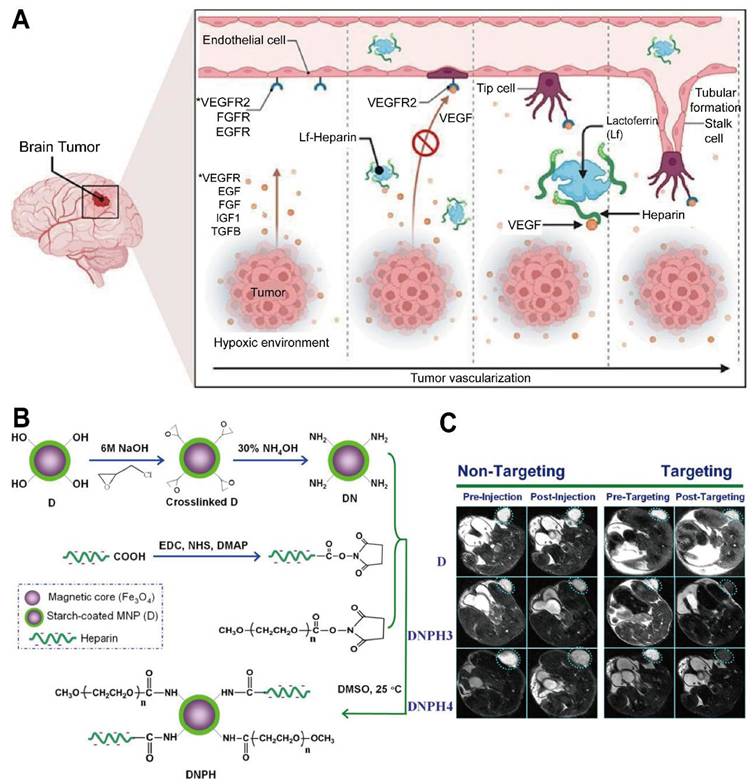
Beyond serving as a carrier, heparin also affects glioma cell signaling and pathogenetic pathways. At therapeutically relevant concentrations, heparin and its derivatives blocked ectonucleotidase activity by inhibiting extracellular adenosine production in an NPP1 (CD203a)-expressing glioma cell line [153]. Conversely, heparin disrupted NP uptake in U87 and GL261 glioma cells in a dose-dependent manner, requiring serum factors for inhibition [154]. In a related in vitro investigation, heparin-mediated obstruction of exosome traffic diminished the ability of irradiated glioma cell-derived exosomes to promote proliferative advantages in recipient cells [155]. These observations suggest a dual role for heparin, as it may safeguard against vesicle-mediated tumor progression but also interfere with nano-delivery strategies. Elucidating precise binding interfaces between heparin and vesicle surfaces could inform more selective interventions, enabling researchers to leverage heparin's biochemical breadth while preserving efficient cargo transfer.
Beyond its use in designing nanocarriers and modulating specific cellular pathways as discussed above, heparin's primary and well-established clinical application is as an anticoagulant. Understanding this systemic role, including its benefits and risks, is crucial when considering any heparin-based therapeutic intervention, especially in the context of GBM, as clinically, GBM patients often face an elevated risk of thrombotic events, prompting explorations of anticoagulant strategies and optimal timing. One retrospective cohort of HGG patients showed that rivaroxaban was associated with a higher incidence of bleeding complications than apixaban and enoxaparin, despite comparable baseline characteristics [156]. Another report found that prophylactic heparin administered within 24 hours postoperatively did not significantly increase major bleeding, suggesting early anticoagulation may be feasible [157]. In an analysis of atrial fibrillation management in GBM or brain metastasis, anticoagulation did not necessarily escalate intracranial hemorrhage risk [158]. A separate series demonstrated encouraging safety and efficacy outcomes for direct oral anticoagulants in GBM patients experiencing postoperative pulmonary embolism, albeit with a need for larger prospective trials [159]. A case report highlighted the rare occurrence of heparin-induced thrombocytopenia (HIT) or related cross-reactivity in a glioma patient, requiring high-dose intravenous immunoglobulin and rivaroxaban after fondaparinux proved ineffective [160]. These findings underscore the delicate balance between achieving effective thromboprophylaxis and limiting bleeding risk in central nervous system malignancies. Further prospective comparisons of heparin formulations, along with research on predictive biomarkers for bleeding or thrombotic complications, may streamline anticoagulation practices in GBM care.
4.6 Cyclodextrin (CD)-based approaches
CD-based inclusion complexes alleviate the challenge of poor water solubility often observed in anticancer agents for GBM therapy (Table 5). These nanomaterials serve as integrated platforms for both diagnosis and treatment, evolving from novel water-soluble MRI contrast agents for in vivo glioma imaging (Figure 8) [161] to advanced drug delivery vehicles. For instance, peptide-modified NPs coated with cyclodextrin can effectively deliver paclitaxel to the glioma site, relieve tumor hypoxia, and enhance chemotherapy in animal models (Figure 9A) [162]. Encapsulating disulfiram in β-cyclodextrin derivatives improves its solubility by approximately 1000-fold, leading to enhanced in vitro efficacy against GBM cells (IC50 around 7000 nM), although it remains somewhat less potent than in melanoma cells [163]. A similar approach using liposomal carriers enriched with CD, a system designed to improve encapsulation efficiency and drug retention (Figure 9B) [164], increased the retention of butylidenephthalide (BP) in GBM cells for over 8 hours, prolonging median survival in animal models bearing TMZ-resistant tumors. Other studies confirm the versatility of CD encapsulation in various chemical contexts, including a cyclodextrin-calixarene-based amphiphilic system that encapsulates docetaxel and TMZ at over 80% efficiency [165]. Moreover, methyl-β-cyclodextrin facilitates perillyl alcohol loading and influences sodium-potassium ATPase-Src signaling in GBM cell lines, despite partial cholesterol depletion complicating mechanistic interpretations [166]. CD-based formulations have also proven beneficial for amphiphilic or macromolecular agents: ferrocenyl tamoxifen analogues incorporated into methylated cyclodextrin frameworks retain cytotoxic potency in vitro in the nanomolar range [167], while β-cyclodextrin conjugation enhances hydrophobic porphyrins used in photodynamic therapy in preclinical models [168]. Although many data derive from in vitro or early in vivo assays, these findings highlight CD complexes as promising vehicles when transitioning to more advanced glioma models.
Nonmetallic magnetic resonance contrast agent based on ORCAs as a CD-based carrier for glioma imaging in vivo. Adapted with permission from reference [161]. Copyright 2020 Wiley-VCH Verlag GmbH & Co. KGaA.
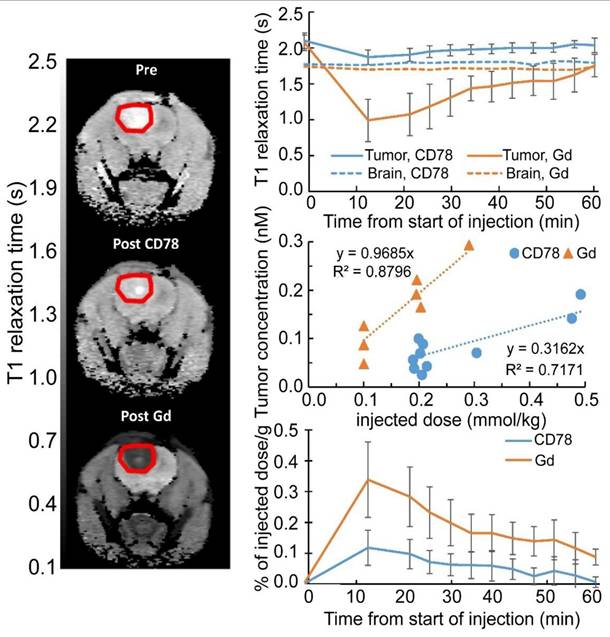
Cyclodextrin-Based approaches
| Approach/Method Name | Cyclodextrin Type | Payload/Guest Molecule | Application/Goal | Validation Model | Key Outcome/Performance | Authors |
|---|---|---|---|---|---|---|
| Disulfiram Encapsulation for Drug Repositioning | HP-β-CD, RAMEB, SBE-β-CD | Disulfiram | Enhance drug solubility and stability for cancer therapy | In vitro (melanoma, GBM cell lines) | ~1000-fold drug solubility enhancement; IC50 ~100 nM on melanoma | Benkő et al. [158] |
| Curcumin-Loaded Polymeric Membrane | β-CD | Curcumin | Local drug delivery system for melanoma and GBM | In vitro (melanoma, GBM cell lines) | Prolonged cytotoxic effect up to 96 h at 50 μg/mL | Gularte et al. [172] |
| Multifunctional Magnetic NPs | Cyclodextrin | Paclitaxel (PTX) | Targeted therapy for drug-resistant GBM | In vitro (GBM and drug-resistant GBM cells) | Enhanced cellular uptake and improved efficacy of PTX in GBM cells | Mu et al. [69] |
| Cyclodextrin-Encapsulated Liposomes | Cyclodextrin | Butylidenephthalide (BP) | Intranasal delivery for drug-resistant brain tumors | In vivo (nude mice with TMZ-resistant GBM) | Encapsulation efficiency up to 95%; 10-fold higher drug accumulation vs. oral | Lin et al. [164] |
| Cyclodextrin-siRNA Conjugates | β-CD | siRNA (luciferase, PLK1) | Targeted siRNA delivery for gene silencing | In vitro (U87, PC3, DU145 cancer cell lines) | Enhanced gene knockdown achieved with ligand-targeted nanoparticle formulations | Malhotra et al. [167] |
| Electrochemical Immunosensor | β-CD (on GO-Fe3O4 nanocomposite) | Anti-5-methylcytosine (5mC) antibody | Detection of MGMT gene methylation for GBM diagnosis | Biological samples (methylated MGMT-DNA) | Detection limit of 0.0825 pM | Yang et al. [170] |
| pH-Sensitive Affinity-Based Delivery System | Cyclodextrin polymer | Adamantane-modified doxorubicin | Tumor-specific, pH-triggered local drug delivery | In vitro (U-87 GBM cells) | Sustained release over 87 days with accelerated release at low pH | Cyphert et al. [173] |
Integrated study of CD-based nanomaterials in the diagnosis and treatment of glioma. (A) A CDD1 for non-invasive intranasal administration, a novel carrier system that significantly improves encapsulation efficiency and drug retention time. (B) tLyP-1 peptide-modified DOPA-beta-CD-coated PTX and supported MnO2 NPs (tLyP-1-CD-DOPA-MnO2@PTX) effectively deliver PTX to the glioma site, while producing Mn2+ and O2 to relieve hypoxia. ROS is produced to kill tumor cells and enhance glioma chemotherapy. Adapted with permission from reference [162, 164]. Copyright 2022 Wiley-VCH GmbH, Weinheim, and 2020 by the authors (open access), respectively.
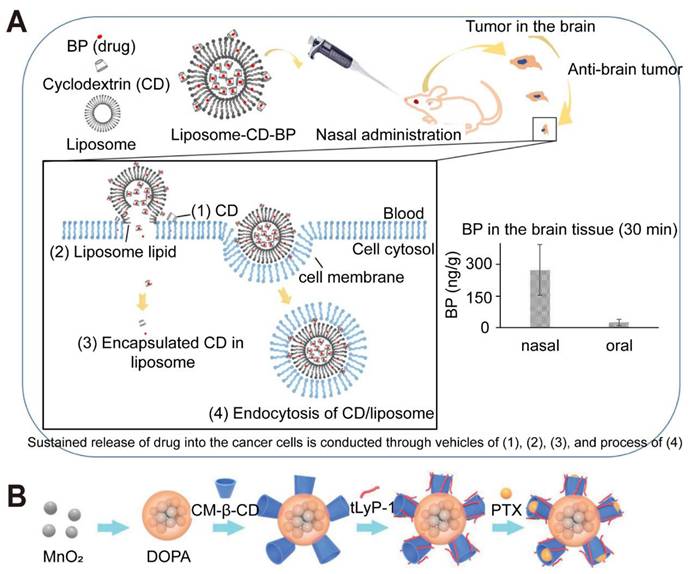
In addition to improving solubility, CD constructs offer opportunities to bypass the BBB through intranasal delivery and ligand-mediated targeting. One study employed a β-cyclodextrin-Chitosan hybrid polymer to coat gold-iron oxide NP loaded with therapeutic microRNAs, achieving significant brain accumulation and improved survival in orthotopic GBM-bearing mice when combined with systemic chemotherapy [19, 105]. This hybrid formulation serves as a quintessential theranostic model, effectively merging diagnostic capabilities (multimodal MRI/optical imaging) with therapeutic action (miRNA gene regulation) into a single, cohesive entity. Intranasal administration of cyclodextrin-encapsulated BP delivered via liposomes resulted in approximately tenfold higher brain drug levels in animal models compared with oral administration. Further targeting efficiencies emerge when CD is paired with specific ligands: a multifunctional NP containing a magnetic iron oxide core, cyclodextrin, fluorescein, PTX, and chlorotoxin (CTX) enhanced intracellular drug delivery and reduced tumor viability in vitro more effectively than the same NP without CTX [69]. Conjugating siRNA to β-cyclodextrin similarly improves knockdown activity in cell culture, particularly when targeting ligands are present on the NP surface [169]. Although limited sample sizes in some experiments, along with heterogeneity in glioma models, complicate broad conclusions, these intranasal and ligand-targeted approaches offer a valuable route for enhanced drug localization in GBM [170].
