13.3
Impact Factor
Theranostics 2024; 14(5):1841-1859. doi:10.7150/thno.89703 This issue Cite
Research Paper
Dual regulation of NEMO by Nrf2 and miR-125a inhibits ferroptosis and protects liver from endoplasmic reticulum stress-induced injury
1. College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea.
2. Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine, Seoul 03080, Republic of Korea.
3. Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
4. College of Pharmacy and Integrated Research Institute for Drug Development, Dongguk University-Seoul, Goyang-si, Kyeonggi-do 10326, Republic of Korea.
Abstract
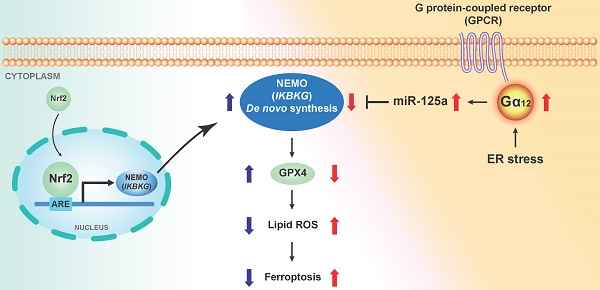
Rationale: The surge of severe liver damage underscores the necessity for identifying new targets and therapeutic agents. Endoplasmic reticulum (ER) stress induces ferroptosis with Gα12 overexpression. NF-κB essential modulator (NEMO) is a regulator of inflammation and necroptosis. Nonetheless, the regulatory basis of NEMO de novo synthesis and its impact on hepatocyte ferroptosis need to be established. This study investigated whether Nrf2 transcriptionally induces IKBKG (the NEMO gene) for ferroptosis inhibition and, if so, how NEMO induction protects hepatocytes against ER stress-induced ferroptosis.
Methods: Experiments were conducted using human liver tissues, hepatocytes, and injury models, incorporating NEMO overexpression and Gα12 gene modulations. RNA sequencing, immunoblotting, immunohistochemistry, reporter assays, and mutation analyses were done.
Results: NEMO downregulation connects closely to ER and oxidative stress, worsening liver damage via hepatocyte ferroptosis. NEMO overexpression protects hepatocytes from ferroptosis by promoting glutathione peroxidase 4 (GPX4) expression. This protective role extends to oxidative and ER stress. Similar shifts occur in nuclear factor erythroid-2-related factor-2 (Nrf2) expression alongside NEMO changes. Nrf2 is newly identified as an IKBKG (NEMO gene) transactivator. Gα12 changes, apart from Nrf2, impact NEMO expression, pointing to post-transcriptional control. Gα12 reduction lowers miR-125a, an inhibitor of NEMO, while overexpression has the opposite effect. NEMO also counters ER stress, which triggers Gα12 overexpression. Gα12's significance in NEMO-dependent hepatocyte survival is confirmed via ROCK1 inhibition, a Gα12 downstream kinase, and miR-125a. The verified alterations or associations within the targeted entities are validated in human liver specimens and datasets originating from livers subjected to exposure to other injurious agents.
Conclusions: Hepatic injury prompted by ER stress leads to the suppression of NEMO, thereby facilitating ferroptosis through the inhibition of GPX4. IKBKG is transactivated by Nrf2 against Gα12 overexpression responsible for the increase of miR-125a, an unprecedented NEMO inhibitor, resulting in GPX4 induction. Accordingly, the induction of NEMO mitigates ferroptotic liver injury.
Keywords: NEMO, Nrf2, miR-125a, Acute liver injury, Ferroptosis
 Global reach, higher impact
Global reach, higher impact