13.3
Impact Factor
Theranostics 2022; 12(18):7788-7803. doi:10.7150/thno.75037 This issue Cite
Research Paper
A Novel Her2/VEGFR2/CD3 trispecific antibody with an optimal structural design showed improved T-cell-redirecting antitumor efficacy
1. State Key Laboratory of Chemical Oncogenomics, Guangdong Provincial Key Laboratory of Chemical Genomics, Peking University Shenzhen Graduate School, Shenzhen, Guangdong, 518055, China.
2. Lunan Pharmaceutical Group Co., Ltd, Feixian County, Shandong, 273400, China.
3. National Engineering Laboratory of High Level Expression in Mammalian Cells, Feixian County, Shandong, 273400, China.
4. Institute of Neurological and Psychiatric Disorders, Shenzhen Bay Laboratory, Shenzhen, Guangdong, 518132, China.
5. Key Laboratory of Protein and Peptide Pharmaceuticals, Beijing Translational Center for Biopharmaceuticals Institute of Biophysics, Chinese Academy of Sciences Beijing 100101, China.
6. Institute of Chemical Biology, Shenzhen Bay Laboratory, Shenzhen, 518132, China.
*These authors contributed equally to this work.
Abstract
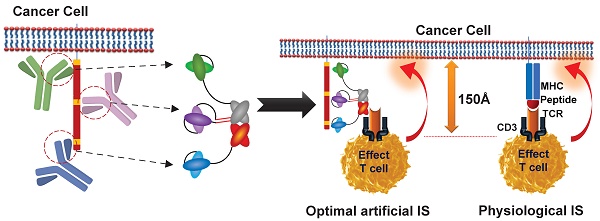
Rationale: T-cell-redirecting bispecific antibodies (bsAbs) and trispecific antibodies (tsAbs) designed to recognize different epitopes or antigens have emerged as promising cancer therapies. Current approaches are all designed to include another antibody specific to the site of the primary antibody, and the molecular structures are generally established. However, the dimensions of target molecule and epitope location play a key role in the efficiency of the immunological synapse (IS) formation and subsequent T-cell-redirecting activities, therefore the connection flexibility of these antibodies determines the geometries of different formats of these molecules and will have a major impact on the efficacy.
Methods: We describe a novel recombination strategy using various linker designs to site-specifically fuse anti-Her2 (2Rs15) or anti-VEGFR2 (3VGR19) nanobodies to different positions of the anti-CD3 antibody fragment (Fab, SP34). Based on the comparison among the various antigen-specific bsAbs, we could determine the desired fusion site of each nanobody to SP34, and further ensure the optimal structure of tsAbs with synergistic dual-antigen enhanced T-cell-redirecting activities.
Results: This approach allows precise control of the formation of IS between Her2- and/or VEGFR2-expressing cancer cells and T cells, to obtain the optimal structure of the Her2/VEGFR2/CD3 tsAb without the need to map antibody-binding epitopes. Optimization of Her2/VEGFR2/CD3 tsAb results in enhanced T-cell-redirecting in vitro and in vivo antitumor efficacy compared with the corresponding bsAbs alone or in combination, and the potency to overcome tumor relapse due to antigen escape or resistance to Herceptin and Cyramza therapy.
Conclusion: The novel design strategy for developing tsAbs using a site-specific recombination approach represents a promising platform for immuno-oncology and in applications other than cancer therapy.
Keywords: Trispecific Antibodies, Protein Engineering, Site-specific Recombination, Immunological Synapse, Therapy Resistance
 Global reach, higher impact
Global reach, higher impact