CD-based systems also lend themselves to multimodal imaging and diagnostic applications in GBM. For instance, β-cyclodextrin functionalized with piperidine or pyrrolidine structures has demonstrated water-soluble MRI contrast capabilities, exhibiting distinct kinetic stabilities and relaxivities at various field strengths. In vivo testing in rats with gliomas documented prolonged T1 relaxation in the tumor region for at least 60 minutes [171]. CD-conjugated iron oxide NP provide parallel opportunities for both imaging and therapeutic payload delivery. Beyond imaging, cyclodextrins serve as building blocks for advanced biosensors: an electrochemical immunosensor platform incorporating graphene oxide, magnetic NPs, and β-cyclodextrin composites directly detected methylated cytosines in the O6-methylguanine-DNA methyltransferase (MGMT) promoter, employing Ru(NH3)63+ as an electrochemical reporter [172]. Although further validation in clinically relevant settings is necessary, these developments demonstrate the feasibility of integrating imaging and diagnostic functions with CD-based carriers.
Controlled and stimuli-responsive release is another key benefit conferred by CD-containing materials. Polymeric membranes or hydrogels often entrap drug-CD complexes to regulate release kinetics, as shown by a cationic starch-derivative/poly(vinyl alcohol) membrane loaded with β-cyclodextrin/curcumin, which prolonged cytotoxic activity against GBM cells for up to 96 hours [173]. Nanoassemblies that incorporate cyclodextrin-calixarene leverage elevated GSH levels in tumor tissue to disassemble and facilitate on-demand drug delivery in preclinical models. Further strategies use pH-sensitive linkages: adamantane-modified DXR conjugated to cyclodextrin-based polymers via hydrazone bonds remains stable under neutral conditions but releases drug in acidic environments [174]. High-affinity binding approaches, such as divinyl sulfone cross-linking in β-cyclodextrin [175] or incorporating antiangiogenic compounds for slow elution [176], underscore the adaptability of CD carriers. Directly conjugating siRNA to cyclodextrin similarly hinges on balanced stability and release, with targeting ligands enabling more efficient intracellular uptake in vitro [169]. These polymeric approaches still require careful optimization of crosslink density and cleavage kinetics, but they reveal considerable potential for sustained, localized dosing of GBM treatments [177].
Collectively, these lines of research highlight the capacity of CD constructs to improve solubility, bioavailability, targeting, imaging, and controlled release in GBM therapy. Although differences in experimental designs, cell models, and dosing protocols have produced some variations in outcome, a consistent underlying principle emerges: cyclodextrin-based systems can integrate multiple functionalities to enhance therapeutic efficacy against GBM in preclinical models. Ongoing studies seeking to optimize CD formulations through standardized procedures and expanded in vivo evaluation will likely accelerate their clinical translation as promising treatment strategies for this challenging disease.
4.7 Other PSCs
PSC-based biomaterial scaffolds have gained prominence in GBM management by leveraging their biocompatibility and adjustable physicochemical features. Multiple studies investigated the potential of pectin hydrogels, which are plant-derived PSCs with variable esterification levels that can be ionically crosslinked with calcium to form robust 3D matrices. One investigation prepared low-esterified pectin films via ionic gelation, culminating in NP measuring 90-115 nm with negative zeta potentials between -8.30 and -7.86 mV [178]. These NP significantly inhibited U87MG human GBM cells, potentially by modifying cell adhesion, yet demonstrated no toxicity towards non-tumor cells. Another approach utilized sprayable pectin-based hydrogels loaded with polylactic acid-PEG nanocrystals, generating localized anticancer drug delivery in ex vivo brain models [179]. In addition, including ECM proteins such as collagen modulates glioma cell morphology. One report showed that pectin hydrogels incorporating different collagen I/IV ratios influenced C6 glioma cell growth patterns and neurite formation [180], while pectin fractions derived from Campomanesia xanthocarpa induced 15.55-37.65% cytotoxicity in human GBM cells and heightened ROS levels without harming normal cells [181]. These observations align with a related study in which collagen films were functionalized with chondroitin sulfate to enhance U87 GBM cell adhesion and proliferation [182, 183]. Despite these promising effects, much of the efficacy data derive from in vitro or limited ex vivo models with small sample sizes or single tumor cell lines. Nonetheless, the collective evidence suggests that pectin-based biomaterials offer customizable physicochemical properties suitable for glioma cell inhibition or targeted drug transport. Future research will likely focus on refining in vivo delivery platforms or implantable systems capable of synchronizing local ECM composition with controlled drug release.
While the aforementioned PSC-based biomaterials offer promise as therapeutic delivery systems and modulators of the tumor environment, attention must also be given to the inherent roles of specific proteoglycans within the glioma milieu, which themselves can be pivotal therapeutic targets. In this regard, research on chondroitin sulfate proteoglycans (CSPGs) emphasizes their crucial role in shaping glioma cell behaviors. Multiple studies have identified elevated levels of chondroitin sulfate proteoglycan 4 (CSPG4) and associated enzymes in high-grade gliomas, where they contribute to tumor maintenance, stemness, and therapy resistance. One study reported that CSPG4 and its glycan chains are essential for glioma-initiating cell (GIC) self-renewal, and their removal triggers GIC differentiation [184]. Mathematical modeling further revealed that CSPG-rich environments facilitate noninvasive tumor growth linked to reactive astrocyte encapsulation, while reduced chondroitin sulfate expression correlates with a more invasive phenotype [185]. In addition, the increased expression of chondroitin sulfate biosynthetic enzymes, such as CHSY1 (chondroitin sulfate synthase 1), predicts poor prognosis, partially through stabilizing PDGFRA and bolstering PDGF-induced signaling [186, 187]. Elevated chondroitin sulfate content is often detected near necrotic and perivascular regions of GBM tumors and is associated with heightened proliferation [188]. Targeting CSPG4 with immunotoxins substantially improved cytotoxicity in vitro, especially when combined with Bcl-2 inhibitors, underscoring the clinical potential of CSPG4-focused therapies [189]. However, large-scale antibody production and heterogeneity pose challenges for translating these therapies to clinical use [190]. Despite methodological limitations, including small patient cohorts and preclinical models, these findings converge to indicate that CSPG4 and the machineries driving aberrant chondroitin sulfate expression serve as attractive therapeutic targets by sustaining critical survival signals within the tumor microenvironment.
Conventional GBM treatments can unintentionally reshape the glycosylated ECM, influencing subsequent tumor regrowth or infiltration [191]. One study in adult Wistar rats demonstrated that extended TMZ therapy reduced chitosan levels in brain tissue by 1.5- to 2.5-fold, in part through downregulating aggrecan core protein, possibly contributing to increased anxiety despite an absence of overt histological damage [192]. Combined TMZ and DXM regimens also modified CSPGs in healthy brain regions, leading to enhanced adhesion and proliferation of GBM cells in organotypic slice models [193]. Ionizing radiation (IR) further disrupts proteoglycan synthesis, with triple irradiation (7 Gy over three days) in an animal model downregulating decorin, biglycan, versican, and brevican, alongside decreases in chondroitin sulfate and heparan sulfate (HS) [194]. Glioma cells cultured with irradiated tissue exhibited intensified adherence and proliferation, indicating that IR-induced glycan remodeling may accelerate tumor recurrence. Although these studies employed small animal cohorts and had limited follow-up, they underscore the need to consider unplanned ECM alterations when administering chemoradiation therapies. Further investigation may clarify whether interventions that maintain healthy chondroitin sulfate profiles can mitigate these unintended effects and slow tumor regeneration.
In parallel with scaffold-based strategies, efforts to disrupt glycosaminoglycan (GAG) biosynthesis have gained momentum as a means of dismantling tumor-promoting ECM elements [195]. One investigation developed small-molecule inhibitors targeting xylosyltransferase (XYLT-1) and β-1,4-galactosyltransferase-7 (β-GALT-7), which catalyze early steps of GAG chain assembly. Using hydrophobic prodrugs bearing 4-deoxy-4-fluoro-2,3-dibenzoyl-xyloside cores, researchers achieved submicromolar to micromolar IC50 values in U251 and U87 GBM lines, presumably by halting GAG elongation. Although these findings suggest that inhibiting GAG biosynthesis can abrogate malignancy-related signaling, challenges persist in delivering these inhibitors across the BBB and avoiding off-target effects, given the importance of GAG pathways in normal tissues. This approach may benefit from combination therapies incorporating GAG inhibition with biomaterial scaffolds that sequester growth factors and precisely deliver anticancer agents. Further in vivo validation could facilitate the integration of GAG inhibitors into broad anticancer regimens while minimizing toxicity to non-tumoral cells.
Collectively, current research on other PSCs in GBM underscores the versatility of PSC-based nanomedicines, scaffold technologies, and ECM-directed therapies. Pectin scaffolds, alone or combined with collagen and chitosan, show promise for local drug delivery and tumor growth modulation, while evidence linking CSPGs to stemness and therapeutic resistance highlights the need to target chitosan pathways. Standard chemotherapy and radiotherapy can inadvertently weaken the ECM through GAG depletion, potentially promoting GBM relapse. Pharmacological inhibition of GAG biosynthesis represents a complementary strategy, as small molecules that interfere with glycan-chain elongation may temper the survival signals maintained by aberrant chitosan expression. Ongoing research integrating scaffold design, targeting of CSPGs, and GAG biosynthesis blockade holds considerable potential to refine GBM treatment and ultimately improve clinical outcomes.
5. Immunotherapeutic Applications of PSC-Based Nanomedicines
PSC-based nanomedicines address key GBM immunotherapy limitations—specifically poor antigen presentation and the immunosuppressive microenvironment, by delivering immunomodulators and leveraging intrinsic immunostimulatory properties (e.g., Chitosan, β-glucans) [5]. Specifically, by enhancing the delivery of tumor-specific cargos to antigen-presenting cells (APCs) and concurrently activating innate immunity, these carriers address critical challenges such as poor antigen presentation and insufficient immune cell activation. Nanoconstructs based on chitosan, HA, or botanical PSCs are being engineered to improve dendritic cell (DC) maturation, boost cytotoxic T lymphocyte (CTL) responses, and favorably modulate cytokine profiles towards pro-inflammatory signals. For instance, dextran can be used for conjugating immunostimulants, and its derivatives can facilitate dual delivery of drugs and immune mediators [81]. HA nanocarriers are often used to co-deliver chemotherapeutics alongside immunologic adjuvants, bolstering immune-mediated antitumor effects. These actions collectively work to dismantle barriers to T cell infiltration, showing promise in transforming GBM from an immunologically 'cold' tumor to one that is responsive to immune-mediated clearance.
