13.3
Impact Factor
Theranostics 2023; 13(5):1520-1544. doi:10.7150/thno.80091 This issue Cite
Review
Strategies targeting PD-L1 expression and associated opportunities for cancer combination therapy
1. Institute of Pharmaceutical Innovation, School of Medicine, Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Wuhan University of Science and Technology, Wuhan, Hubei, China.
2. State Key Laboratory of Biogeology and Environmental Geology, Faculty of Materials Science and Chemistry, China University of Geosciences, Wuhan 430074, China.
3. Key Laboratory for Green Chemical Process of Ministry of Education, School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan 430205, China.
4. Wuhan Asia Heart Hospital, Wuhan University of Science and Technology, Wuhan, Hubei, China.
5. Department of Pathology, Medical College, Wuhan University of Science and Technology, Wuhan, Hubei, China.
# These authors contributed equally
Abstract
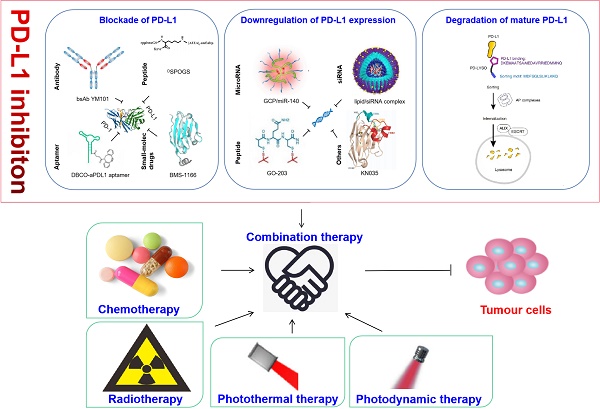
Immunotherapy has achieved great success recently and opened a new avenue for anti-tumor treatment. Programmed cell death 1/programmed cell death ligand 1 (PD-1/PD-L1) are typical immune checkpoints that transmit coinhibitory signals, muting the host immunity. Monoclonal antibodies that block PD-1/PD-L1 axis have benefited many patients with different tumor diseases. However, the objective response rate is still unsatisfactory. In this review, we summarize three strategies targeting PD-L1 based on different forms of PD-L1 and various regulating mechanisms to enhance the therapeutic effect, including blockade of the interaction between PD-L1 and PD-1, downregulation of PD-L1 expression and degradation of mature PD-L1. Thereinto, we describe a variety of materials have been designed to target PD-L1, including antibodies, nanoparticle, peptide, aptamer, RNA, and small molecule. Additionally, we list the drugs with PD-L1 regulation capacity used in clinical and ongoing studies to explore other alternatives for targeting PD-L1 besides anti-PD-L1 monoclonal antibodies. Moreover, we discuss associated opportunities for cancer combination therapy with other modalities such as chemotherapy, radiotherapy, photodynamic therapy (PDT) and photothermal therapy (PTT), as these conventional or emerging modalities are capable of increasing the immune response of tumor cells by altering the tumor microenvironment (TME), and would display synergistic effect. At last, we give a brief summary and outlook regarding the research status and future prospect of immunotherapy.
Keywords: PD-L1, immune checkpoint blockade, targeted therapy, immunotherapy, combination therapy
 Global reach, higher impact
Global reach, higher impact