13.3
Impact Factor
Theranostics 2018; 8(9):2565-2582. doi:10.7150/thno.22878 This issue Cite
Research Paper
MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress.
1. Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital and Institutes of Biomedical Science, Fudan University, Shanghai 200032, China
2. Department of Cardiology, East Hospital, Tongji University School of Medicine, Shanghai 200120, China
3. Department of kinesiology, Institute of physical education, Shanghai Normal University, Shanghai 200234, China
* equal contribution
Abstract
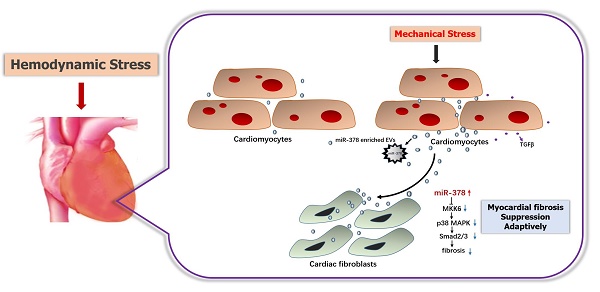
Rationale: Excessive myocardial fibrosis is the main pathological process in the development of cardiac remodeling and heart failure; therefore, it is important to prevent excessive myocardial fibrosis. We determined that microRNA-378 (miR-378) is cardiac-enriched and highly repressed during cardiac remodeling. We therefore proposed that miR-378 has a critical role in regulation of cardiac fibrosis, and examined the effects of miR-378 on cardiac fibrosis after mechanical stress.
Methods: Mechanical stress was respectively imposed on mice through a transverse aortic constriction (TAC) procedure and on cardiac fibroblasts by stretching silicon dishes. A chemically modified miR-378 mimic (Agomir) or an inhibitor (Antagomir) was administrated to mice by intravenous injection and to cells by direct addition to the culture medium. MiR-378 knockout mouse was constructed. Cardiac fibroblasts were cultured in the conditioned media from the cardiomyocytes with either miR-378 depletion or treatment with sphingomyelinase inhibitor GW4869. Quantitative real-time polymerase chain reaction analysis of gene and miRNA expression, Western blot analysis, immunochemistry and electron microscopy were performed to elucidate the mechanisms.
Results: Mechanical stress induced significant increases in fibrotic responses, including myocardial fibrosis, fibroblast hyperplasia, and protein and gene expression of collagen and matrix metalloproteinases (MMPs) both in vivo and in vitro. All these fibrotic responses were attenuated by treatment with a chemically modified miR-378 mimic (Agomir) but were exaggerated by treatment with an inhibitor (Antagomir). MiR-378 knockout mouse models exhibited aggravated cardiac fibrosis after TAC. Media from the cardiomyocytes with either miR-378 depletion or treatment with sphingomyelinase inhibitor GW4869 enhanced the fibrotic responses of stimulated cardiac fibroblasts, confirming that miR-378 inhibits fibrosis in an extracellular vesicles-dependent secretory manner. Mechanistically, the miR-378-induced anti-fibrotic effects manifested partially through the suppression of p38 MAP kinase phosphorylation by targeting MKK6 in cardiac fibroblasts.
Conclusions: miR-378 is secreted from cardiomyocytes following mechanical stress and acts as an inhibitor of excessive cardiac fibrosis through a paracrine mechanism.
Keywords: mechanical overload, cardiac fibrosis, microRNA-378, extracellular vesicles, p38, MKK6
 Global reach, higher impact
Global reach, higher impact