To address challenges in delivering and sustaining immunotherapeutic effects within the GBM microenvironment, PSC-based hydrogels offer solutions for localized and prolonged activity. For instance, an injectable oxidized high-amylose starch hydrogel was developed to overcome the difficulty of effectively delivering and supporting immune cells locally. By serving as a scaffold for macrophages and co-delivering polarizing agents, this hydrogel bypassed the BBB and fostered a supportive niche that modulated macrophage behavior towards an anti-tumor phenotype, thereby boosting therapeutic efficacy [196]. This system tackled the issue of poor immune cell infiltration and survival by providing an interconnected porous structure. Similarly, to counteract the rapid clearance and systemic toxicity associated with conventional chemo-immunotherapy, a chemically crosslinked chitosan hydrogel was engineered for sustained local co-delivery of doxorubicin (DXR) and an immune checkpoint inhibitor (BMS-1). This approach solved the problem of insufficient drug retention in GBM lesions, achieving prolonged local release and significantly enhancing chemo-immunotherapy outcomes by preventing uncontrolled polymer swelling and maintaining therapeutic concentrations [197]. Chitosan's utility in chemo-immunotherapy is further exemplified by systems where Dox-loaded bovine serum albumin NPs crosslinked with chitosan alongside anti-PD-1 checkpoint inhibition prompt immunogenic cell death and stimulate a durable anti-tumor immune response [100]. Dextran-based hydrogels can also be coupled with immunomodulatory strategies; for example, an injectable system combining Dox-loaded ferritin with oxidized dextran gradually discharged intact ferritin particles, promoting antitumor responses, and has been explored with immune checkpoint inhibitors. A remaining challenge for such platforms is optimizing the hydrogel design to ensure the long-term viability and functional efficacy of embedded immune cells or labile immunomodulators, requiring a delicate balance between mechanical integrity and nutrient diffusion.
PSC scaffolds are also being engineered to solve issues of suboptimal immune stimulation by conventional immunotherapies. For example, to overcome the poor delivery and systemic toxicity of STING (Stimulator of Interferon Genes) agonists, a HA-STING agonist conjugate (HA-MSA2) was developed. This local polymer-drug delivery system effectively bolstered immunogenic cell death and STING-related cytokine production, thereby addressing the challenge of inadequate innate immune activation and successfully reshaping the immune microenvironment by recruiting NK and CD8+ T cells in murine GBM models [198]. Another strategy to tackle insufficient tumor immunogenicity and promote T cell responses involves a chitosan-based hydrogel. This system, by co-delivering DXR-loaded bovine serum albumin NPs with anti-PD-1 checkpoint blockade, addressed the need for sustained induction of immunogenic cell death and broader immune activation. The prolonged DXR release, coupled with chitosan's intrinsic immunostimulatory properties, effectively promoted tumor-specific T cell responses [100]. The versatility of HA-based systems extends to their use with immunostimulatory agents. For instance, cationic polyethylenimine coated with selenocysteine and sodium hyaluronate has been combined with natural killer cells to cross the BBB and exhibit potent GBM cytotoxicity, highlighting the potential of HA for multimodal immunotherapeutic approaches. HA-based NPs can enable synergistic treatments combining photothermal, photodynamic, or immunotherapeutic approaches [112]. Such PSC-based approaches demonstrate the capacity to overcome distinct hurdles in immunotherapy, with the HA-STING system targeting specific pathway activation and the DXR-chitosan design offering multi-faceted immune engagement.
Further innovations in PSC hydrogels aim to address the critical challenges of off-target toxicity and insufficient spatiotemporal control in GBM immunotherapy. An alginate-based injectable prodrug hydrogel (APN) employing sonodynamic therapy (SDT)-responsive NPs was designed to tackle these issues by enabling on-demand release of an IDO inhibitor, a key immunosuppressive pathway target [142]. Ultrasound-triggered singlet oxygen generation not only provoked immunogenic cell death but also cleaved oxygen-sensitive linkers to release the IDO (immunosuppressive pathway) inhibitor precisely at the tumor site. This strategy directly addresses the problem of systemic exposure and non-specific immunomodulation, although optimizing ultrasound penetration in brain tissue remains a hurdle. Another approach to overcome the limitations of systemic toxicity and achieve potent, localized immune activation involves HA-drug conjugates. For instance, an HA matrix co-delivering DXR and the Toll-like receptor-9 agonist CpG successfully induced robust immunogenic cell death and CD8+ T cell responses at reduced drug dosages, thereby mitigating systemic side effects often seen with free-drug administrations [199]. Despite these delivery successes, a recurring reason for therapeutic failure in PSC-based immunotherapy is the profound immunosuppression of the GBM microenvironment, which nanocarriers alone cannot always reverse. While carriers like alginate or HA successfully deliver immune agonists, they do not inherently alter the physical stiffness of the extracellular matrix (ECM) that physically excludes T-cells. Consequently, inconsistent findings across studies often correlate with the density of the tumor ECM, indicating that delivery efficiency does not linearly translate to immune activation in "cold" GBM tumors.
Alginate formulations have also shown the capacity to reprogram macrophages or T cells within the glioma microenvironment, and engineered alginate NP or hydrogels can exhibit efficient macrophage uptake, modulating immune cell function for combined chemo-immunotherapy approaches [200]. Future research on alginate-based capture platforms should explore combinations with immunotherapies, examining how pore geometry and biochemical cues shape immune responses in vivo. Collectively, these PSC hydrogel systems demonstrate significant potential in solving key problems in GBM immunotherapy: they function as localized immunomodulatory reservoirs that ensure controlled drug release, enhance immune activation specifically at the tumor site, and reduce systemic toxicity, potentially improving patient compliance. Future work will focus on refining hydrogel properties to further sustain immunotherapeutic action and minimize physical impacts on brain tissue, thereby advancing therapeutic precision for GBM.
Beyond hydrogels, PSC nanoparticle platforms offer distinct advantages for tackling immunotherapy challenges in GBM. Their inherent tunability allows for the incorporation of responsive elements (e.g., pH-, enzyme-, or redox-sensitivity) that can solve problems related to non-specific drug release and off-target effects of potent immunomodulators [178]. Dextran NPs, for example, can leverage intrinsic immunostimulatory effects. Dextran derivatives can be engineered to amplify or modulate immune responses, supporting combination therapies geared toward eliminating malignant cells and invigorating antitumor immunity. Dextran's immunomodulatory capability complements cytotoxic regimens designed to overcome the immunosuppressive tumor milieu. Although unmodified dextran has low immunogenicity, specialized derivatives can prompt beneficial immune activation by enhancing antigen presentation [125]. Such systems can deliver antigens or immune-stimulating molecules to antigen-presenting cells in glioma, strengthening T cell infiltration and function and fortifying the immunosurveillance network [201]. Dextran can also consolidate immunogenic cell death in tandem with DAMPs released during chemotherapy. Combining triggered release approaches with immunotherapeutic elements boosts dextran's versatility; immunostimulatory ligands, adjuvants, or monoclonal antibodies can be conjugated or co-encapsulated to stimulate dendritic cell maturation and augment cytotoxic T cell expansion. By pairing targeted therapy with immune activation, dextran-based formulations address resistance mechanisms and dampen immunosuppression, notably through M2-to-M1 macrophage polarization. Key design refinements in PSC NPs are crucial for enhancing immunotherapeutic efficacy. For instance, controlling particle size can address the poor penetration of immunotherapies into deep tumor tissues, while appending immunostimulatory ligands directly onto NP surfaces can solve the problem of insufficient local immune activation. Such modifications aim to ensure that immunotherapeutic payloads are delivered precisely to immune cells or the tumor microenvironment, thereby amplifying desired anti-tumor immune responses. By strategically designing PSC NPs to overcome specific hurdles in GBM immunotherapy, such as poor bioavailability of immune agents or inadequate engagement of immune effector mechanisms, these systems hold the potential to significantly improve treatment outcomes [202, 203]. Rigorous preclinical and clinical validation will be crucial to translate these sophisticated NP designs into effective GBM immunotherapies.
Beyond their role as delivery vehicles, certain PSCs possess inherent immunomodulatory properties that can be harnessed to directly address the immunosuppressive nature of GBM. Heparins, for example, tackle the problem of adenosine-mediated immunosuppression by allosterically inhibiting the ectonucleotidase CD203a. This action limits extracellular adenosine accumulation (thereby reducing immunosuppressive effects) and curtails the development of immunosuppressive regulatory T cell (Treg) phenotypes, thus synergizing with T cell-based immunotherapies [153]. Heparin-based NPs have also been studied for their role in regulating tumor vascularization and immunosuppression. A significant challenge, however, is heparin's systemic anticoagulant activity, which complicates its therapeutic application. PSC-based formulation strategies, such as controlled-release systems or structural modifications to heparin itself, are being explored to solve this issue by segregating its tumor-specific immunomodulatory benefits from its systemic effects on coagulation. Investigating these refined approaches is key to unlocking heparin's clinical potential in GBM immunotherapy.
Fungal β-glucans exemplify another category of PSCs with intrinsic immunostimulatory capabilities, offering a means to counteract immune coldness in the GBM microenvironment. These agents have been shown to activate microglia, leading to the secretion of pro-inflammatory cytokines that inhibit tumor proliferation and promote apoptosis [204]. However, their application faces immunotherapy-specific challenges, including the translational complexities of ex vivo microglial conditioning and the poor BBB penetration of high molecular weight β-glucans, which restricts their efficacy upon direct administration. To overcome these limitations, PSC-based formulation strategies, particularly nanoparticle encapsulation, are being investigated. Such approaches aim to solve the problems of targeted delivery and BBB traversal, thereby enhancing the therapeutic window and reducing dosage requirements for β-glucans. These efforts underscore the utility of leveraging both the intrinsic immunomodulatory effects of PSCs and advanced delivery systems to augment their impact, especially in combination with other immunotherapies like checkpoint inhibitors.
In conclusion, PSC-based nanomedicines and inherently immunomodulatory PSCs offer multifaceted solutions to critical challenges hampering effective immunotherapy for GBM. Whether by acting as sophisticated carriers that overcome delivery barriers and improve the targeting of immunostimulatory agents, or by exerting direct immunomodulatory effects, these biopolymers are pivotal in enhancing immune system engagement against brain tumors [203, 204]. Strategies employing materials like heparin and β-glucans demonstrate how PSCs can directly counteract specific immunosuppressive mechanisms within the GBM microenvironment. Dextran's mild immunostimulatory properties can enhance immune responses, and ongoing research explores combining dextran scaffolds with immunotherapies. As immuno-oncology evolves, dextran's chemical modifiability may expand its role in uniting targeted drug delivery with robust immune activation. Similarly, the immunotherapeutic targeting of chondroitin sulfate proteoglycans (CSPGs) is an active area of research for other PSC-based strategies. The success of these approaches hinges on innovative engineering to solve inherent limitations, such as systemic side effects (e.g., anticoagulation) or poor bioavailability (e.g., restricted BBB penetration). By focusing on how PSC-based systems can resolve existing problems in immunotherapy—such as poor drug localization, insufficient immune activation, significant off-target toxicities, and the immunosuppressive tumor niche—research is paving the way for transformative treatment regimens. Continued refinement of their biochemical properties and delivery mechanisms promises to shift the balance in GBM from immune evasion towards a pro-inflammatory state amenable to robust and sustained anti-tumor immunity.
6. Main Challenges for PSC-Based Nanomedicines Applied Clinically
Despite the extensive library of preclinical successes described in this review, the translation of PSC-based nanomedicines to the clinic remains stagnant. Currently, no PSC-specific nanocarriers have received FDA approval for Glioblastoma, and extremely few have advanced to Phase I/II trials, in stark contrast to the clinical success of lipid-based nanoparticles (e.g., Onpattro® or COVID-19 vaccines). This translational gap is not due to a lack of efficacy, but rather the unique barriers imposed by natural polymer sourcing. A fundamental barrier to clinical progression is the challenge of ensuring manufacturing reproducibility and achieving Good Manufacturing Practice (GMP) compliance [205]. The transition of multifunctional PSC nanoplatforms from bench-scale synthesis to industrial-scale production is hampered by the complex, often bottom-up processes required for creating theranostic systems [206]. PSCs sourced from natural materials often vary in molecular weight, branching structure, and purity due to environmental factors, which introduces raw material variability. Unlike synthetic polymers (e.g., PLGA) which have defined stoichiometries, natural PSCs like chitosan and alginate suffer from batch-to-batch inconsistencies driven by seasonal and species-specific variations in the raw source material (e.g., crustacean shells or algae type). Furthermore, a critical but often overlooked barrier is endotoxin (lipopolysaccharide) contamination. Cationic PSCs bind avidly to negatively charged endotoxins, making purification notoriously difficult. This is a "deal-breaker" for clinical translation, as the regulatory limit for endotoxins in intrathecal/intracranial products is significantly stricter than for intravenous drugs to prevent aseptic meningitis.
