13.3
Impact Factor
Theranostics 2020; 10(25):11637-11655. doi:10.7150/thno.48064 This issue Cite
Research Paper
ROS production and mitochondrial dysfunction driven by PU.1-regulated NOX4-p22phox activation in Aβ-induced retinal pigment epithelial cell injury
1. Department of Ophthalmology, Shanghai General Hospital (Shanghai First People's Hospital), Shanghai Jiao Tong University School of Medicine, Shanghai, China.
2. National Clinical Research Center for Ophthalmic Diseases, Shanghai, China.
3. Shanghai Engineering Center for Visual Science and Photomedicine, Shanghai, China.
4. Center for Advanced Vision Science, University of Virginia School of Medicine, Charlottesville, VA, USA.
5. Department of Ophthalmology, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
6. Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida, USA.
7. Department of Dermatology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
8. Shanghai Key Laboratory of Fundus Diseases, Shanghai, China.
9. Shanghai Engineering Center for precise diagnosis and treatment of eye disease.
10. Geriatric Institute of Chinese Medicine, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
11. Department of Pathology, Yale University School of Medicine, New Haven, CT, USA.
#These authors contributed equally to this paper.
Abstract
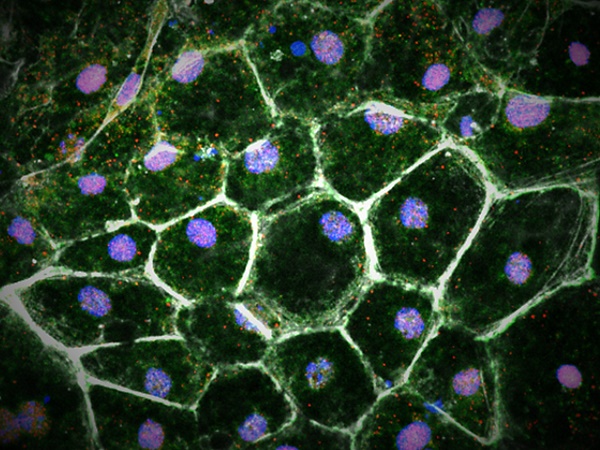
Rationale: Amyloid β (Aβ) deposition, an essential pathological process in age-related macular degeneration (AMD), causes retinal pigment epithelium (RPE) degeneration driven mostly by oxidative stress. However, despite intense investigations, the extent to which overoxidation contributes to Aβ-mediated RPE damage and its potential mechanism has not been fully elucidated.
Methods: We performed tandem mass-tagged (TMT) mass spectrometry (MS) and bioinformatic analysis of the RPE-choroid complex in an Aβ1-40-induced mouse model of retinal degeneration to obtain a comprehensive proteomic profile. Key regulators in this model were confirmed by reactive oxygen species (ROS) detection, mitochondrial ROS assay, oxygen consumption rate (OCR) measurement, gene knockout experiment, chromatin immunoprecipitation (ChIP), and luciferase assay.
Results: A total of 4243 proteins were identified, 1069 of which were significantly affected by Aβ1-40 and found to be enriched in oxidation-related pathways by bioinformatic analysis. Moreover, NADPH oxidases were identified as hub proteins in Aβ1-40-mediated oxidative stress, as evidenced by mitochondrial dysfunction and reactive oxygen species overproduction. By motif and binding site analyses, we found that the transcription factor PU.1/Spi1 acted as a master regulator of the activation of NADPH oxidases, especially the NOX4-p22phox complex. Also, PU.1 silencing impeded RPE oxidative stress and mitochondrial dysfunction and rescued the retinal structure and function.
Conclusion: Our study suggests that PU.1 is a novel therapeutic target for AMD, and the regulation of PU.1 expression represents a potentially novel approach against excessive oxidative stress in Aβ-driven RPE injury.
Keywords: retinal pigment epithelial cells, amyloid β, age-related macular degeneration, PU.1, NADPH oxidases
 Global reach, higher impact
Global reach, higher impact