13.3
Impact Factor
Theranostics 2024; 14(1):283-303. doi:10.7150/thno.90370 This issue Cite
Research Paper
Neutrophil-like pH-responsive pro-efferocytic nanoparticles improve neurological recovery by promoting erythrophagocytosis after intracerebral hemorrhage
1. Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Zhejiang University, Hangzhou, 310016, China.
2. Key Laboratory of Precise Treatment and Clinical Translational Research of Neurological Diseases, Hangzhou, 310016, China.
3. MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027, China.
# Linfeng Fan, Lulu Jin and Tianchi Tang contributed equally to this work.
Abstract
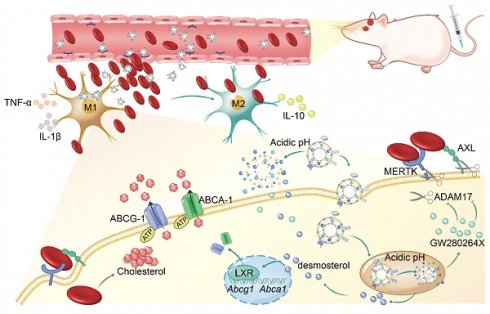
Rationale: Intracerebral hemorrhage (ICH) is a devastating cerebrovascular disease resulting from blood extravasating into the brain parenchyma. Escalation of erythrophagocytosis (a form of efferocytosis), avoiding the consequent release of the detrimental erythrocyte lysates, may be a promising target of ICH management. The ADAM17 inhibitor and liver X receptor (LXR) agonist could promote efficient efferocytosis and injury repair. Nevertheless, the poor bioavailability and restriction of the blood-brain barrier (BBB) hinder their application. Therefore, it is needed that biocompatible and smart nanoplatforms were designed and synthesized to realize effective therapy targeting erythrophagocytosis.
Methods: We first assessed the synergistic effect of therapeutic GW280264X (an ADAM17 inhibitor) and desmosterol (an LXR agonist) on erythrophagocytosis in vitro. Then a pH-responsive neutrophil membrane-based nanoplatform (NPEOz) served as a carrier to accurately deliver therapeutic GW280264X and desmosterol to the damaged brain was prepared via co-extrusion. Afterwards, their pH-responsive performance was valued in vitro and targeting ability was assessed through fluorescence image in vivo. Finally, the pro-erythrophagocytic and anti-neuroinflammatory ability of the nanomedicine and related mechanisms were investigated.
Results: After the synergistical effect of the above two drugs on erythrophagocytosis was confirmed, we successfully developed neutrophil-disguised pH-responsive nanoparticles to efficiently co-deliver them. The nanoparticles could responsively release therapeutic agents under acidic environments, and elicit favorable biocompatibility and ability of targeting injury sites. D&G@NPEOz nanoparticles enhanced erythrophagocytosis through inhibiting shedding of the efferocytotic receptors MERTK/AXL mediated by ADAM17 and accelerating ABCA-1/ABCG-1-mediated cholesterol efflux regulated by LXR respectively. In addition, the nano-formulation was able to modulate the inflammatory microenvironment by transforming efferocytes towards a therapeutic phenotype with reducing the release of proinflammatory cytokines while increasing the secretion of anti-inflammatory factors, and improve neurological function.
Conclusions: This biomimetic nanomedicine is envisaged to offer an encouraging strategy to effectively promote hematoma and inflammation resolution, consequently alleviate ICH progression.
Keywords: intracranial hemorrhage, erythrophagocytosis, pH-responsive, neutrophil-like nanoparticles, neurological recovery
 Global reach, higher impact
Global reach, higher impact