This, combined with complex synthesis strategies, can lead to significant batch-to-batch inconsistencies in critical quality attributes such as particle size, surface charge, and drug-loading capacity [207]. This variability is particularly problematic for multi-functional PSCs (e.g., those combining targeting ligands, drugs, and imaging agents). The stochastic nature of chemical conjugation often results in "polydisperse" batches where some nanoparticles are heavily targeted while others are naked. This heterogeneity complicates the establishment of the structure-activity relationships required by regulatory bodies like the FDA, representing a primary bottleneck preventing these promising academic studies from entering Phase I trials. Such variability can drastically affect stability, biodistribution, and therapeutic performance, hindering the comparability of preclinical and clinical data [208]. To overcome these challenges, robust engineering solutions are essential. Standardized synthesis protocols, supported by automated quality control measures, are crucial for minimizing variability. Furthermore, modular manufacturing platforms, such as microfluidic synthesis, are increasingly being adopted to allow for the precise, continuous, and highly reproducible fabrication of NPs, often maintaining superior control over particle size compared to traditional bulk methods [209]. Simplifying the chemical synthesis pathways for complex carriers also enhances the potential for large-scale, cost-effective GMP production.
A related challenge is the scalability and consistency of ligand conjugation, which is necessary to achieve specific targeting and successful BBB penetration [210]. The attachment of targeting moieties (e.g., peptides, antibodies, aptamers) must be highly efficient and reproducible without compromising nanoparticle integrity or biological activity [211]. Given the high structural complexity and functional variability of GBM tumors, where receptor expression is heterogeneous, robust and adaptable conjugation methods are required [212]. Efficient bio-orthogonal chemistries, such as copper-free click reactions, offer an attractive solution, as they enable the precise incorporation of targeting ligands onto nanoparticle surfaces in a manner that is both reproducible and scalable [213]. Research also indicates that tuning the density and affinity of targeting ligands—with intermediate affinities often yielding enhanced BBB transport—must be consistently maintained throughout the manufacturing scale-up process to guarantee predictable biological outcomes [214].
Furthermore, the path from promising preclinical investigation to approved GBM treatments is significantly obstructed by regulatory hurdles. Agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) impose rigorous demands for comprehensive safety and efficacy data, including thorough evaluations of pharmacokinetics (PK), biodistribution, potential toxicity, and immunogenicity. A critical challenge in this domain is bridging the "preclinical translation gap." While rodent models are essential for initial screening, their physiological differences from humans often lead to poor prediction of safety and efficacy. Consequently, there is a growing regulatory expectation for robust preclinical studies in large animal models, such as canines or non-human primates, particularly for CNS-targeted nanomedicine. These models provide more relevant data on complex pharmacology, neuro-toxicology, and immunogenic responses due to their closer similarities in brain structure, immune systems, and metabolic pathways. However, conducting such extensive pharmacology and toxicology studies is resource-intensive, ethically complex, and presents significant logistical challenges, thereby slowing the translational pipeline. This scrutiny is particularly intense for novel nanomaterials targeting the central nervous system (CNS), where off-target effects and long-term accumulation pose unique neurotoxicity risks [215, 216]. Regulatory considerations for CNS delivery go beyond standard toxicology. Agencies require specialized neurotoxicology assessments, specifically monitoring for CNS inflammation (gliosis), brain edema, and lowering of the seizure threshold—adverse events that standard systemic toxicity studies often miss. Additionally, demonstrating the stability of the PSC-nanocarrier complex in cerebrospinal fluid (CSF), which has a different ionic strength and protein composition than plasma, is a mandatory but frequently neglected step in the Investigational New Drug (IND) enabling process. Although naturally derived PSCs generally exhibit low immunogenicity, the complex chemical modifications required for theranostic functionality—including PEGylation, cationization, or ligand integration—can inadvertently alter their biological identity and toxicological profiles, potentially triggering unforeseen immune responses or enhancing clearance by the reticuloendothelial system (RES). To mitigate these risks and meet regulatory thresholds, strategies include shifting towards biodegradable materials, such as specific PSC derivatives and lipid-based formulations, which offer improved clearance profiles. The development of "self-destructing" nanocarriers that degrade upon drug delivery is also being explored to reduce the potential for long-term tissue retention, addressing a primary safety concern for CNS applications [217].
Finally, the vast, high-dimensional design space of PSC-based nanomaterials presents a fundamental challenge that traditional, low-throughput experimental methods cannot efficiently address. This is where AI-driven discovery and design represent a new frontier. The optimization of a nanocarrier for GBM involves a complex interplay of variables, including polymer source, molecular weight, branching, chemical modifications, ligand density, and particle morphology. Machine learning (ML) models, trained on emerging datasets, can accelerate the prediction of structure-activity relationships (SAR) and critical quality attributes. These models can screen virtual libraries of PSC derivatives to identify novel materials with optimized properties for BBB penetration, tumor-specific targeting, and payload delivery. Furthermore, generative AI approaches may enable the de novo design of bespoke PSC-based nanostructures with precisely tailored theranostic functions. The primary hurdle, however, lies in the generation and curation of large, high-quality, and standardized datasets required to train reliable predictive models—a task compounded by the very manufacturing inconsistencies that plague the field. Integrating "closed-loop" systems, which combine AI-driven design with automated, high-throughput synthesis platforms (like microfluidics), is a formidable but necessary challenge to accelerate the rational design and clinical translation of next-generation PSC nanomaterials for GBM.
7. Conclusions
In summary, PSC-based nanoplatforms possess excellent biocompatibility, inherent stimuli-responsiveness, and versatile chemical modifiability. These properties underpin promising advancements, but realizing their full clinical potential requires a structured and visionary approach. The future perspective for engineering PSC nanoplatforms in GBM (GBM) theranostics centers on overcoming key translational challenges—specifically, bypassing the formidable BBB and navigating tumor heterogeneity—through the synergistic integration of advanced methodologies. This new generation of multifunctional nanoplatforms must be intelligent, precisely targeted, and capable of personalized adaptation. The convergence of bio-orthogonal chemistry, artificial intelligence (AI)-driven design, and patient-derived models is poised to revolutionize the rational development, preclinical testing, and clinical readiness of PSC-based delivery systems.
A crucial opportunity lies in leveraging bio-orthogonal chemistry for enhanced nanoplatform functionalization and targeted delivery. Bio-orthogonal reactions, such as the Inverse Electron-Demand Diels-Alder (IEDDA) reaction or copper-free click chemistry, enable the highly specific and rapid conjugation of targeting ligands (e.g., peptides or antibodies), therapeutic payloads, or imaging agents onto PSC carriers. Crucially, this occurs under mild physiological conditions. This precision is vital for directing nanoplatforms toward GBM cells or stromal components, thereby enhancing specificity toward the tumor microenvironment (TME), minimizing off-target effects, and overcoming the long-standing challenge of stabilizing conjugations without diminishing bioactivity. Furthermore, these chemical tools facilitate advanced strategies, such as pre-targeting, where a modified PSC carrier first accumulates at the tumor site, followed by a second component that rapidly "clicks" into place, enhancing local drug activation. Bio-orthogonal strategies can also be employed for cellular engineering, allowing the introduction of artificial receptors onto immune or stem cells to create highly specific cellular vehicles that guide nanoplatforms directly to the tumor.
The complexity inherent in optimizing nanoplatform design—including composition, size, morphology, surface charge, and stimuli-responsive drug release kinetics—necessitates the adoption of AI-driven strategies. Machine learning (ML) and deep learning algorithms, such as convolutional neural networks (CNNs), are becoming indispensable for predicting optimal synthesis parameters, which can accelerate the development cycle, reduce costs, and refine large-scale manufacturing. Computational modeling and AI can be utilized to predict the binding affinity of nanocarriers to specific receptor targets, forecast BBB permeability, and assess potential toxicity (ADMET parameters) in silico before laboratory synthesis. Beyond nanocarrier optimization, AI-enabled platforms are crucial for analyzing vast data sets from multi-omics profiling and radiomics, enabling the stratification of GBM patients and the customization of nanomedicine compositions to match individual tumor molecular signatures. AI-assisted algorithms are also essential for optimizing the material properties of PSC-based biomaterials, predicting how variations in structure influence therapeutic efficacy.
To ensure translational success, these intelligently designed and precisely functionalized nanoplatforms must be rigorously validated in human-relevant preclinical models. Patient-derived models (PDMs), including GBM organoids (GBOs), patient-derived cancer organoids (PDCOs), and advanced microfluidic BBB chips, are crucial for this purpose. Unlike traditional two-dimensional cultures, GBOs and PDCOs retain the histopathological, genomic, and molecular heterogeneity of the original tumor and facilitate the study of TME interactions. The integration of GBOs into organ-on-a-chip (OOC) devices, particularly those incorporating perfusable, patient-specific microvascular systems, is promising. These devices provide an accurate platform for high-throughput screening to assess nanoplatform transport across the BBB and evaluate patient-specific treatment responses. Moving forward, the fusion of PDM technology with AI will allow for timely analysis of treatment response, generating predictive digital twins that simulate therapeutic futures and accelerate the clinical pipeline for highly customized PSC nanotheranostics.
Finally, the versatility of PSC nanoplatforms makes them ideal vehicles for synergistic combination therapies. Certain PSCs can intrinsically activate immune cells, suggesting powerful opportunities to combine immunomodulators, adjuvants, or cytokines with chemotherapy for synergistic effects. The integration of PSC nanoplatforms with radiotherapy could also enhance treatment outcomes by co-delivering radiosensitizers. Moreover, these carriers are promising for advanced genetic medicine; co-delivery of gene-editing tools, such as CRISPR-Cas9 plasmids or siRNA, could disrupt oncogenes, although ensuring high specificity in GBM cells remains a formidable challenge that may be addressed by the targeting strategies discussed above.
In conclusion, addressing the multifaceted challenges in production, standardization, and safety is critical for clinical translation. Aligning research methods and establishing uniform testing conditions using the advanced models described will be vital for moving PSC-based approaches from the laboratory to the clinic. With continued innovation and cooperation among academia, industry, and regulators, the intelligent design and application of PSC-based nanomedicines hold considerable promise as a future therapeutic modality for GBM.
