13.3
Impact Factor
Theranostics 2022; 12(7):3007-3023. doi:10.7150/thno.71815 This issue Cite
Research Paper
Time-restricted feeding modulates the DNA methylation landscape, attenuates hallmark neuropathology and cognitive impairment in a mouse model of vascular dementia
1. Memory Aging and Cognition Centre, Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
2. Integrative Sciences and Engineering Programme, NUS Graduate School, National University of Singapore.
3. Cardiovascular Research Institute, National University Health System, Singapore.
4. Cardiovascular Translational Research Programme, National University of Singapore, Singapore.
5. Genome Institute of Singapore, Agency for Science, Technology and Research, Singapore.
6. Department of Biochemistry, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
7. Healthy Longevity Translational Research Program, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
8. Centre for Healthy Longevity, National University Health System (NUHS), Singapore.
9. Department of Psychological Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
10. Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore.
11. School of Pharmacy, Sungkyunkwan University, Suwon, Republic of Korea.
12. Centre for Cardiovascular Biology and Disease Research, Department of Microbiology, Anatomy, Physiology and Pharmacology, School of Agriculture, Biomedicine and Environment, La Trobe University, La Trobe University, Bundoora, VIC, Australia.
Abstract
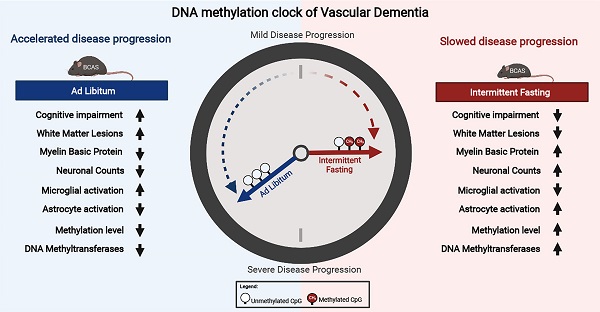
Objective: Vascular dementia (VaD) is the second most common cause of dementia worldwide. The increasing contribution of lifestyle-associated risk factors to VaD has pointed towards gene-environment interactions (i.e. epigenetics). This study thus aims to investigate the DNA methylation landscape in a chronic cerebral hypoperfusion (CCH) mouse model of VaD. As a nexus between the gene-environment interaction, intermittent fasting (IF) was introduced as a prophylactic intervention.
Methods: Bilateral common carotid artery stenosis (BCAS) was used to induce CCH by placing micro-coils of 0.18 mm in each common carotid artery of the mice. The coils were left in the mice for 7, 15 and 30 days to study temporal differences. IF was introduced for 16 h daily for 4 months prior to BCAS. Reduced Representation Bisulfite Sequencing (RRBS) was used to study the DNA methylation landscape. Cognitive impairment was measured using Barnes Maze Test. White matter lesions (WML) and neuronal loss were measured using Luxol fast blue staining and cresyl violet staining respectively.
Results: IF mice subjected to CCH displayed significantly better cognitive learning ability and memory, improved neuropathological alterations with reduced WMLs and neuronal loss. Modulation of DNA methylation patterns in the cortex of AL CCH mice was re-modelled and signs of reversal was observed in IF CCH mice across all three timepoints.
Conclusions: These findings provide an understanding of how IF may protect the brain against damage caused by CCH and show promise in offering potential beneficial effects in mitigating the neuropathology and cognitive deficits in VaD.
Keywords: Intermittent Fasting, DNA Methylation, White Matter Lesion, Chronic Cerebral Hypoperfusion, Vascular Cognitive Impairment, Vascular Dementia
 Global reach, higher impact
Global reach, higher impact