Acknowledgements
The authors thank their colleagues at the Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital, Sichuan University and West China Xiamen Hospital of Sichuan University, for their cooperation and support. This work was supported by the National Natural Science Foundation of China (82202227).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Xing Y, He M, Su Z, Yasinjan F, Liu J, Wang H. et al. Emerging trends and research foci of epithelial-mesenchymal transition in gliomas: A scientometric analysis and review. Front Oncol. 2022;12:1015236
2. Caprifico AE, Foot PJ, Polycarpou E, Calabrese G. Overcoming the blood-brain barrier: Functionalised chitosan nanocarriers. Pharmaceutics. 2020;12(11):1013
3. Zhang T-T, Li W, Meng G, Wang P, Liao W. Strategies for transporting Nanoparticles across the blood-brain barrier. Biomater Sci. 2016;4(2):219-229
4. Zhang J, Yang J, Chen Y, Mao Q, Li S, Xiong W. et al. Genetic variants of VEGF (rs201963 and rs3025039) and KDR (rs7667298, rs2305948, and rs1870377) are associated with glioma risk in a Han Chinese population: a case-control study. Mol Neurobiol. 2016;53:2610-2618
5. Wickström M, Dyberg C, Milosevic J, Einvik C, Calero R, Sveinbjörnsson B. et al. Wnt/β-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat Commun. 2015;6(1):8904
6. Zhong H, Liu S, Cao F, Zhao Y, Zhou J, Tang F. et al. Dissecting tumor antigens and immune subtypes of glioma to develop mRNA vaccine. Front Immunol. 2021;12:709986
7. Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M. et al. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. 2018;9(1):2336
8. Taylor OG, Brzozowski JS, Skelding KA. GBM multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:963
9. Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136-151
10. Toy R, Peiris PM, Ghaghada KB, Karathanasis E. Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of Nanoparticles. Nanomedicine. 2014;9(1):121-134
11. Rock K, McArdle O, Forde P, Dunne M, Fitzpatrick D, O'Neill B. et al. A clinical review of treatment outcomes in GBM multiforme—the validation in a non-trial population of the results of a randomised Phase III clinical trial: has a more radical approach improved survival? Br J Radiol. 2012;85(1017):e729-e733
12. Munoz JL, Walker ND, Scotto KW, Rameshwar P. Temozolomide competes for P-glycoprotein and contributes to chemoresistance in GBM cells. Cancer Lett. 2015;367(1):69-75
13. Nowak M, Brown TD, Graham A, Helgeson ME, Mitragotri S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng Transl Med. 2020;5(2):e10153
14. Säälik P, Lingasamy P, Toome K, Mastandrea I, Rousso-Noori L, Tobi A. et al. Peptide-guided Nanoparticles for GBM targeting. J Control Release. 2019;308:109-118
15. Branco F, Cunha J, Mendes M, Vitorino C, Sousa JJ. Peptide-hitchhiking for the development of nanosystems in GBM. ACS nano. 2024;18(26):16359-16394
16. Ahmadi M, Ashoub MH, Heydarian K, Abolghasemi S, Dawi EA, Amiri M. Magnetic and pH sensitive nanocomposite microspheres for controlled temozolomide delivery in GBM cells. Sci Rep. 2024;14(1):1-15
17. Zhou B-BS, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8(10):806-823
18. Ahmad S, Khan I, Pandit J, Emad NA, Bano S, Dar KI. et al. Brain targeted delivery of carmustine using chitosan coated Nanoparticles via nasal route for GBM treatment. Int J Biol Macromol. 2022;221:435-445
19. Sukumar UK, Bose RJ, Malhotra M, Babikir HA, Afjei R, Robinson E. et al. Intranasal delivery of targeted polyfunctional gold-iron oxide Nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of GBM to temozolomide. Biomaterials. 2019;218:119342
20. Bărăian A-I, Raduly L, Zănoagă O, Iacob B-C, Barbu-Tudoran L, Dinte E. et al. Targeting JAK/STAT3 in GBM cells using an alginate-PNIPAm molecularly imprinted hydrogel for the sustained release of ruxolitinib. Int J Biol Macromol. 2025;298:140025
21. Belyaeva AA, Averchuk AS, Rozanova NA, Alexandrova OP, Solomakha OA, Nashchekina YA. et al. Thermosensitive injectable fibrillar gels based on cellulose nanocrystals grafted with poly (N-isopropylacrylamide) as biocompatible brain implants. Carbohydr Polym. 2024;346:122596
22. Bellary A, Nowak C, Iwanicki I, Flores-Guzman F, Wu L, Kandel JJ. et al. Non-viral nitric oxide-based gene therapy improves perfusion and liposomal doxorubicin sonopermeation in neuroblastoma models. Theranostics. 2023;13(10):3402
23. Lu G, Wang X, Li F, Wang S, Zhao J, Wang J. et al. Engineered biomimetic Nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against GBM. Nat Commun. 2022;13(1):4214
24. Shen Z, Liu T, Li Y, Lau J, Yang Z, Fan W. et al. Fenton-reaction-acceleratable magnetic Nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS nano. 2018;12(11):11355-11365
25. Li D, Ren T, Wang X, Xiao Z, Sun G, Zhang N. et al. A Tween-80 modified hypoxia/esterase dual stimulus-activated nanomicelle as a delivery platform for carmustine-Design, synthesis, and biological evaluation. Int J Biol Macromol. 2024;274:133404
26. Kim BD, Mondal SK, Kenyon E, Chen M, Mallett CL, deCarvalho AC. et al. Nanoparticle Delivery of an Oligonucleotide Payload in a GBM Multiforme Animal Model. J Vis Exp. 2024(211):e66986
27. Dash BS, Lu Y-J, Huang Y-S, Chen J-P. Chitosan-coated magnetic graphene oxide for targeted delivery of doxorubicin as a nanomedicine approach to treat GBM. Int J Biol Macromol. 2024;260:129401
28. Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104(8):2224-2234
29. Choi M-R, Bardhan R, Stanton-Maxey KJ, Badve S, Nakshatri H, Stantz KM. et al. Delivery of Nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer Nanotechnol. 2012;3:47-54
30. Niu W, Xiao Q, Wang X, Zhu J, Li J, Liang X. et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based Nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484-1492
31. Harder BG, Blomquist MR, Wang J, Kim AJ, Woodworth GF, Winkles JA. et al. Developments in blood-brain barrier penetrance and drug repurposing for improved treatment of GBM. Front Oncol. 2018;8:462
32. Sarkar S, Greer J, Marlowe NJ, Medvid A, Ivan ME, Kolishetti N. et al. Stemness, invasion, and immunosuppression modulation in recurrent GBM using nanotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024;16(4):e1976
33. Bors LA, Erdő F. Overcoming the blood-brain barrier. challenges and tricks for CNS drug delivery. Sci Pharm. 2019;87(1):6
34. Yu D, Ding Q, Xiang C, Wang D, Hu L, Wang J. et al. NIR-II Engineered Exosome Nanotheranostic Probes for “Oriented Blasting” in Orthotopic GBM. ACS nano. 2025;19(25):22900-22913
35. Su X, Liu Y, Zhong Y, Shangguan P, Liu J, Luo Z. et al. A Brain-Targeting NIR-II Polymeric Phototheranostic Nanoplatform toward Orthotopic Drug-Resistant GBM. Nano Lett. 2025;25(9):3445-3454
36. Huang R, Harmsen S, Samii JM, Karabeber H, Pitter KL, Holland EC. et al. High precision imaging of microscopic spread of GBM with a targeted ultrasensitive SERRS molecular imaging probe. Theranostics. 2016;6(8):1075
37. Kawak P, Sawaftah NMA, Pitt WG, Husseini GA. Transferrin-targeted liposomes in GBM therapy: a review. Int J Mol Sci. 2023;24(17):13262
38. Ruan S, Yuan M, Zhang L, Hu G, Chen J, Cun X. et al. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold Nanoparticles. Biomaterials. 2015;37:425-435
39. Zhou Y, Zhu F, Liu Y, Zheng M, Wang Y, Zhang D. et al. Blood-brain barrier-penetrating siRNA nanomedicine for Alzheimer's disease therapy. Sci Adv. 2020;6(41):eabc7031
40. Garcia-Bennett A, Nees M, Fadeel B. In search of the Holy Grail: folate-targeted Nanoparticles for cancer therapy. Biochem Pharmacol. 2011;81(8):976-984
41. Petri B, Bootz A, Khalansky A, Hekmatara T, Müller R, Uhl R. et al. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly (butyl cyanoacrylate) Nanoparticles: revisiting the role of surfactants. J Control Release. 2007;117(1):51-58
42. Gonçalves DP, Rodriguez RD, Kurth T, Bray LJ, Binner M, Jungnickel C. et al. Enhanced targeting of invasive GBM cells by peptide-functionalized gold nanorods in hydrogel-based 3D cultures. Acta Biomater. 2017;58:12-25
43. Liu Y, Luo J, Chen X, Liu W, Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nanomicro Lett. 2019;11(1):100
44. Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34-47
45. Soares TB, Loureiro L, Carvalho A, Oliveira MECR, Dias A, Sarmento B. et al. Lipid nanocarriers loaded with natural compounds: Potential new therapies for age related neurodegenerative diseases? Prog Neurobiol. 2018;168:21-41
46. Peng X-H, Qian X, Mao H, Wang AY, Chen Z, Nie S. et al. Targeted magnetic iron oxide Nanoparticles for tumor imaging and therapy. Int J Nanomedicine. 2008;3(3):311-321
47. Stummer W, Reulen H-J, Meinel T, Pichlmeier U, Schumacher W, Tonn J-C. et al. Extent of resection and survival in GBM multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564-576
48. Blau R, Epshtein Y, Pisarevsky E, Tiram G, Dangoor SI, Yeini E. et al. Image-guided surgery using near-infrared Turn-ON fluorescent nanoprobes for precise detection of tumor margins. Theranostics. 2018;8(13):3437
49. Gerritsen JKW, Broekman MLD, De Vleeschouwer S, Schucht P, Nahed BV, Berger MS. et al. Safe surgery for GBM: Recent advances and modern challenges. Neurooncol Pract. 2022;9(5):364-379
50. Polyak A, Ross TL. Nanoparticles for SPECT and PET imaging: towards personalized medicine and theranostics. Curr Med Chem. 2018;25(34):4328-4353
51. Ozgür B, Puris E, Brachner A, Appelt-Menzel A, Oerter S, Balzer V. et al. Characterization of an ipolysaccharide -based barrier model for blood-brain barrier investigations using the SBAD0201 stem cell line. Fluids Barriers CNS. 2023;20(1):96
52. Niccoli Asabella A, Di Palo A, Altini C, Ferrari C, Rubini G. Multimodality imaging in tumor angiogenesis: present status and perspectives. Int J Mol Sci. 2017;18(9):1864
53. Ren L, Xu P, Yao J, Wang Z, Shi K, Han W. et al. Targeting the mitochondria with pseudo-stealthy nanotaxanes to impair mitochondrial biogenesis for effective cancer treatment. ACS nano. 2022;16(7):10242-10259
54. Luk BT, Zhang L. Current advances in polymer-based nanotheranostics for cancer treatment and diagnosis. ACS Appl Mater Interfaces. 2014;6(24):21859-21873
55. Liu J, Zheng J, Nie H, Zhang D, Cao D, Xing Z. et al. Molybdenum disulfide-based hyaluronic acid-guided multifunctional theranostic nanoplatform for magnetic resonance imaging and synergetic chemo-photothermal therapy. J Colloid Interface Sci. 2019;548:131-144
56. Zhuang D, Zhang H, Hu G, Guo B. Recent development of contrast agents for magnetic resonance and multimodal imaging of GBM. J Nanobiotechnology. 2022;20(1):284
57. Czarnywojtek A, Borowska M, Dyrka K, Van Gool S, Sawicka-Gutaj N, Moskal J. et al. GBM multiforme: the latest diagnostics and treatment techniques. Pharmacology. 2023;108(5):423-431
58. Majós C, Cos M, Castañer S, Pons A, Gil M, Fernández-Coello A. et al. Preradiotherapy MR imaging: a prospective pilot study of the usefulness of performing an MR examination shortly before radiation therapy in patients with GBM. AJNR Am J Neuroradiol. 2016;37(12):2224-2230
59. Maiter A, Butteriss D, English P, Lewis J, Hassani A, Bhatnagar P. Assessing the diagnostic accuracy and interobserver agreement of MRI perfusion in differentiating disease progression and pseudoprogression following treatment for GBM in a tertiary UK centre. Clin Radiol. 2022;77(8):e568-e575
60. Buckland BC. The process development challenge for a new vaccine. Nat Med. 2005;11(Suppl 4):S16-S19
61. Swierczewska M, Han HS, Kim K, Park J, Lee S. polysaccharide-based Nanoparticles for theranostic nanomedicine. Advanced drug delivery reviews. 2016;99:70-84
62. Kasprzyk M, Opiła G, Hinz A, Stankiewicz S, Bzowska M, Wolski K. et al. Hyaluronic acid-coated SPIONs with attached folic acid as potential T2 MRI contrasts for anticancer therapies. ACS Appl Mater Interfaces. 2025;17(6):9059-9073
63. Huang R-Y, Lin Y-H, Lin S-Y, Li Y-N, Chiang C-S. et al. Magnetic ternary nanohybrids for nonviral gene delivery of stem cells and applications on cancer therapy. Theranostics. 2019;9(8):2411
64. Chang Y-L, Liao P-B, Wu P-H, Chang W-J, Lee S-Y, Huang H-M. Cancer cytotoxicity of a hybrid hyaluronan-superparamagnetic iron oxide nanoparticle material: An in-vitro evaluation. Nanomaterials. 2022;12(3):496
65. Huang H-M, Wu P-H, Chou P-C, Hsiao W-T, Wang H-T, Chiang H-P. et al. Enhancement of T2* weighted MRI imaging sensitivity of U87MG GBM cells using γ-ray irradiated low molecular weight hyaluronic acid-conjugated iron Nanoparticles. Int J Nanomedicine. 2021;16:3789-3802
66. Shevtsov M, Nikolaev B, Marchenko Y, Yakovleva L, Skvortsov N, Mazur A. et al. Targeting experimental orthotopic GBM with chitosan-based superparamagnetic iron oxide Nanoparticles (CS-DX-SPIONs). Int J Nanomedicine. 2018:1471-1482
67. Chauhan A, Midha S, Kumar R, Meena R, Singh P, Jha SK. et al. Rapid tumor inhibition via magnetic hyperthermia regulated by caspase 3 with time-dependent clearance of iron oxide Nanoparticles. Biomater Sci. 2021;9(8):2972-2990
68. Siow WX, Chang Y-T, Babič M, Lu Y-C, Horák D, Ma Y-H. Interaction of poly-l-lysine coating and heparan sulfate proteoglycan on magnetic nanoparticle uptake by tumor cells. Int J Nanomedicine. 2018:1693-1706
69. Mu Q, Jeon M, Hsiao MH, Patton VK, Wang K, Press OW. et al. Stable and efficient Paclitaxel Nanoparticles for targeted GBM therapy. Adv Healthc Mater. 2015;4(8):1236-1245
70. Mansur AA, Caires AJ, Carvalho SM, Capanema NS, Carvalho IC, Mansur HS. Dual-functional supramolecular nanohybrids of quantum dot/biopolymer/chemotherapeutic drug for bioimaging and killing brain cancer cells in vitro. Colloids Surf B Biointerfaces. 2019;184:110507
71. Carvalho IC, Mansur AA, Carvalho SM, Florentino RM, Mansur HS. L-cysteine and poly-L-arginine grafted carboxymethyl cellulose/Ag-In-S quantum dot fluorescent nanohybrids for in vitro bioimaging of brain cancer cells. Int J Biol Macromol. 2019;133:739-753
72. Sabouri Z, Akbari A, Hosseini HA, Khatami M, Darroudi M. Tragacanth-mediate synthesis of NiO nanosheets for cytotoxicity and photocatalytic degradation of organic dyes. Bioprocess Biosyst Eng. 2020;43:1209-1218
73. Unnikrishnan B, Preethi G, Sreelekha T. A comprehensive study on 2D, 3D and solid tumor environment to explore a multifunctional biogenic nanoconjugate. Sci Rep. 2021;11(1):8721
74. Zhao Z, Shen J, Zhang L, Wang L, Xu H, Han Y. et al. Injectable postoperative enzyme-responsive hydrogels for reversing temozolomide resistance and reducing local recurrence after glioma operation. Biomater Sci. 2020;8(19):5306-5316
75. June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361-1365
76. Qu H, Zhang Y, Chen M, Shao S, Chen J, Wu Y. et al. NIR-II photo-and sono-responsive hyaluronic acid-capped nanozymes for GBM-targeting theranostics. Int J Biol Macromol. 2025;306:141737
77. Mansur AA, Carvalho SM, Lobato ZI, Leite MF, Krambrock K, Mansur HS. Bioengineering stimuli-responsive organic-inorganic nanoarchitetures based on carboxymethylcellulose-poly-l-lysine nanoplexes: Unlocking the potential for bioimaging and multimodal chemodynamic-magnetothermal therapy of brain cancer cells. Int J Biol Macromol. 2025;290:138985
78. John JS, Metild P. Fluorescent probe materials based on curcumin derivatives for anti-tumor studies: A review. Results Chem. 2025;18:102831
79. Mazarei M, Mohammadi Arvejeh P, Mozafari M, Khosravian P, Ghasemi S. Anticancer potential of temozolomide-loaded Eudragit-chitosan coated selenium Nanoparticles: In vitro evaluation of cytotoxicity, apoptosis and gene regulation. Nanomaterials. 2021;11(7):1704
80. Novak U, Kaye AH. Extracellular matrix and the brain: components and function. J Clin Neurosci. 2000;7(4):280-290
81. Bruinsmann FA, Alves AdCS, de Fraga Dias A, Silva LFL, Visioli F, Pohlmann AR. et al. Nose-to-brain delivery of simvastatin mediated by chitosan-coated lipid-core nanocapsules allows for the treatment of GBM in vivo. Int J Pharm. 2022;616:121563
82. Turabee MH, Jeong TH, Ramalingam P, Kang JH, Ko YT. N, N, N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr Polym. 2019;203:302-309
83. Long W, Li S, Yang Y, Chen A, Xu M, Zhai H. et al. Self-cross-linked chitosan/albumin-bound nanoparticle hydrogel for inhibition of postsurgery malignant glioma recurrence. ACS Appl Mater Interfaces. 2023;15(49):56774-56785
84. Naves VdML, Bruzadelli RFD, Ionta M, Gremião MPD, Pedreiro LN, Pereira GR. et al. Uptake and Inhibition of P-Glycoprotein-Mediated Efflux Evaluation of Encapsulated Methotrexate Chitosan and Hypromellose Phthalate Nanoparticles for Potential GBM Treatment. Pharmaceutics. 2025;17(2):239
85. Gil-Gonzalo R, Durante-Salmerón DA, Pouri S, Doncel-Pérez E, Alcántara AR, Aranaz I. et al. Chitosan-coated liposome formulations for encapsulation of ciprofloxacin and etoposide. Pharmaceutics. 2024;16(8):1036
86. Chen YM, Chambon J, Moquin A, Hashimoto M, Perrino S, Leibovitch M. et al. Nanoparticle encapsulation enables systemic IGF-Trap delivery to inhibit intra-cerebral glioma growth. Neuro Oncol. 2025;27(5):1227-1240
87. Shirvalilou S, Khoei S, Khoee S, Raoufi NJ, Karimi MR, Shakeri-Zadeh A. Development of a magnetic nano-graphene oxide carrier for improved glioma-targeted drug delivery and imaging: in vitro and in vivo evaluations. Chem Biol Interact. 2018;295:97-108
88. Baati T, Chaabani I, Salek A, Njim L, Selmi M, Al-Kattan A. et al. Chitosan-coated ultrapure silicon Nanoparticles produced by laser ablation: biomedical potential in nano-oncology as a tumor-targeting nanosystem. Nanoscale Adv. 2023;5(11):3044-3052
89. Taheri A, Cheniany M, Ganjeali A, Arefi-Oskouie A. ICP-OES assessment of trace and toxic elements in Ziziphora clinopodioides Lam. from Iran by chemometric approaches. Biometals. 2022;35(6):1169-1186
90. Janát-Amsbury M, Ray A, Peterson C, Ghandehari H. Geometry and surface characteristics of gold Nanoparticles influence their biodistribution and uptake by macrophages. Eur J Pharm Biopharm. 2011;77(3):417-423
91. Dahifale A, Agnihotri TG, Jain A, Jain A. Quality-by-design-engineered mitochondrial targeted Nanoparticles for GBM therapy. RSC Adv. 2024;14(46):34100-34118
92. de Paula GA, de Paula MC, Paes Dutra JA, Carvalho SG, Di Filippo LD, Villanova JCO. et al. Targeted polymeric Nanoparticles as a strategy for the treatment of GBM: a review. Curr Drug Deliv. 2025;22(4):413-430
93. Di Cintio F, Argenziano M, Scomparin A, Capolla S, Busato D, Steffè A. et al. The anti-glypican 1 AT101 antibody as targeting agent to effectively deliver chitosan nanobubbles to GBM cells. Nanomedicine. 2025;20(1):23-36
94. De Capua A, Vecchione R, Sgambato C, Chino M, Lagreca E, Lombardi A. et al. Peptide Functionalization of Emulsion-Based Nanocarrier to Improve Uptake across Blood-Brain Barrier. Pharmaceutics. 2024;16(8):1010
95. Abadi B, Khazaeli P, Forootanfar H, Ranjbar M, Ahmadi-Zeidabadi M, Nokhodchi A. et al. Chitosan-sialic acid Nanoparticles of selenium: Statistical optimization of production, characterization, and assessment of cytotoxic effects against two human GBM cell lines. Int J Pharm. 2023;637:122884
96. Ragelle H, Vandermeulen G, Préat V. Chitosan-based siRNA delivery systems. J Control Release. 2013;172(1):207-218
97. King JL, Valdivia A, Hingtgen SD, Benhabbour SR. Injectable Tumoricidal Neural Stem Cell-Laden Hydrogel for Treatment of GBM Multiforme-An In Vivo Safety, Persistence, and Efficacy Study. Pharmaceutics. 2024;17(1):3
98. Adiguzel S, Karamese M, Kugu S, Kacar EA, Esen MF, Erdogan H. et al. Doxorubicin-loaded liposome-like particles embedded in chitosan/hyaluronic acid-based hydrogels as a controlled drug release model for local treatment of GBM. Int J Biol Macromol. 2024;278:135054
99. Parès L, Naasri S, Delattre L, Therriault H, Liberelle B, De Crescenzo G, Lauzon M-A, Faucheux N, Paquette B, Virgilio N. Macroporous chitosan/alginate hydrogels crosslinked with genipin accumulate and retain GBM cancer cells. RSC Adv. 2024;14(48):35286-35304
100. Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30(12):2180-2198
101. Seo B, Lim K, Kim SS, Oh KT, Lee ES, Choi H-G. et al. Small gold nanorods-loaded hybrid albumin Nanoparticles with high photothermal efficacy for tumor ablation. Colloids Surf B Biointerfaces. 2019;179:340-351
102. Caro C, Gámez F, Quaresma P, Páez-Muñoz JM, Domínguez A, Pearson JR. et al. Fe3O4-Au core-shell Nanoparticles as a multimodal platform for in vivo imaging and focused photothermal therapy. Pharmaceutics. 2021;13(3):416
103. Ramalho MJ, Serra É, Lima J, Loureiro JA, Pereira MC. Chitosan-PLGA mucoadhesive Nanoparticles for gemcitabine repurposing for GBM therapy. Eur J Pharm Biopharm. 2024;200:114326
104. Liu S, Liu J, Li H, Mao K, Wang H, Meng X. et al. An optimized ionizable cationic lipid for brain tumor-targeted siRNA delivery and GBM immunotherapy. Biomaterials. 2022;287:121645
105. Chen H-A, Lu Y-J, Dash BS, Chao Y-K, Chen J-P. Hyaluronic acid-modified cisplatin-encapsulated poly (lactic-co-glycolic acid) magnetic Nanoparticles for dual-targeted NIR-responsive chemo-photothermal combination cancer therapy. Pharmaceutics. 2023;15(1):290
106. Abolfathi S, Zare M. The evaluation of chitosan hydrogel based curcumin effect on DNMT1, DNMT3A, DNMT3B, MEG3, HOTAIR gene expression in GBM cell line. Mol Biol Rep. 2023;50(7):5977-5989
107. Sathiyaseelan A, Saravanakumar K, Mariadoss AVA, Wang M-H. pH-controlled nucleolin targeted release of dual drug from chitosan-gold based aptamer functionalized nano drug delivery system for improved GBM treatment. Carbohydr Polym. 2021;262:117907
108. Zhou Y, Pereira G, Tang Y, James M, Zhang M. 3D porous scaffold-based high-throughput platform for cancer drug screening. Pharmaceutics. 2023;15(6):1691
109. Gherardini L, Vetri Buratti V, Maturi M, Inzalaco G, Locatelli E, Sambri L. et al. Loco-regional treatment with temozolomide-loaded thermogels prevents GBM recurrences in orthotopic human xenograft models. Sci Rep. 2023;13(1):4630
110. Liu X, Li W, Chen T, Yang Q, Huang T, Fu Y. et al. Hyaluronic acid-modified micelles encapsulating Gem-C12 and HNK for GBM Multiforme Chemotherapy. Mol Pharm. 2018;15(3):1203-1214
111. Dash BS, Lu Y-J, Pejrprim P, Lan Y-H, Chen J-P. Hyaluronic acid-modified, IR780-conjugated and doxorubicin-loaded reduced graphene oxide for targeted cancer chemo/photothermal/photodynamic therapy. Biomater Adv. 2022;136:212764
112. Catania G, Rodella G, Vanvarenberg K, Préat V, Malfanti A. Combination of hyaluronic acid conjugates with immunogenic cell death inducer and CpG for GBM local chemo-immunotherapy elicits an immune response and induces long-term survival. Biomaterials. 2023;294:122006
113. Fernandes J, Ghate MV, Mallik SB, Lewis SA. Amino acid conjugated chitosan Nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int J Pharm. 2018;547(1-2):563-571
114. Yang Y, Zhang X, Wu S, Zhang R, Zhou B, Zhang X. et al. Enhanced nose-to-brain delivery of siRNA using hyaluronan-enveloped nanomicelles for glioma therapy. J Control Release. 2022;342:66-80
115. Peura L, Malmioja K, Huttunen K, Leppänen J, Hämäläinen M, Forsberg MM. et al. Design, synthesis and brain uptake of LAT1-targeted amino acid prodrugs of dopamine. Pharm Res. 2013;30:2523-2537
116. Ahmadi Nasab N, Hassani Kumleh H, Beygzadeh M, Teimourian S, Kazemzad M. Delivery of curcumin by a pH-responsive chitosan mesoporous silica Nanoparticles for cancer treatment. Artif Cells Nanomed Biotechnol. 2018;46(1):75-81
117. Safarians G, Sohrabi A, Solomon I, Xiao W, Bastola S, Rajput BW. et al. GBM Spheroid Invasion through Soft, Brain-Like Matrices Depends on Hyaluronic Acid-CD44 Interactions. Adv Healthc Mater. 2023;12(14):2203143
118. Xiao W, Wang S, Zhang R, Sohrabi A, Yu Q, Liu S. et al. Bioengineered scaffolds for 3D culture demonstrate extracellular matrix-mediated mechanisms of chemotherapy resistance in GBM. Matrix Biol. 2020;85:128-146
119. Martinez-Quintanilla J, He D, Wakimoto H, Alemany R, Shah K. Encapsulated stem cells loaded with hyaluronidase-expressing oncolytic virus for brain tumor therapy. Mol Ther. 2015;23(1):108-118
120. HPS AK, Saurabh CK, Fazita MN, Syakir M, Davoudpour Y, Rafatullah M. et al. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr Polym. 2016;150:216-226
121. Periayah MH, Halim AS, Saad AZM. Chitosan: A promising marine polysaccharide for biomedical research. Pharmacogn Rev. 2016;10(19):39-42
122. Wasiak I, Kulikowska A, Janczewska M, Michalak M, Cymerman IA, Nagalski A. et al. Dextran nanoparticle synthesis and properties. PLoS One. 2016;11(1):e0146237
123. Zhang W, An M, Xi J, Liu H. Targeting CpG adjuvant to lymph node via dextran conjugate enhances antitumor immunotherapy. Bioconjug Chem. 2017;28(7):1993-2000
124. Zhang J, Hao G, Yao C, Hu S, Hu C, Zhang B. Paramagnetic albumin decorated CuInS 2/ZnS QDs for CD133+ glioma bimodal MR/fluorescence targeted imaging. J Mater Chem B. 2016;4(23):4110-4118
125. Kim S-S, Rait A, Kim E, Pirollo KF, Nishida M, Farkas N. et al. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes GBM to chemotherapy and improves survival. ACS nano. 2014;8(6):5494-5514
126. van Solinge TS, Nieland L, Chiocca EA, Broekman ML. Advances in local therapy for GBM—taking the Figureht to the tumour. Nat Rev Neurol. 2022;18(4):221-236
127. Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y. et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12(7):692-700
128. Belykh E, Shaffer KV, Lin C, Byvaltsev VA, Preul MC, Chen L. Blood-brain barrier, blood-brain tumor barrier, and fluorescence-guided neurosurgical oncology: delivering optical labels to brain tumors. Front Oncol. 2020;10:739
129. Arora A, Somasundaram K. GBM vs temozolomide: can the red queen race be won? Cancer Biol Ther. 2019;20(8):1083-1090
130. Barrios-Esteban S, Reimóndez-Troitiño S, Cabezas-Sainz P, de la Fuente M, Sánchez L, Rahman R. et al. Protamine-Based Nanotherapeutics for Gene Delivery to GBM Cells. Mol Pharm. 2025;22(5):2466-2481
131. Hu M, Liu H, Zhang Y, Lu D, Zheng L, Wang Y. et al. Preparation and evaluation of the PD0721-DOX antibody-drug conjugate targeting EGFRvIII to inhibit GBM. Exp Ther Med. 2024;27(6):254
132. He W, Li X, Morsch M, Ismail M, Liu Y, Rehman FU. et al. Brain-targeted codelivery of Bcl-2/Bcl-xl and Mcl-1 inhibitors by biomimetic Nanoparticles for orthotopic GBM therapy. ACS nano. 2022;16(4):6293-6308
133. Van Straten D, Bimbo JF, Hennink WE, Vermonden T, Schiffelers RM. Nanoparticle-in-Hydrogel Delivery System for the Sequential Release of Two Drugs. Pharmaceutics. 2025;17(1):127
134. Liu R, Liang Q, Luo JQ, Li YX, Zhang X, Fan K. et al. Ferritin-Based Nanocomposite Hydrogel Promotes Tumor Penetration and Enhances Cancer Chemoimmunotherapy. Adv Sci. 2024;11(3):2305217
135. Wang Y, Wei Y, Wu Y, Zong Y, Song Y, Pu S. et al. Multifunctional nano-realgar hydrogel for enhanced GBM synergistic chemotherapy and radiotherapy: a new paradigm of an old drug. Int J Nanomedicine. 2023;18:743-763
136. Graham-Gurysh EG, Murthy AB, Moore KM, Hingtgen SD, Bachelder EM, Ainslie KM. Synergistic drug combinations for a precision medicine approach to interstitial GBM therapy. J Control Release. 2020;323:282-292
137. Graham-Gurysh EG, Moore KM, Schorzman AN, Lee T, Zamboni WC, Hingtgen SD. et al. Tumor responsive and tunable polymeric platform for optimized delivery of paclitaxel to treat GBM. ACS Appl Mater Interfaces. 2020;12(17):19345-19356
138. Liu S, Yang S, Ho PC. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan Nanoparticles for drug delivery to the brain. Asian J Pharm Sci. 2018;13(1):72-81
139. Bahraminasab M, Asgharzade S, Doostmohamadi A, Satari A, Hasannejad F, Arab S. Development of a hydrogel-based three-dimensional (3D) GBM cell lines culture as a model system for CD73 inhibitor response study. Biomed Eng Online. 2024;23(1):127
140. Wang C, Li J, Sinha S, Peterson A, Grant GA, Yang F. Mimicking brain tumor-vasculature microanatomical architecture via co-culture of brain tumor and endothelial cells in 3D hydrogels. Biomaterials. 2019;202:35-44
141. Li Q, Lin H, Rauch J, Deleyrolle LP, Reynolds BA, Viljoen HJ. et al. Scalable culturing of primary human GBM tumor-initiating cells with a cell-friendly culture system. Sci Rep. 2018;8(1):3531
142. Delattre L, Naasri S, Solano AG, Therriault H, Bergeron-Fortier S, Moreau V. et al. The role of pore size and mechanical properties on the accumulation, retention and distribution of F98 GBM cells in macroporous hydrogels. Biomed Mater. 2024;19(4):041-045
143. Solano AG, Dupuy J, Therriault H, Liberelle B, Faucheux N, Lauzon M-A. et al. An alginate-based macroporous hydrogel matrix to trap cancer cells. Carbohydr Polym. 2021;266:118115
144. Dragoj M, Stojkovska J, Stanković T, Dinić J, Podolski-Renić A, Obradović B. et al. Development and validation of a long-term 3D GBM cell culture in alginate microfibers as a novel bio-mimicking model system for preclinical drug testing. Brain Sci. 2021;11(8):1025
145. Musah-Eroje A, Watson S. A novel 3D in vitro model of GBM reveals resistance to temozolomide which was potentiated by hypoxia. J Neurooncol. 2019;142(2):231-240
146. Hwang HH, Kim HS, Lee DY. Gastrointestinally absorbable lactoferrin-heparin conjugate with anti-angiogenic activity for treatment of brain tumor. J Control Release. 2023;355:730-744
147. Zhang J, Shin MC, Yang VC. Magnetic targeting of novel heparinized iron oxide Nanoparticles evaluated in a 9L-glioma mouse model. Pharm Res. 2014;31:579-592
148. Wang F, Zhang S, Sun F, Chen W, Liu C, Dong H. et al. Anti-angiogenesis and anti-immunosuppression gene therapy through targeting COUP-TFII in an in situ GBM mouse model. Cancer Gene Ther. 2024;31(8):1135-1150
149. Kim HS, Seol JH, Hwang HH, Lee DY. Nanoarchitectured conjugates targeting angiogenesis: investigating heparin-taurocholate acid conjugates (LHT7) as an advanced anti-angiogenic therapy for brain tumor treatment. Biomater Res. 2023;27(1):89
150. Fang JH, Chiu TL, Huang WC, Lai YH, Hu SH, Chen YY. et al. Dual-Targeting Lactoferrin-Conjugated Polymerized Magnetic Polydiacetylene-Assembled Nanocarriers with Self-Responsive Fluorescence/Magnetic Resonance Imaging for In Vivo Brain Tumor Therapy. Adv Healthc Mater. 2016;5(6):688-695
151. Zhao Y-Z, Lin Q, Wong HL, Shen X-T, Yang W, Xu H-L. et al. Glioma-targeted therapy using Cilengitide Nanoparticles combined with UTMD enhanced delivery. J Control Release. 2016;224:112-125
152. Lopez V, Schuh HM, Mirza S, Vaaßen VJ, Schmidt MS, Sylvester K. et al. Heparins are potent inhibitors of ectonucleotide pyrophosphatase/phospho-diesterase-1 (NPP1)-a promising target for the immunotherapy of cancer. Front Immunol. 2023;14:1173634
153. van Solinge TS, Friis KP, O'Brien K, Verschoor RL, van Aarle J, Koekman A. et al. Heparin interferes with the uptake of liposomes in glioma. Int J Pharm X. 2023;6:100191
154. Mrowczynski OD, Madhankumar AB, Sundstrom JM, Zhao Y, Kawasawa YI, Slagle-Webb B. et al. Exosomes impact survival to radiation exposure in cell line models of nervous system cancer. Oncotarget. 2018;9(90):36083
155. Drouet L, Harenberg J, Torri G. The multiple faces of heparin: opportunities in COVID-19 infection and beyond. Thromb Haemost. 2020;120(10):1347-1350
156. Wang P, Chi L, Zhang Z, Zhao H, Zhang F, Linhardt RJ. Heparin: An old drug for new clinical applications. Carbohydr Polym. 2022;295:119818
157. Woods RR, Lesser GJ. Management of Thromboembolic Disease in patients with primary and metastatic brain tumors. Current Treatment Options in Oncology. 2023;24(9):1293-1303
158. Benkő B-M, Tóth G, Moldvai D, Kádár S, Szabó E, Szabó Z-I. et al. Cyclodextrin encapsulation enabling the anticancer repositioning of disulfiram: Preparation, analytical and in vitro biological characterization of the inclusion complexes. Int J Pharm. 2024;657:124187
159. Omuro A, DeAngelis LM. GBM and other malignant gliomas: a clinical review. Jama. 2013;310(17):1842-1850
160. Kim S-S, Rait A, Kim E, DeMarco J, Pirollo KF, Chang EH. Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anti-cancer efficacy and reduces toxicity in a mouse model of GBM. Cancer Lett. 2015;369(1):250-258
161. Melone L, Bach A, Lamura G, Canepa F, Nivajärvi R, Olsson V. et al. Cyclodextrin-Based Organic Radical Contrast Agents for in vivo Imaging of Gliomas. ChemPlusChem. 2020;85(6):1171-1178
162. Jiang SQ, Li XH, Zhang F, Mao JJ, Cao MH, Zhang XN. et al. Manganese dioxide-based nanocarrier delivers paclitaxel to enhance chemotherapy against orthotopic glioma through hypoxia relief. Small Methods. 2022;6(7):2101531
163. Bastiancich C, Molinar C, Tchoghandjian A, Cavalli R, Scomparin A. Treating glioblastoma with dextrin-based polymers. Expert Opin Drug Deliv. 2025;22(12):1915-1935
164. Lin E-Y, Chen Y-S, Li Y-S, Chen S-R, Lee C-H, Huang M-H. et al. Liposome consolidated with cyclodextrin provides prolonged drug retention resulting in increased drug bioavailability in brain. Int J Mol Sci. 2020;21(12):4408
165. Najlaoui F, Pigeon P, Abdelkafi Z, Leclerc S, Durand P, El Ayeb M. et al. Phthalimido-ferrocidiphenol cyclodextrin complexes: Characterization and anticancer activity. Int J Pharm. 2015;491(1-2):323-334
166. Tran NH, Ryzhov V, Volnitskiy A, Amerkanov D, Pack F, Golubev AM. et al. Radiosensitizing Effect of Dextran-Coated Iron Oxide Nanoparticles on Malignant Glioma Cells. Int J Mol Sci. 2023;24(20):15150
167. Malhotra M, Gooding M, Evans JC, O'Driscoll D, Darcy R, O'Driscoll CM. Cyclodextrin-siRNA conjugates as versatile gene silencing agents. Eur J Pharm Sci. 2018;114:30-37
168. Zhang H, Zhang W, Zhou Y, Jiang Y, Li S. Dual functional mesoporous silicon Nanoparticles enhance the radiosensitivity of VPA in GBM. Transl Oncol. 2017;10(2):229-240
169. Franco L, Isse AA, Barbon A, Altomare L, Hyppönen V, Rosa J. et al. Redox Properties and in Vivo Magnetic Resonance Imaging of Cyclodextrin-Polynitroxides Contrast Agents. ChemPhysChem. 2023;24(19):e202300100
170. Yang H, Ren J, Zhao M, Chen C, Wang F, Chen Z. Novel electrochemical immunosensor for O6-methylguanine-DNA methyltransferase gene methylation based on graphene oxide-magnetic Nanoparticles-β-cyclodextrin nanocomposite. Bioelectrochemistry. 2022;146:108111
171. Qiu JR, Kong LD, Cao XY, Li AJ, Wei P, Wang L. et al. Enhanced delivery of therapeutic siRNA into GBM cells using dendrimer-entrapped gold Nanoparticles conjugated with β-cyclodextrin. Nanomaterials. 2018;8(3):131
172. Gularte MS, Quadrado RF, Pedra NS, Soares MS, Bona NP, Spanevello RM. et al. Preparation, characterization and antitumor activity of a cationic starch-derivative membrane embedded with a β-cyclodextrin/curcumin inclusion complex. Int J Biol Macromol. 2020;148:140-152
173. Cyphert EL, Fu AS, von Recum HA. Featured Article: Chemotherapeutic delivery using pH-responsive, affinity-based release. Exp Biol Med. 2017;242(7):692-699
174. Mohamed MH, Wang C, Peru KM, Headley JV, Wilson LD. Characterization of the Physicochemical Properties of β-Cyclodextrin-Divinyl Sulfone Polymer Carrier-Bile Acid Systems. Mol Pharm. 2017;14(8):2616-2623
175. McCrorie P, Mistry J, Taresco V, Lovato T, Fay M, Ward I. et al. Etoposide and olaparib polymer-coated Nanoparticles within a bioadhesive sprayable hydrogel for post-surgical localised delivery to brain tumours. Eur J Pharm Biopharm. 2020;157:108-120
176. Wu M, Frieboes HB, Chaplain MA, McDougall SR, Cristini V, Lowengrub JS. The effect of interstitial pressure on therapeutic agent transport: coupling with the tumor blood and lymphatic vascular systems. J Theor Biol. 2014;355:194-207
177. da Costa Amaral S, Barbieri SF, Ruthes AC, Bark JM, Winnischofer SMB, Silveira JLM. Cytotoxic effect of crude and purified pectins from Campomanesia xanthocarpa Berg on human GBM cells. Carbohydr Polym. 2019;224:115140
178. Barbugian F, Cadamuro F, Nicotra F, Riccardi C, Russo L. Plasma-treated collagen functionalized with chondroitin sulfate as bioactive and nanostructured extracellular matrix mimics. Nanomedicine. 2024;19(9):799-810
179. Niibori-Nambu A, Yamasaki Y, Kobayashi D, Angata K, Kuno A, Panawan O. et al. Chondroitin sulfate modification of CSPG4 regulates the maintenance and differentiation of glioma-initiating cells via integrin-associated signaling. J Biol Chem. 2024;300(3):105706
180. Kim Y, Kang H, Powathil G, Kim H, Trucu D, Lee W. et al. Role of extracellular matrix and microenvironment in regulation of tumor growth and LAR-mediated invasion in GBM. PLoS One. 2018;13(10):e0204865
181. Liao W-C, Liao C-K, Tseng T-J, Ho Y-J, Chen Y-R, Lin K-H. et al. Chondroitin sulfate synthase 1 enhances proliferation of GBM by modulating PDGFRA stability. Oncogenesis. 2020;9(2):9
182. Tsidulko AY, Kazanskaya GM, Volkov AM, Suhovskih AV, Kiselev RS, Kobozev VV. et al. Chondroitin sulfate content and decorin expression in GBM are associated with proliferative activity of glioma cells and disease prognosis. Cell Tissue Res. 2020;379:147-155
183. Yu X, Dobrikov M, Keir ST, Gromeier M, Pastan IH, Reisfeld R. et al. Synergistic antitumor effects of 9.2. 27-PE38KDEL and ABT-737 in primary and metastatic brain tumors. PLoS One. 2019;14(1):e0210608
184. Spiesberger K, Paulfranz F, Egger A, Reiser J, Vogl C, Rudolf-Scholik J. et al. Large-scale purification of r28M: A bispecific scFv antibody targeting human melanoma produced in transgenic cattle. PLoS One. 2015;10(10):e0140471
185. Wolf KJ, Chen J, Coombes JD, Aghi MK, Kumar S. Dissecting and rebuilding the GBM microenvironment with engineered materials. Nat Rev Mater. 2019;4(10):651-668
186. Strokotova AV, Sokolov DK, Molodykh OP, Koldysheva EV, Kliver EE, Ushakov VS. et al. Prolonged use of temozolomide leads to increased anxiety and decreased content of aggrecan and chondroitin sulfate in brain tissues of aged rats. Biomed Rep. 2023;20(1):7
187. Tsidulko AY, Shevelev OB, Khotskina AS, Kolpakova MA, Suhovskih AV, Kazanskaya GM. et al. Chemotherapy-Induced degradation of glycosylated components of the brain extracellular matrix promotes GBM relapse development in an animal model. Front Oncol. 2021;11:713139
188. Politko MO, Tsidulko AY, Pashkovskaya OA, Kuper KE, Suhovskih AV, Kazanskaya GM. et al. Multiple irradiation affects cellular and extracellular components of the mouse brain tissue and adhesion and proliferation of GBM cells in experimental system in vivo. Int J Mol Sci. 2021;22(24):13350
189. Kalita M, Villanueva-Meyer J, Ohkawa Y, Kalyanaraman C, Chen K, Mohamed E. et al. Synthesis and Screening of α-Xylosides in Human GBM Cells. Mol Pharm. 2020;18(1):451-460
190. Ma C, Wang J, Li Q, Wu Y, Yu Z, Chao Y. et al. Injectable Oxidized High-Amylose Starch Hydrogel Scaffold for Macrophage-Mediated GBM Therapy. Biomaterials. 2025: 123128.
191. Chuang S-H, Chen K-J, Cheng Y-T, Chen Y-S, Lin S-Y, Chou H-Y. et al. A thermo-responsive chemically crosslinked long-term-release chitosan hydrogel system increases the efficiency of synergy chemo-immunotherapy in treating brain tumors. Int J Biol Macromol. 2024;280:135894
192. Chellen T, Bausart M, Maus P, Vanvarenberg K, Limaye N, Préat V. et al. In situ administration of STING-activating hyaluronic acid conjugate primes anti-GBM immune response. Materials Today Bio. 2024;26:101057
193. Zhu L, Ge F, Yang L, Li W, Wei S, Tao Y. et al. Alginate particles with ovalbumin (OVA) peptide can serve as a carrier and adjuvant for immune therapy in B16-OVA cancer model. Med Sci Monit Basic Res. 2017;23:166-172
194. Sousa F, Lee H, Almeida M, Bazzoni A, Rothen-Rutishauser B, Petri-Fink A. Immunostimulatory Nanoparticles delivering cytokines as a novel cancer nanoadjuvant to empower GBM immunotherapy. Drug Deliv Transl Res. 2024;14(10):2655-2667
195. Alifieris C, Trafalis DT. GBM multiforme: Pathogenesis and treatment. Pharmacol Ther. 2015;152:63-82
196. Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J. et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(suppl_4):iv1-iv63
197. Folgado-Dorado C, Caracena-De La Corte J, Aguilera-Velázquez JR, Santana-Villalona R, Rivera-Ramos A, Carbonero-Aguilar MP. et al. Isolation and Purification of Fungal β-Glucan as an Immunotherapy Strategy for GBM. J Vis Exp. 2023(196):e64924
198. Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6(3):317-333
199. Liu X, Xie J, Jia S, Huang L, Wang Z, Li C, Xie M. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264. 7. Int J Biol Macromol. 2017;98:576-581
200. Jatyan R, Singh P, Sahel DK, Karthik Y, Mittal A, Chitkara D. Polymeric and small molecule-conjugates of temozolomide as improved therapeutic agents for GBM multiforme. J Control Release. 2022;350:494-513
201. Nirmala MJ, Kizhuveetil U, Johnson A, Nagarajan R, Muthuvijayan V. Cancer nanomedicine: a review of nano-therapeutics and challenges ahead. RSC advances. 2023;13(13):8606-8629
202. Shayegh F, Türk Z, Armani A, Zarghami N. New insights into polysaccharide-based nanostructured delivery systems in breast cancer: Possible application of antisense oligonucleotides in breast cancer therapy. Int J Biol Macromol. 2024;272:132890
203. Liu Y, Zhou Z, Feng Y, Zhao X-G, Vaidyanathan G, Zalutsky MR. et al. Gold nanostars: a novel platform for developing 211At-labeled agents for targeted alpha-particle therapy. Int J Nanomedicine. 2021;16:7297-7305
204. Erel-Akbaba G, Carvalho LA, Tian T, Zinter M, Akbaba H, Obeid PJ. et al. Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy. ACS nano. 2019;13(4):4028-4040
205. Miao T, Wang J, Zeng Y, Liu G, Chen X. Polysaccharide-based controlled release systems for therapeutics delivery and tissue engineering: from bench to bedside. Adv Sci (Weinh). 2018;5(4):1700513
206. Yuan C, Xing R, Cui J, Fan W, Li J, Yan X. Multistep desolvation as a fundamental principle governing peptide self-assembly through liquid-liquid phase separation. CCS Chemistry. 2024;6(1):255-265
207. Louros N, Schymkowitz J, Rousseau F. Mechanisms and pathology of protein misfolding and aggregation. Nat Rev Mol Cell Biol. 2023;24(12):912-933
208. Patil YP, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014;177:8-18
209. Yoo J, Park C, Yi G, Lee D, Koo H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers. 2019;11(5):640
210. Mattheolabakis G, Rigas B, Constantinides PP. Nanodelivery strategies in cancer chemotherapy: biological rationale and pharmaceutical perspectives. Nanomedicine. 2012;7(10):1577-1590
211. Perrin SL, Samuel MS, Koszyca B, Brown MP, Ebert LM, Oksdath M. et al. GBM heterogeneity and the tumour microenvironment: implications for preclinical research and development of new treatments. Biochem Soc Trans. 2019;47(2):625-638
212. Taiariol L, Chaix C, Farre C, Moreau E. Click and bioorthogonal chemistry: the future of active targeting of Nanoparticles for nanomedicines? Chem Rev. 2021;122(1):340-384
213. Johnsen KB, Bak M, Melander F, Thomsen MS, Burkhart A, Kempen PJ. et al. Modulating the antibody density changes the uptake and transport at the blood-brain barrier of both transferrin receptor-targeted gold Nanoparticles and liposomal cargo. J Control Release. 2019;295:237-249
214. Shabaninejad Z, Pourhanifeh MH, Movahedpour A, Mottaghi R, Nickdasti A, Mortezapour E. et al. Therapeutic potentials of curcumin in the treatment of glioblstoma. Eur J Med Chem. 2020;188:112040
215. Sood A, Gupta A, Agrawal G. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydr Polym Tech. 2021;2:100067
216. Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12(3):267-277
217. Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X. Anti-GBM efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL Nanoparticles. Biomaterials. 2012;33(32):8167-8176
Author contact
![]() Corresponding author: E-mail: tzeng92com.
Corresponding author: E-mail: tzeng92com.
 Global reach, higher impact
Global reach, higher impact